Nested Stromal and Epithelial Tumor of the Liver: An Unusual Nonhepatocytic Entity
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(02): 235-237
DOI: DOI: 10.4103/ijmpo.ijmpo_58_20
Introduction
Nested stromal-epithelial tumor (NSET) of the liver is an extremely rare, nonhepatocytic, nonbiliary tumor of the liver. To the best of our knowledge, 41 cases have been reported in literature. We report the 42nd case. The reported tumors have been found predominantly in females and more commonly in children, and most arose from the right lobe of the liver. In a number of cases, an association between these tumors and Cushing syndrome has also been described. Here, we report NSET of the liver in a 39-year-old female patient. To the best of our knowledge, this case is the first to be reported from India to have undergone transarterial chemoembolization (TACE).
Publication History
Received: 12 February 2020
Accepted: 09 April 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Introduction
Nested stromal-epithelial tumor (NSET) of the liver is an extremely rare, nonhepatocytic, nonbiliary tumor of the liver. To the best of our knowledge, 41 cases have been reported in literature. We report the 42nd case. The reported tumors have been found predominantly in females and more commonly in children, and most arose from the right lobe of the liver. In a number of cases, an association between these tumors and Cushing syndrome has also been described. Here, we report NSET of the liver in a 39-year-old female patient. To the best of our knowledge, this case is the first to be reported from India to have undergone transarterial chemoembolization (TACE).
Case Presentation
A 39-year-old patient presented with dyspepsia, jaundice, and weight loss of 3 months’ duration. Liver function tests showed serum bilirubin of 3.6 mg/dL, aspartate aminotransferase (AST) of 127 IU/L, and alanine aminotransferase (ALT) of 58 IU/L. Alpha fetoprotein (AFP), carbohydrate antigen19.9 (CA 19-9), and carcinoembryonic antigen (CEA) were normal. Viral markers were nonreactive. Contrast-enhanced computed tomography (CECT) of the abdomen as shown in [Figure 1]a revealed a space-occupying lesion in the liver measuring 11.2 cm × 11.5 cm × 6.7 cm involving segment IVB, V, VI, and VII of the right lobe. Initial liver biopsy was suspicious of metastatic squamous cell carcinoma with neuroendocrine differentiation on routine H and E staining. The patient was evaluated in lines of metastasis of unknown origin; however, the primary could not be identified even with a fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET-CT).
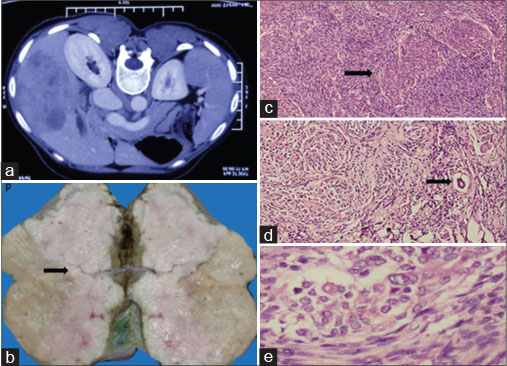
| Figure 1: (a) Computed tomography of the abdomen showing a large solitary, heterogeneous enhancing mass in segments IVB, V, VI, and VII of the liver, (b) gross appearance showing nodular gray-white tumor with attached gallbladder, (c) neoplastic cells arranged in organoid whorls surrounded by desmoplastic stroma shown by arrow (H and E, ×40), (d) proliferating bile ducts shown by arrow (H and E, ×100), (e) neoplastic cells with vesicular nuclei and inconspicuous nucleoli (H and E, ×400)
Question 1
What are the most common malignant causes of such a presentation?
Answer
Fibrolamellar hepatocellular carcinoma (HCC)HCCMetastasisHepatoblastoma.
