Newly established stem cell transplant program: 100 days follow-up of patients and its comparison with published Indian literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 168-173
DOI: DOI: 10.4103/0971-5851.190362
Abstract
Background: Hematopoietic progenitor stem cell transplantation (HPSCT) is used as a standard treatment option to improve outcome in hematological and nonhematological disorders. It is important for new HPSCT program to look at its patient outcome data and compare it with the published data to evaluate the efficacy of program. Aims: The aim was to compile and collate the patient outcome data of HPSCT and compare with published reports. Materials and Methods: Patient demographics, indications, stem cell harvest by apheresis, dose collected, infusion, engraftment, and follow-up data were collected from hospital information system from 2010 to 2013 in a tertiary care hospital. HPSCs were mobilized with granulocyte colony-stimulating factor, and harvests were done on the 5th day. Engraftment was decided for neutrophil when counts were 0.5 × 109/L and for platelets when counts were 20 × 109/L on two consecutive days without any transfusion support. Results: There were 133 harvests for 95 patients with various disorders; multiple myeloma was most common in autologous and acute lymphoblastic leukemia in allogeneic group. One hundred harvests were done for autologous and 33 for allogeneic HPSCT. In autologous group, of 66 patients, 60 (90.9%) received stem cell infusion at median dose of 4.63 × 106 CD34+ cells/kg. Similarly, in allogeneic group, of 29 patients, 27 (93.10%) received infusion at median dose of 5.8 × 106 CD34+ cells/kg. 58 (96.9%) patients and 25 (92.6%) engrafted in autologous and allogeneic group, respectively. The median time for neutrophils engraftment was 11 days in autologous group and 12 days in allogeneic group. The median time for platelet engraftment was 11.5 days in autologous group and 13 days in allogeneic group. The 100-day survival rate was 95% n = 57) in autologous group and 77.8% n = 21) in allogeneic group. Conclusion: This data analysis shows reasonably good results of HPSCTs with majority of patients surviving at 100-day follow-up.
Keywords
Follow-up - hematopoietic progenitor stem cell transplants - mortality - peripheral blood stem cells - stem cell transplant program - survival - transplantPublication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Hematopoietic progenitor stem cell transplantation (HPSCT) is used as a standard treatment option to improve outcome in hematological and nonhematological disorders. It is important for new HPSCT program to look at its patient outcome data and compare it with the published data to evaluate the efficacy of program.
Aims:
The aim was to compile and collate the patient outcome data of HPSCT and compare with published reports.
Materials and Methods:
Patient demographics, indications, stem cell harvest by apheresis, dose collected, infusion, engraftment, and follow-up data were collected from hospital information system from 2010 to 2013 in a tertiary care hospital. HPSCs were mobilized with granulocyte colony-stimulating factor, and harvests were done on the 5th day. Engraftment was decided for neutrophil when counts were 0.5 × 109/L and for platelets when counts were 20 × 109/L on two consecutive days without any transfusion support.
Results:
There were 133 harvests for 95 patients with various disorders; multiple myeloma was most common in autologous and acute lymphoblastic leukemia in allogeneic group. One hundred harvests were done for autologous and 33 for allogeneic HPSCT. In autologous group, of 66 patients, 60 (90.9%) received stem cell infusion at median dose of 4.63 × 106 CD34+ cells/kg. Similarly, in allogeneic group, of 29 patients, 27 (93.10%) received infusion at median dose of 5.8 × 106 CD34+ cells/kg. 58 (96.9%) patients and 25 (92.6%) engrafted in autologous and allogeneic group, respectively. The median time for neutrophils engraftment was 11 days in autologous group and 12 days in allogeneic group. The median time for platelet engraftment was 11.5 days in autologous group and 13 days in allogeneic group. The 100-day survival rate was 95% (n = 57) in autologous group and 77.8% (n = 21) in allogeneic group.
Conclusion:
This data analysis shows reasonably good results of HPSCTs with majority of patients surviving at 100-day follow-up.
