mTOR inhibition in management of advanced breast cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(02): 89-94
DOI: DOI: 10.4103/0971-5851.99737
Abstract
The mTOR pathway is becoming increasingly important in several cancers including breast cancer. This review will focus on the role of its inhibition in the management of advanced breast cancer.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The mTOR pathway is becoming increasingly important in several cancers including breast cancer. This review will focus on the role of its inhibition in the management of advanced breast cancer.
INTRODUCTION TO mTOR INHIBITORS
Mammalian target of rapamycin (mTOR) is well recognized as serine/threonine protein kinase important for cellular growth, proliferation, motility and survival. It belongs to the family of phosphatidylinositol 3-kinase-related kinases. Its pathway is stimulated by several ligands including insulin and related growth factors (IGF-1 and IGF-2). Its dysregulation in associated with several diseases including several malignancies.[1]
Sirolimus: The first mTOR inhibitor identified was sirolimus (also known as rapamycin). It was first discovered as a product of the bacterium Streptomyces hygroscopicus in a soil sample from Easter Island, an island also known as Rapa Nui, hence the name.[1] It was approved by US FDA in 1999. It was initially developed as an antifungal drug. However its unique properties made it effective as an immunosuppressant drug used to prevent rejection in organ transplantation (marketed as Rapamune). Later it was found to prevent activation of T cells and B-cells by inhibiting their response to interleukin-2 (IL-2), a fact initally exploited for the benefit of cancer patients.
Temsirolimus (Torisel): Also known as CCI-779 (cell cycle inhibitor-779), it is a derivative of sirolimus and goes after the same target. It went through a large phase 2 trial for the treatment of multiple sclerosis (MS), at whose completion, Wyeth decided to shelve further development. This was due to serious but rare cases of interstitial lung disease, bowel perforation, and acute renal failure. On May 30, 2007, US FDA dispelled these fears by approving it for the treatment of advanced renal cell carcinoma (RCC).[2]
Everolimus: Also called RAD001, is the new kid on the block! The rest of this paper shall focus on everolimus.(Ridaforolimus/Deforolimus is also currently being evaluated.)
EVEROLIMUS
As an oral inhibitor of mammalian target of rapamycin (mTOR), it is also a derivative of and functions like Rapamycin (sirolimus). It does this by allosterically binding with high affinity to the FK506 binding protein-12 (FKBP- 12). This drug complex in turn inhibits the activation of mTOR. Like other mTOR inhibitors, action of everolimus is solely on the mTORC1 protein (leaving the mTORC2 protein unaffected).[3]
This inhibition has cascading effect on the downstream effectors, resulting in arrest of cell between G1 and S phase of the cell cycle. This ultimately leads to inhibition of hypoxia-inducible factor, cell proliferation and angiogenesis as well as reduction in vascular endothelial growth factor and glucose uptake - leading to cell growth arrest and apoptosis. In breast cancer cells, this effect is synergistically enhanced when combined with aromatase inhibition.[4]
Everolimus was first approved by US FDA in 2009.[5] It is available as tablets of various strengths (2.5 mg, 5 mg and 10 mg). It is recommended to be taken once daily at the same time, consistently with or without food. Severe or intolerable adverse reactions may require temporary dose reduction (to 5 mg) and/or interruption of treatment. For moderate hepatic impairment (Child-Pugh class B), reduce the dose to 5 mg daily. The use of strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, nefazodone, saquinavir, telithromycin, ritonavir, indinavir, nelfinavir, voriconazole) should be avoided. When co-administered with moderate CYP3A4 and/or PgP inhibitors (e.g., amprenavir, fosamprenavir, aprepitant, erythromycin, fluconazole, verapamil, diltiazem), the dose of everolimus needs to be reduced to 2.5 mg daily. If this moderate inhibitor is discontinued, a washout period of approximately 2 to 3 days should be allowed before the everolimus dose is increased. If it is unavoidable to use concomitantly with strong CYP3A4 inducers (e.g., phenytoin, carbamazepine, rifampin, rifabutin, rifapentine, phenobarbital), dose of everolimus needs to be increased from 10 mg daily up to 20 mg daily (based on pharmacokinetic data), using 5 mg increments. Grapefruit and grapefruit juice (foods known to inhibit cytochrome P450 and PgP activity) may increase everolimus exposures and should be avoided. Peak everolimus concentrations are reached 1 to 2 hours after administration of oral doses. High fat meals reduced systemic absorption and circulating blood levels. Major route of excretion from the body is through the feaces (80%). An additional small amount (5%) is eliminated in the urine (5%). Elimination half life is 30 hours.
Everolimus is already approved in more than 80 countries for four indications (the oncology ones being advanced renal cell carcinoma, giant cell astrocytoma and neuroendocrine tumors of pancreas). Breast cancer will be the fifth indication.[6]
It is marketed under various trade names: Zortress (USA) and Certican (Europe and other countries) in transplantation medicine; Afinitor and Votubia in oncology.