In view of a single liver lesion, the patient underwent right hepatectomy and cholecystectomy. Histopathology showed neoplastic cells arranged in cords, nests, and whorls, with vesicular nuclei and eosinophilic nucleoli. Foci of squamous differentiation and dense fibrous stroma was noted with lymphocytic infiltrate as in [Figure 1]b, [Figure 1]c, [Figure 1]d, [Figure 1]e. Since the morphology was not suggestive of squamous cell carcinoma, immunohistochemistry (IHC) was done. It was positive for cytokeratin, vimentin, CD56, β-catenin, smooth muscle actin (SMA), Wilms tumor 1 (WT1), and CD68. Ki67 was 12%–15% [Figure 2].
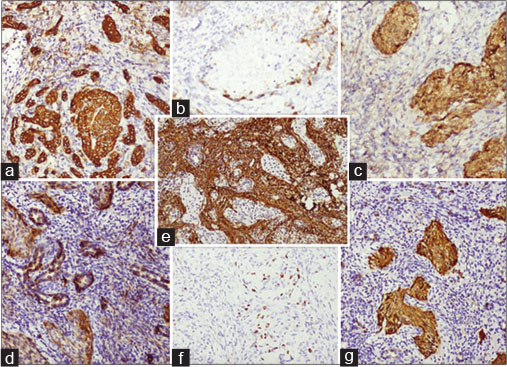
| Figure 2: (a) Positive immunostaining with pancytokeratin, (b) focal epithelial membrane antigen positivity, (c) nuclear and nonspecific cytoplasmic expression of β-catenin, (d) membranous staining with CD56, (e) stroma highlighted by smooth muscle actin, (f) low Ki67, (g) nuclear staining with p16
Question 2
What are the histologic differentials?
Answer
Desmoplastic small round cell tumor (DSRCT)Synovial sarcomaHepatoblastoma.
In view of the morphology and associated IHC, a diagnosis of “nested epithelial and stromal tumor of liver” was made.
Question 3
How common is NSET of the liver?
Answer
NSET of the liver is a rare entity. We reviewed the literature. To our knowledge, 41 cases have been reported till date. We report 42nd case and this is the first case to be reported from India to have undergone TACE.
The patient was on regular follow-up with yearly CECT of the abdomen. CECT of the abdomen done after 18 months showed a 4.8 cm × 4.1 cm lesion in the left lobe of the liver. Fine-needle aspiration cytology of the lesion was suggestive of recurrence. The patient defaulted for the next 7 months, and at this juncture, CECT of the abdomen showed two liver lesions; the larger one being 7.4 cm × 7.6 cm × 7.9 cm in the right lobe and the other being 6 cm × 6.6 cm × 6.5 cm in the left lobe. The patient underwent TACE. Emulation lipiodol and doxorubicin 50 mg were slowly injected into the branches of the right and left hepatic arteries [Figure 3]. Reassessment triphasic CECT of the abdomen after 3 months was suggestive of partial response. The patient is currently on regular follow-up, and after 7 months of the procedure, she is asymptomatic and CECT is suggestive of stable disease.
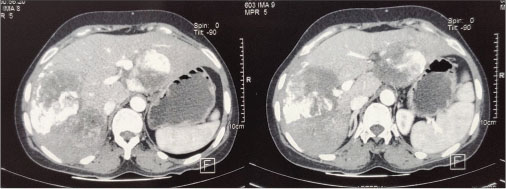
| Figure 3: Post transarterial chemoembolization contrast-enhanced computed tomography of the abdomen showing multiple lesions in both liver lobes showing heterogeneous enhancement with lipiodol deposition within
Discussion
NSET of the liver is an extremely rare, recently described nonhepatocytic, nonbiliary tumor of the liver.[1]
In 2001, it was first described by Ishak et al.[2] It is also called as desmoplastic nested spindle cell tumor of the liver and ossifying malignant mixed epithelial and stromal tumor. It consists of nests of the epithelial and spindle cells with associated myofibroblastic stroma and variable intralesional calcification and ossification.[3]
The reported tumors have been found predominantly in females consistent with our case and commonly in children. Most arose from the right lobe of the liver as in our case. To our knowledge, few cases of NSETs have been reported;[3] [4] [5] [6] [7] of these, only one has been described in India,[8] ours being the first case from India to undergo TACE.