INTRODUCTION
Hematopoietic progenitor stem cell transplants (HPSCTs) are an established therapy to treat several benign and malignant disorders, with hematological malignancies being the most common indications for HPSCT.[1,2,3,4,5] High-dose chemotherapy along with autologous HPSCT results in better survival rates and remission as compared with “only” standard multi-agent chemotherapy in multiple myeloma (MM).[6] In other hematological conditions such as acute myeloid leukemia (AML), acute lymphoid leukemia, and Hodgkin's lymphoma requiring allogeneic HPSCT, such transplants were earlier considered as the second line of treatment.[7,8,9] However, over the last few years, use of allogeneic HPSCT has become the first line of treatment for these disorders. Many studies on allogeneic HPSCT have recognized the advantages of the transplant procedure in these disorders.[9,10,11,12,13,14,15,16,17]
As per published data in JAMA 2006,[18] 50,417 transplants were done all over the world comprised 57% of autologous and 43% of allogeneic transplants while in India, only 1540 transplants were done from October 1986 to December 2006 in nine centers across the country, with an average of around 77 transplants per year which works out to be miniscule (0.002%) portion of global transplants pie.[19] Since then, HPSCT has increased several folds with obvious increase in numbers of transplant centers in India as evident in several published reports from India.[19,20,21,22,23,24] We would like to report a single-center experience of patient outcome in autologous and allogeneic transplants including but not limited to 30 and 100 days patient follow-up data. These results have been compared with published data from India.
MATERIALS AND METHODS
New hematopoietic progenitor stem cell transplantation program
In 2010, the HPSCT program was started at 1000-bedded tertiary care hospital with high efficiency particulate air (HEPA)- filter rooms. The institute organized trained personnel, systems, processes, and all ancillary services for initiating HPSCT program.
Data collection
The data were retrieved from hospital information system (HIS) for the 3-year period, 2010–2013. These data included patient and donor epidemiological data, disease condition, chemotherapy regimen, mobilization, harvest data, dose collected, cryopreservation if any, infusion data, engraftment, and follow-up. Data for the patients who succumbed to their illness before stem cell infusion were also collected.
Chemotherapy
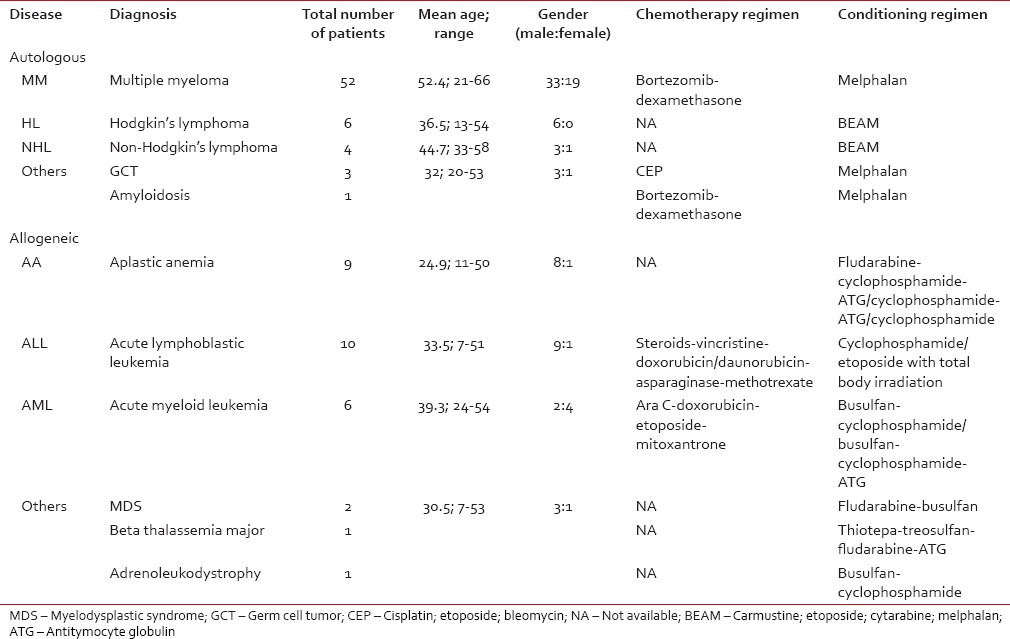
Chemotherapy and conditioning regimens were given to patients of both groups [Table 1].