EVEROLIMUS CLINICAL TRIALS AND BREAST CANCER
BOLERO-2 was an international, double-blind, phase 3 study in which patients were randomised in a 2:1 ratio between oral everolimus and matching placebo (at a dose of 10 mg daily).[7] All patients received exemestane in the dose of 25 mg daily. Randomization was stratified for visceral metastasis and sensitivity to endocrine therapy (adjuvant treatment for at least 24 months with endocrine therapy before recurrence or a response/ stabilization for at least 24 weeks of endocrine therapy for advanced disease). The primary end point of the study was progression-free survival (PFS).
This trial was conducted at 189 global sites (24 countries) and enrolled 724 women having a median age of 62 years. Their baseline characteristics were well balanced between the two study groups. The key inclusion criteria are shown in Table 1.
Table 1
Key inclusion criteria of BOLERO-2 study

In hormone receptor positive breast cancer cells, endocrine resistance develops as a result of aberrant signaling through the phosphatidylinositol 3-kinase (PI3K)-Akt-mTOR pathway.[8–10] One of S6 kinase 1 is the substrates of the mTOR complex 1 (mTORC1) that phosphorylates the activation function domain 1 of the ER and leads to ligand-independent receptor activation. Thus, resistance to endocrine therapy is associated with activation of the mTOR intracellular signaling pathway.
As of February 11, 2011, a total of 296 patients were still receiving study treatment: 227 (47%) in the combination-therapy group and 69 (29%) in the exemestane-alone group. The median duration of exposure to everolimus was 14.6 weeks in study arm as compared with 12.0 weeks of exposure to placebo. Disease progression led to discontinuation in 37% patients among the combination-therapy group as compared to 66% in the exemestane-alone group [Table 2].
Table 2
Results of BOLERO-2 study (International, double-blind, phase 3 study conducted at 189 global sites (24 countries) involving 724 postmenopausal HR +ve Her2 -ve advanced breast cancer women)
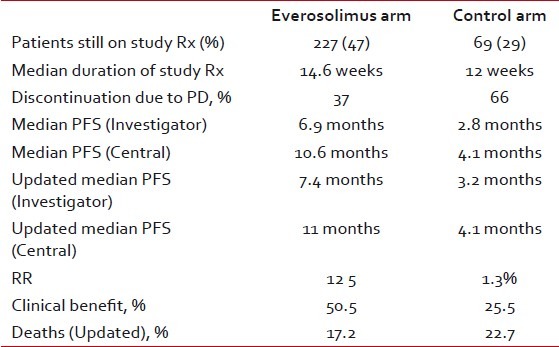
The primary end point was the median progression-free survival. It was 6.9 months for everolimus plus exemestane versus 2.8 months for placebo plus exemestane (hazard ratio for progression or death, 0.43; 95% confidence interval (CI), 0.35 to 0.54; P < 0.001) as per investigator's assessment. On central assessment, these figures were 10.6 months and 4.1 months respectively (hazard ratio, 0.36; 95% CI, 0.27 to 0.47; P < 0.001). Interestingly the central assessment actually led to increase in the progression free times in both the arms. At the San Antonio Breast Cancer Symposium (SABCS) last year and the Early Breast Cancer Congress (EBCC) meeting earlier this year, the updated findings showed a PFS of 7.4 months for the everolimus arm as compared to 3.2 months for the control arm.[11,12] Response rates and clinical benefit rates (patients with complete response, partial response, or stable disease for greater than six months) were higher in the combination arms (12.0% vs. 1.3% and 50.5% vs. 25.5%; P < 0.0001), respectively. The benefit was seen regardless of the presence of visceral disease, prior use of chemotherapy or number of prior therapies. In addition, patients with only bone metastases benefited from the combination. These results represent an additional five months of follow-up (median duration of follow-up of 17.5 months)
These results are similar to the benefit seen with chemotherapy (without their toxicity). For instance, the median PFS with capecitabine, taxanes or anthracyclines also ranges between 6.2 months and 8.2 months.[13]
Corresponding figures for overall response rates (ORR) were 9.5% and 0.4% respectively (P < 0.001). It is still too early to discuss overall survival (OS) as longer follow up and greater number of events are awaited. As per the cut off date for these results, 83 deaths have occurred of which 10.7% of patients were in the combination-therapy group and 13.0% in the exemestane-placebo group.
Important grade 3 or 4 adverse events were stomatitis (8% in the everolimus-plus-exemestane group vs. 1% in the placebo-plus-exemestane group), anemia (6% vs. < 1%), dyspnea (4% vs. 1%), hyperglycemia (4% vs. < 1%), fatigue (4% vs. 1%), and pneumonitis (3% vs. 0%).