In a number of cases, an association between these tumors and Cushing syndrome[9] and Beckwith–Wiedemann syndrome[10] has also been described. However, in majority, it was detected incidentally on imaging studies, and the patients were completely asymptomatic. Some of them present with abdominal symptoms. Ours was peculiar with jaundice as the presenting symptom.
The pathogenesis is unclear. One hypothesis is that WT1 expression is associated with impaired mesenchymal–epithelial transition. Another hypothesis is that it represents an epithelial tumor that has undergone dedifferentiation. Origin from a hepatic mesenchymal precursor cell with primitive differentiation along the bile duct lineage has also been suggested.[11]
From a clinical standpoint, one of the main differential diagnoses is a mixed epithelial and mesenchymal hepatoblastoma, although very few cases have been reported in adults.[12] Fibrolamellar HCC, synovial sarcomas, DSRCTs, and metastatic WT are other differentials.
NSET begins as a gradually enlarging small calcified lesion.[6] In our case, calcification of the liver was not observed. In blood tests, serum levels of AFP and CEA were in the normal range in almost all investigated cases as in our case. Imaging shows a typically large and well-circumscribed lesion with a lobulated margin, with heterogeneous enhancement and dense calcification on CT and similar features with predominant T1 hypointensity and T2 hyperintensity on magnetic resonance imaging. Histologic analysis shows typical characteristics of well-demarcated nests of the spindle and epithelioid cells surrounded by a desmoplastic stroma. Stroma has morphologic characteristics of myofibroblasts, and the surrounding liver parenchyma largely shows no remarkable finding. Individual cell psammomatous calcification and regions of ossification have frequently been described. IHC shows tumor cells positive for vimentin, pancytokeratin, and CD57. Staining for WT1 protein in tumor cells can be varied, with weak-to-moderate nuclear staining, dot-like paranuclear to diffuse cytoplasmic staining. Nest cells are focally positive for NSE, CD56, and sometimes S-100. Stromal components are consistently immunoreactive for α-SMA.
There is no consensus about the treatment protocol. Surgery seems to be the best option associated with better prognosis.[4] [5] Liver transplant can be useful in patients who have unresectable liver mass and no extrahepatic disease. A few cases received chemotherapy using a soft-tissue sarcoma or hepatoblastoma protocol. However, the effect of using chemotherapy or radiotherapy has not been proved. To our knowledge, till date, in only one case, TACE was done as described by Tsuruta et al., in Japan and ours is the second case in the world, however being the first case reported from India.[13]
NSET prognosis remains unclear. However, based on the current information, it seems to have a low proliferation rate and an indolent course, behaving as a low-grade malignancy. However, specific tumoral features, such as large size and vascular invasion, are associated with high probability of recurrence. Since these tumors have a known metastatic potential, postoperative follow-up with PET can be helpful.[14] [15] In our case, the patient had a relatively shorter recurrence-free interval compared to the previously reported cases.