Table 1
Chemotherapy and conditioning regimens followed at our center
Donor selection (patient-donor and allogeneic donor)
In autologous patients, the HPSC harvest was done when the patient-donor was in complete remission (minimal residual disease of < 0.01% for acute lymphoblastic leukemia [ALL] and < 0.1% for AML performed on three-color flow cytometry; FACSCalibur, Becton Dickinson, Heidelberg, Germany).[25] In allogeneic HPSCT, donor selection was based on human leukocyte antigen (HLA) typing and donor with 6/6 match (HLA A, HLA B, and DRB1) for matched siblings and 10/10 for unmatched unrelated donors (HLA A, B, C, DRB1, and DQB1). All donors underwent mandatory test for infectious disease markers (anti-HIV, anti-hepatitis C virus, anti-hepatitis B core [total], anti-cytomegalovirus [CMV], hepatitis B surface antigen, tests for syphilis and malaria, and CMV polymerase chain reaction). Patient-donors (autologous) and patients (allogeneic) were screened and cleared for transplant after normal liver function tests (LFTs), renal function tests (RFTs), echocardiography, pulmonary function tests, ENT, and dental and psychiatric evaluation. ABO and Rh blood group was done for both donors and recipients.
Mobilization and harvest
Mobilization was done using granulocyte colony-stimulating factor at a dose of 10 µg/kg body weight in two divided doses given subcutaneously for 4 days and in a single dose on the 5th day before harvest.[26] Harvest was carried out on the 5th day. An 18-gauge double-lumen hemodialysis type intravenous catheter in jugular or femoral vein was used for venous access in adults (8/11 F double-lumen in children below 12 years). All procedures were done by apheresis machine (COM.TEC® Fresenius Kabi, Germany). The P1YA kit was used, and the collection program was set to auto-mononuclear cells. A dose of 5–6 million CD34+ cells/kg body weight [27,28] was targeted with minimum of 2 million CD34+ cells/kg body weight for both groups. A dose of < 2 million CD34+ cells/kg body weight was considered as “inadequate dose.”
Enumeration
The CD34+ cells enumeration was done on FACSCalibur. CD34+ count was done before harvest (to enter the precount value of stem cell in apheresis machine) and for the product (to know the final dose in collection bag). The enumeration was done as per the standard International Society of Haematology and Graft Engineering protocol.[29]
Cryopreservation
In some cases, HPSCs were harvested and cryopreserved. For cryopreservation procedure, volume depletion of product was done by removing red blood cells and plasma. In 100 ml bag, 50 ml of product was mixed with 50 ml cocktail of saline, albumin dimethyl sulfoxide, and dextran-40. Storage was done in liquid nitrogen (−196°C).[30,31]
Stem cell infusion
The HPSCT infusion was carried out in rooms with high-efficiency particulate air (HEPA) filters under positive pressure. The infusion was done through a central line (Hickman), and all the patients were prophylactically hydrated and given antihistamines before infusion. They were monitored for blood pressure, pulse, respiratory rate, and oxygen saturation during the procedure. Reverse barrier nursing was practiced according to the institutional protocol. Patient monitoring was done for critical parameters at defined frequency (complete blood counts and electrolytes once a day; LFT, RFT, and blood glucose twice a week; blood culture as and when deemed necessary). Antimicrobial prophylaxis included levofloxacin, fluconazole, and acyclovir.
Engraftment
Neutrophil engraftment was defined as absolute neutrophil count of 500 cells/mm3 (0.5 × 109/L) or greater for two consecutive days. Platelet engraftment was defined by the achievement of a continued platelet count of 20,000/mm3 (20 × 109/L) or greater for two consecutive days, at least 7 days from the last platelet infusion.[32] Those who did not engraft up till the 28th day were considered as “engraftment failure.”[32] Standard protocols were followed in deciding blood component transfusion for all ABO-compatible transplants and major, minor, and bidirectional ABO-incompatible transplants.[33] The threshold for initiating RBC transfusion was hemoglobin of 7 g/dl and 10,000/µl platelet transfusions. After successful engraftment, patients were shifted to non-HEPA rooms and discharged once they were clinically stable.
Follow-up
Patients were followed up after discharge daily for 1 week, every alternate day for 2 weeks, then weekly till posttransplant day 100. Mortality data of all patients at 30 and 100 days were also collected. The follow-up included engraftment failure, complications, transplant-related death, and all-causes death.
Statistical analysis
The data were censored on December 31, 2013. Data were analyzed and mean, median, and range were calculated using Microsoft Excel software and SPSS Statistical Package for the Social Sciences version 20 (SPSS Inc., Chicago, IL, USA).