At the time of updated analysis, 137 patients died, constituting 17.2% of patients in the everolimus plus exemestane arm and 22.7% of those in the exemestane-only arm.
Backelot T et al. reported results of a trial of everolimus plus tamoxifen for ER-positive, HER2-negative, metastatic disease at last year's SABCS.[14]
In 2009, Dr. Baselga and colleagues reported results of the study in newly diagnosed breast cancer patients given neoadjuvant everolimus combined with letrozole. They showed that there was improvement in the clinical response rate and reduction in the tumor-cell proliferation as compared to letrozole alone.[15]
In a randomized phase 2 study with similar patient group (postmenopausal women with ER-positive advanced breast cancer) but previously treated with an aromatase inhibitor, everolimus - tamoxifen combination led to improved PFS as compared to tamoxifen alone (8.6 months vs. 4.5 months, P=0.002). In addition the study arm led to significantly better OS as well (median not reached vs. 24.4 months, P=0.01).[16]
Another interesting study presented at SABCS Dec 2011, combined everolimus with cisplatin and paclitaxel in heavily pretreated Her2 negative patients with metastatic breast cancer.[17] Members of the p53 gene family are involved in development, differentiation, and response to cellular stress. It is known that p63 and p73 work in opposite directions. So increasing p73 activity and/or decreasing p63 expression will increase apoptosis and reduce tumorigenesis. Since cisplatin and mTOR increase p73 activity whereas paclitaxel decreases p63 expression, this seems to be an ideal three drug combination to be evaluated. Besides p53 and p73 promote apoptosis in response to DNA damage and p53 mutations are seen in about a third of breast cancers (usually in aggressive, hormone receptor negative patients).
This single arm, open-label, multicenter phase II trial used cisplatin 25 mg/m2 plus paclitaxel 80 mg/m2 on Days 1, 8, and 15 every 28 days alongwith everolimus 5 mg/day orally. The treatment was continued until disease progression or unacceptable toxicity. The primary aim was to measure TTP. More than half (62.5%) of patients were triple negative and in 54% of cases metastasis was detected at diagnosis or within 24 months of previous treatment. It resulted in an ORR 23.4 % and median TTP of 5 months. The most common grade 3 and4 toxicities were anemia (16%) and neutropenia (28%), febrile neutropenia occurring in only 1%; even among heavily pretreated patients, only 13% discontinued treatment due to toxicity.
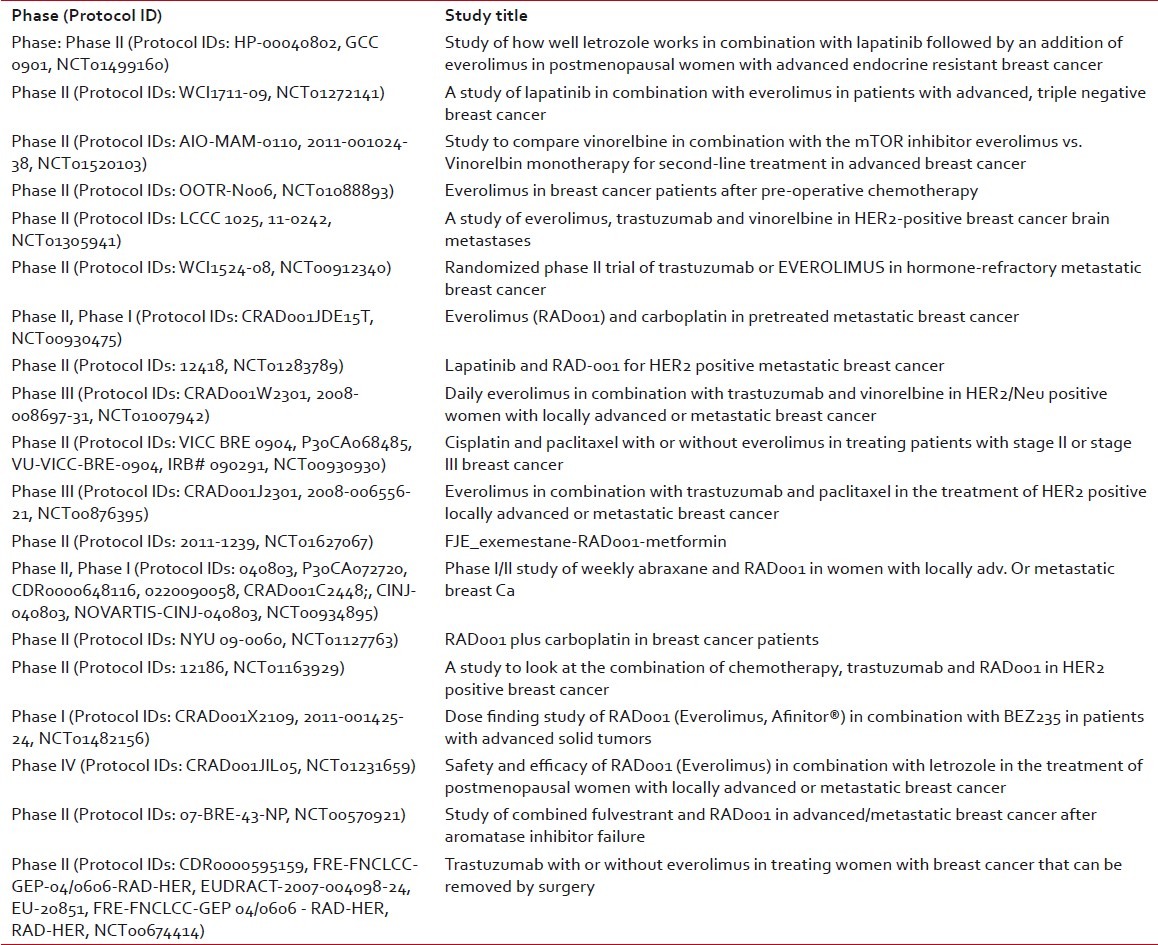
Table 3 summarises the current trials using Everolimus in Breast Cancer. Besides breast cancer, phase III trials have been done/ are under way in gastric cancer, hepatocellular carcinoma and lymphoma.[18–21]
Table 3
Current breast cancer trials with everolimus
SIDE EFFECTS