Conclusion
Awareness of hepatic NSET may help identify additional cases, expanding knowledge about NSETs’ clinical behavior and prognosis, thus limiting the possibility of being misdiagnosed and confused with other aggressive liver malignancies. It can further aid in risk stratification, patient management, and follow-up. From our case, it is evident that TACE is a feasible option for recurrence.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that her name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Benedict M, Zhang X. Calcifying nested stromal-epithelial tumor of the liver: An update and literature review. Arch Pathol Lab Med 2019; 143: 264-8
- Ishak KG, Goodman ZD, Stocker JT. Tumors of the Liver and Intrahepatic Bile Ducts. Ch. 11. Washington, DC: Armed Forces Institute of Pathology; 2001: 276-78
- Heywood G, Burgart LJ, Nagorney DM. Ossifying malignant mixed epithelial and stromal tumor of the liver: A case report of a previously undescribed tumor. Cancer 2002; 94: 1018-22
- Grazi GL, Vetrone G, D’Errico A, Caprara G, Ercolani G, Cescon M. et al. Nested stromal-epithelial tumor (NSET) of the liver: A case report of an extremely rare tumor. Pathol Res Pract 2010; 206: 282-6
- Heerema-McKenney A, Leuschner I, Smith N, Sennesh J, Finegold MJ. Nested stromal epithelial tumor of the liver: Six cases of a distinctive pediatric neoplasm with frequent calcifications and association with cushing syndrome. Am J Surg Pathol 2005; 29: 10-20
- Makhlouf HR, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal-epithelial tumors of the liver: A clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol 2009; 33: 976-83
- Wang Y, Zhou J, Huang WB, Rao Q, Ma HH, Zhou XJ. Calcifying nested stroma-epithelial tumor of the liver: A case report and review of literature. Int J Surg Pathol 2011; 19: 268-72
- Venus A, Mehta SS, Natarajan S. A rare nonhepatocytic primary liver neoplasm with an unusual presentation: Ossifying malignant mixed epithelial and stromal tumor. Indian J Med Paediatric Oncol 2018; 39: 237-40
- Rod A, Voicu M, Chiche L, Bazille C, Mittre H, Louiset E. et al. Cushing’s syndrome associated with a nested stromal epithelial tumor of the liver: Hormonal, immunohistochemical, and molecular studies. Eur J Endocrinol 2009; 161: 805-10
- Khoshnam N, Robinson H, Clay MR, Schaffer LR, Gillespie SE, Shehata BM. Calcifying nested stromal-epithelial tumor (CNSET) of the liver in Beckwith-Wiedemann syndrome. Eur J Med Genet 2017; 60: 136-9
- Assmann G, Kappler R, Zeindl-Eberhart E, Schmid I, Häberle B, Graeb C. et al. β-Catenin mutations in 2 nested stromal epithelial tumors of the liver – A neoplasia with defective mesenchymal-epithelial transition. Hum Pathol 2012; 43: 1815-27
- Remes-Troche JM, Montaño-Loza A, Meza-Junco J, García-Leiva J, Torre-Delgadillo A. Hepatoblastoma in adult age. A case report and literature review. Ann Hepatol 2006; 5: 179-81
- Tsuruta S, Kimura N, Ishido K, Kudo D, Sato K, Endo T. et al. Calcifying nested stromal epithelial tumor of the liver in a patient with Klinefelter syndrome: A case report and review of the literature. World J Surg Oncol 2018; 16: 227
- Brodsky SV, Sandoval C, Sharma N, Yusuf Y, Facciuto ME, Humphrey M. et al. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: Case report and review of literature. Pediatr Dev Pathol 2008; 11: 469-73
- Hommann M, Kaemmerer D, Daffner W, Prasad V, Baum RP, Petrovitch A. et al. Nested stromal epithelial tumor of the liver – Liver transplantation and follow-up. J Gastrointest Cancer 2011; 42: 292-5
Address for correspondence
Publication History
Received: 12 February 2020
Accepted: 09 April 2020
Article published online:
23 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: (a) Computed tomography of the abdomen showing a large solitary, heterogeneous enhancing mass in segments IVB, V, VI, and VII of the liver, (b) gross appearance showing nodular gray-white tumor with attached gallbladder, (c) neoplastic cells arranged in organoid whorls surrounded by desmoplastic stroma shown by arrow (H and E, ×40), (d) proliferating bile ducts shown by arrow (H and E, ×100), (e) neoplastic cells with vesicular nuclei and inconspicuous nucleoli (H and E, ×400)