Ethical approval
No permission from the Ethics Committee was required since the analysis was limited to retrospective HIS data. Personal identifiers such as name were not used.
RESULTS
There were 133 harvests for 95 patients (autologous: 66, allogeneic: 29) with various disorders; MM was most common in autologous and ALL in allogeneic group [Table 1]. There were 67 men and 28 women, with the mean age of 40 years. Chemotherapy and conditioning regimens were used as per the patient's disease condition [Table 1].
In autologous group, 54 (81.8%) patient-donors required only one apheresis session while 12 (18.2%) patient-donors required more than one apheresis session (mean = 1.52). In allogeneic group, 26 (89.6%) donors required one session and three (10.4%) required two or more apheresis sessions (mean = 1.14) to obtain adequate dose.
Table 2
Comparison of present study with other published Indian literature
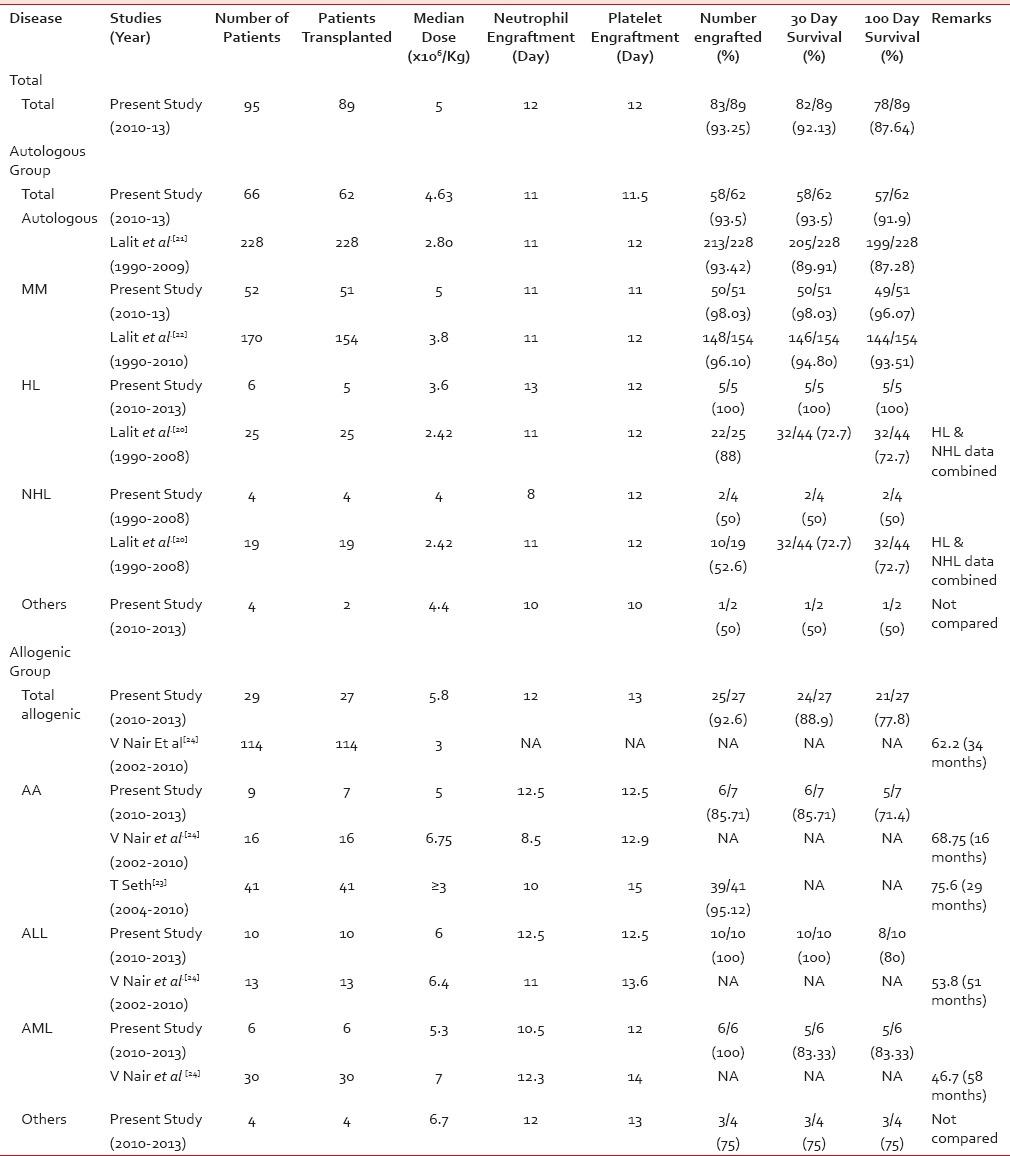 In 62 patients who were infused in the autologous group, 58 (93.5%) engrafted while four (6.5%) had engraftment failure. Similarly, in allogeneic group, of 27 patients, 25 (92.6%) engrafted and 2 (7.40%) had engraftment failure [Table 2].