Side effects include hematological, metabolic, dermatological, gastrointestinal, respiratory, hypersensitivity, musculoskeletal, cardiovascular, ocular, renal and related to the nervous system. However most of them are minor and/or infrequent. Grade 3 or 4 side effects seen in more than 4 % of the patients included stomatitis, anemia, fatigue, hyperglycemia and elevated liver enzymes. They can be an impediment in for prolonged use of this drug in affected patients.[7]
FUTURE OF EVEROLIMUS IN BREAST CANCER
On June 22, 2012 it was announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has adopted a positive opinion for everolimus tablets, in combination with exemestane, for the treatment of hormone receptor-positive (HR+), HER2/neu-negative (HER2-) advanced breast cancer, in postmenopausal women without symptomatic visceral disease after recurrence or progression following a non-steroidal aromatase inhibitor.[22,23] The European Commission generally follows the recommendations of the CHMP and usually delivers its final decision within three months of the CHMP recommendation. The decision will be applicable to all 27 European Union member states plus Iceland and Norway. Everolimus is currently being considered in this patient population for approval by the US Food and Drug Administration, the Swiss Agency for Therapeutic Products (Swissmedic) and by health authorities worldwide.
Everolimus is also being studied in HER2-positive breast cancer in two Phase III trials [Figure 1].[24]
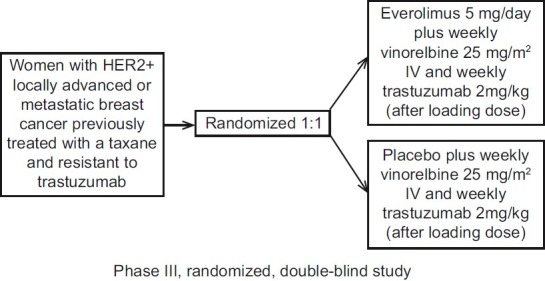
| Fig. 1 BOLERO-3 study design
Agents inhibiting the phosphoinositide 3-kinase-Akt-mammalian target of rapamycin (PAM) pathway have been associated with metabolic toxicities of hyperlipidemia and hyperglycemia. The PAM Task Force of the National Cancer Institute Investigational Drug Steering Committee convened an interdisciplinary expert panel to review the pathophysiology of such hyperlipidemia and hyperglycemia.[25] Their conclusion is that Dose modifications or discontinuation of PAM pathway inhibitors should only be considered if such metabolic toxicities are severe and persists even after their treatment has been administered for sufficiently long time.
It is well established that hormonal therapy is the key to successful treatment of advanced breast cancer. Yet a significant number of patients with metastatic disease do not respond to initial treatment and the vast majority, in fact, will ultimately develop resistance. Our approach to counter this was the sequential use of as all possible hormone therapies available to us with the aim of keeping longest possible control over the malignancy.
Everolimus has the potential to disrupt this thinking, be a game changer. It represents a major innovation in HR+/HER2-advanced breast cancer, something we have been waiting for since aromatase inhibitors were introduced more than 15 years ago. This approach promises to address an important unmet need for hundreds of thousands of women with hormone receptor expressing advanced disease.[26]
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 BOLERO-3 study design


 PDF
PDF  Views
Views  Share
Share