| Figure 2: (a) Positive immunostaining with pancytokeratin, (b) focal epithelial membrane antigen positivity, (c) nuclear and nonspecific cytoplasmic expression of β-catenin, (d) membranous staining with CD56, (e) stroma highlighted by smooth muscle actin, (f) low Ki67, (g) nuclear staining with p16

| Figure 3: Post transarterial chemoembolization contrast-enhanced computed tomography of the abdomen showing multiple lesions in both liver lobes showing heterogeneous enhancement with lipiodol deposition within
- 1 Benedict M, Zhang X. Calcifying nested stromal-epithelial tumor of the liver: An update and literature review. Arch Pathol Lab Med 2019; 143: 264-8
- 2 Ishak KG, Goodman ZD, Stocker JT. Tumors of the Liver and Intrahepatic Bile Ducts. Ch. 11. Washington, DC: Armed Forces Institute of Pathology; 2001: 276-78
- 3 Heywood G, Burgart LJ, Nagorney DM. Ossifying malignant mixed epithelial and stromal tumor of the liver: A case report of a previously undescribed tumor. Cancer 2002; 94: 1018-22
- 4 Grazi GL, Vetrone G, D’Errico A, Caprara G, Ercolani G, Cescon M. et al. Nested stromal-epithelial tumor (NSET) of the liver: A case report of an extremely rare tumor. Pathol Res Pract 2010; 206: 282-6
- 5 Heerema-McKenney A, Leuschner I, Smith N, Sennesh J, Finegold MJ. Nested stromal epithelial tumor of the liver: Six cases of a distinctive pediatric neoplasm with frequent calcifications and association with cushing syndrome. Am J Surg Pathol 2005; 29: 10-20
- 6 Makhlouf HR, Abdul-Al HM, Wang G, Goodman ZD. Calcifying nested stromal-epithelial tumors of the liver: A clinicopathologic, immunohistochemical, and molecular genetic study of 9 cases with a long-term follow-up. Am J Surg Pathol 2009; 33: 976-83
- 7 Wang Y, Zhou J, Huang WB, Rao Q, Ma HH, Zhou XJ. Calcifying nested stroma-epithelial tumor of the liver: A case report and review of literature. Int J Surg Pathol 2011; 19: 268-72
- 8 Venus A, Mehta SS, Natarajan S. A rare nonhepatocytic primary liver neoplasm with an unusual presentation: Ossifying malignant mixed epithelial and stromal tumor. Indian J Med Paediatric Oncol 2018; 39: 237-40
- 9 Rod A, Voicu M, Chiche L, Bazille C, Mittre H, Louiset E. et al. Cushing’s syndrome associated with a nested stromal epithelial tumor of the liver: Hormonal, immunohistochemical, and molecular studies. Eur J Endocrinol 2009; 161: 805-10
- 10 Khoshnam N, Robinson H, Clay MR, Schaffer LR, Gillespie SE, Shehata BM. Calcifying nested stromal-epithelial tumor (CNSET) of the liver in Beckwith-Wiedemann syndrome. Eur J Med Genet 2017; 60: 136-9
- 11 Assmann G, Kappler R, Zeindl-Eberhart E, Schmid I, Häberle B, Graeb C. et al. β-Catenin mutations in 2 nested stromal epithelial tumors of the liver – A neoplasia with defective mesenchymal-epithelial transition. Hum Pathol 2012; 43: 1815-27
- 12 Remes-Troche JM, Montaño-Loza A, Meza-Junco J, García-Leiva J, Torre-Delgadillo A. Hepatoblastoma in adult age. A case report and literature review. Ann Hepatol 2006; 5: 179-81
- 13 Tsuruta S, Kimura N, Ishido K, Kudo D, Sato K, Endo T. et al. Calcifying nested stromal epithelial tumor of the liver in a patient with Klinefelter syndrome: A case report and review of the literature. World J Surg Oncol 2018; 16: 227
- 14 Brodsky SV, Sandoval C, Sharma N, Yusuf Y, Facciuto ME, Humphrey M. et al. Recurrent nested stromal epithelial tumor of the liver with extrahepatic metastasis: Case report and review of literature. Pediatr Dev Pathol 2008; 11: 469-73
- 15 Hommann M, Kaemmerer D, Daffner W, Prasad V, Baum RP, Petrovitch A. et al. Nested stromal epithelial tumor of the liver – Liver transplantation and follow-up. J Gastrointest Cancer 2011; 42: 292-5


 PDF
PDF  Views
Views  Share
Share