In 62 patients who were infused in the autologous group, 58 (93.5%) engrafted while four (6.5%) had engraftment failure. Similarly, in allogeneic group, of 27 patients, 25 (92.6%) engrafted and 2 (7.40%) had engraftment failure [Table 2].The median time for neutrophil and platelet engraftment in autologous group was 11 and 11.5 days, respectively, while in allogeneic group was 12 and 13 days, respectively [Table 2].
The 30-day disease-free survival was 93.5% (n = 58) in autologous HPSCT and 88.9% (n = 24) in allogeneic HPSCT group. The 100-day survival rate was 91.9% (n = 57) in autologous group and 77.8% (n = 21) in allogeneic group [Table 2].
DISCUSSION
This was a retrospective study done in single center to find the patient survival outcomes in recipients of autologous and allogeneic HPSCTs. In this study, mean age of the patients was 40 years, and the indications for which HPSCT was done in this study follow the pattern of other transplants studies from India.[20,21,22,23,24] This study also acknowledges the fact that a few patients (4 in autologous and 2 in allogeneic group) succumb to their disease even before they are transplanted (before infusion). This brings forth the fact that these patients with hematological conditions have quite an aggressive disease and the disease per se, the chemotherapy, or waiting for the transplant (during conditioning) or a combination of these can take a toll. This is also supported by other published report.[21]
The median dose of 4.63 × 106 CD34+ cells/kg of recipient body weight in autologous group and 5.8 × 106 CD34+ cells/kg in allogeneic group came in mean 1.52 and 1.14 harvests per donor, respectively. This was due to improved collection efficiency of harvest procedures in this study. Mean of 1.52 procedures in autologous group is lower than the mean procedures (2) as reported by Kumar et al. per patient to obtain CD34+ yield of 2.42 × 106 cells/kg of recipient body weight undergoing autologous blood stem cell transplantation.[22] The improved collection efficiency in the present study was possibly because of large-volume leukapheresis (mean of 18.9 L in autologous and 6.9 L in allogeneic group) and better CD34+ cell monitoring before and during harvest.
The median CD34+ dose of 5 million cells/kg in the present study resulted in early engraftment in majority (83/89) of the cases. The median time for neutrophil and platelet engraftment in autologous group was 11 and 11.5 days, respectively, which was similar to Kumar et al.[21] while median time for neutrophil and platelet engraftment in allogeneic group was 12 and 13 days, respectively, which was comparable to Nair et al. and Seth et al.[23,24] Four of 89 patients had engraftment failure.
The overall survival at day-30 and day-100 close to 90% shows that with physical infrastructure such as HEPA rooms, trained personnel, and multidisciplinary approach in a new transplant program can have good patient survival outcomes. Since this is a new HPSCT program, better patient selection and newer drug regimens would have contributed to relatively good results. Five patients succumbed after engraftment, one in autologous group because of cardiac arrest on day 45, four in allogeneic group because of veno-occlusive disease (one patient) on day 14 and graft-versus-host disease (three patients) between days 30 and 100. The cause of mortality is similar to the published report.[24]
In the autologous group, the patient survival in MM, HL, and NHL was similar to the publications by Kumar et al.[20,21,22] Likewise, in the allogeneic group, the patient survival in aplastic anemia (AA), AML, and ALL is comparable with Nair et al.[24] In addition, the patient survival in AA is in line with the publication of Seth et al.[23]
CONCLUSION
This data analysis shows reasonably good results of HPSCTs with majority of patients surviving at 30-day and 100-day follow-up. These results and its comparison with existing published reports reassure the robustness of the new HPSCT program and provide framework for designing patient counseling/education material and future outcome research.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

