Modulated Radiotherapy with Concurrent and Adjuvant Temozolomide for Anaplastic Gliomas: Indian Single center Data
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 495-501
DOI: DOI: 10.4103/ijmpo.ijmpo_200_16
Abstract
Objective: To evaluate early clinical outcome for anaplastic gliomas (AG) treated in the era of modulated radiotherapy (RT) and concurrent plus adjuvant temozolomide (TMZ) in an Indian setting. Materials and Methods: Fifty-three patients with AGs treated with modulated RT and concurrent (95%) and adjuvant TMZ (90%) were analyzed. About 80% of patients had Karnofsky performance status (KPS) at least 90 with 30% seizure at presentation. Postoperative magnetic resonance imaging was available in 65%-cases and RT dose was 60 Gy in 30 fractions. First posttreatment imaging was performed at 1 month and then at 3 and 6 months post-RT and then every 3 months. Kaplan–Meier analysis was used to estimate disease-free survival (DFS) and overall survival (OS), and analysis was done using SPSS version 18.0. Results: With median follow-up of 25 months, 2-year DFS and OS were 75% and 88%. There were only 5% symptomatic central nerves system and 8% symptomatic hematological toxicities. At the 1st evaluation, 30.4% hadcomplete response (CR), at 3 months 40%, and at 6 months 43%. At 6 months, only 4% had progressive disease. Forty-six patients were evaluable till the last follow-up with and 55% had stable to CR. On univariate analysis for DFS, KPS at presentation >90 (P = 0.001) and response at 6 months (P = 0.02) were significant and for OS KPS at presentation (P = 0.004) alone. Conclusion: Modulated RT with TMZ among Grade III glioma patients resulted in minimum treatment-related toxicities and encouraging survival. Molecular prognostic markers will determine most favorable groups in future.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Objective:
To evaluate early clinical outcome for anaplastic gliomas (AG) treated in the era of modulated radiotherapy (RT) and concurrent plus adjuvant temozolomide (TMZ) in an Indian setting.
Materials and Methods:
Fifty-three patients with AGs treated with modulated RT and concurrent (95%) and adjuvant TMZ (90%) were analyzed. About 80% of patients had Karnofsky performance status (KPS) at least 90 with 30% seizure at presentation. Postoperative magnetic resonance imaging was available in 65%-cases and RT dose was 60 Gy in 30 fractions. First posttreatment imaging was performed at 1 month and then at 3 and 6 months post-RT and then every 3 months. Kaplan–Meier analysis was used to estimate disease-free survival (DFS) and overall survival (OS), and analysis was done using SPSS version 18.0.
Results:
With median follow-up of 25 months, 2-year DFS and OS were 75% and 88%. There were only 5% symptomatic central nerves system and 8% symptomatic hematological toxicities. At the 1st evaluation, 30.4% had complete response (CR), at 3 months 40%, and at 6 months 43%. At 6 months, only 4% had progressive disease. Forty-six patients were evaluable till the last follow-up with and 55% had stable to CR. On univariate analysis for DFS, KPS at presentation >90 (P = 0.001) and response at 6 months (P = 0.02) were significant and for OS KPS at presentation (P = 0.004) alone.
Conclusion:
Modulated RT with TMZ among Grade III glioma patients resulted in minimum treatment-related toxicities and encouraging survival. Molecular prognostic markers will determine most favorable groups in future.
Introduction
The primary brain tumors comprise 2% of all cancers.[1] As per the World Health Organization classification, anaplastic gliomas (AGs) are Grade III malignant tumors and are managed on the line of glioblastoma multiforme (GBM) with maximal safe resection (MSR) followed by adjuvant radiotherapy (RT). Adjuvant RT has demonstrated improved survival both in terms of local control and overall survival (OS).[2,3,4,5] Following the trend in management of GBM with RT with concurrent and adjuvant temozolomide (TMZ), the protocol has been adopted gradually to manage anaplastic Grade III gliomas.[6,7] Till date, there are no prospective randomized data to validate the role of RT and TMZ for Grade III gliomas although retrospective single-center experiences have been encouraging.
Outside clinical trials, anaplastic astrocytomas (AA) are treated as GBM with RT and TMZ. AA lack 1p19q co-deletion is considered to be as high risk as GBM. On the other hand, anaplastic oligodendrogliomas (AODG) that express 1p19q co-deletion can be managed with adjuvant procarbazine, carmustine, and vincristine (PCV) chemotherapy.[8,9] TMZ has been favored keeping in mind the favorable toxicity profile and tolerability. Although there is no differential activity between the two, TMZ has been preferred due to reduced toxicity, tolerability, and ease of administration.[10,11,12]
We evaluated our cohort of AG patients (AA, AODG, and anaplastic oligoastrocytoma [AOA]) treated uniformly with MSR followed by adjuvant RT plus concurrent TMZ and adjuvant TMZ. The survival outcomes and prognostic factors were assessed.
Materials and Methods
Between November 2011 and November 2014 consecutively treated 71 AG patients, data were evaluated. The uniform criteria of MSR (gross-total resection [GTR] or near-total resection [NTR] or stereotactic [STS] biopsy) followed by adjuvant RT plus TMZ and adjuvant TMZ was applied, and at least 1 year of follow-up, posttreatment was also considered before analysis. Finally, 53 patients were analyzed after careful evaluation of clinical follow-up data from these network systems. The treatment protocol was the consensus decision of institutional neuro-oncology tumor board comprising of neurosurgeons, radiation oncologists, pathologists, and neuroradiologists and medical oncologists. Informed consent was taken for all patients before initiation of treatment.
Surgical characteristics
GTR was defined as no residual enhancement on postoperative magnetic resonance imaging (MRI) scans. NTR/near-total excision was defined as having thin rim of enhancement in resection cavity only. Subtotal resection was as having residual nodular enhancement. Few patients also had STS biopsy alone in view of location of the tumor and medical decompression. Whatever may be the surgery, postoperative biopsy report other than grade of tumor and molecular markers such as 1p19q co-deletion status was also ordered.
Radiotherapy protocol
Postoperative RT was carried out 1 week after stich removal, and in majority, the commencement was between 3 and 4 weeks after surgery. A postoperative contrast-enhanced MRI (CEMRI) scan of the brain was taken before RT planning. All patients were immobilized with thermoplastic head mask, and a 3-mm slice-thickness computed tomography (CT) scan was acquired from vertex to mid neck in supine position. If there were no contraindications, all patients had IV contrast injection while RT planning CT scan was done. The CT images were then transferred through DICOM to Focal Sim planning software (Elekta Crawley, UK) system. Planning CT was fused with MRI and target delineation was done according to Radiotherapy Oncology Group (RTOG) and European Society for Therapeutic Radiation Oncology- Advisory Committee on Radiation Oncology Practice guideline. A neuroradiologist was also called to verify the volumes. Gross tumor volume (GTV) included postoperative cavity, residual disease, and edema based on both T1 postcontrast and T2FLAIR sequences of MRI. Clinical target volume (CTV) was created by giving a 2-cm margin to GTV and then edited from natural barriers, and planning target volume was created by giving 0.5 mm margin to CTV as a standard departmental protocol. Besides target volumes, organs at risk including bilateral eyes, optic nerves, lenses, optic chiasm, brain stem, temporal lobes, normal brain, and hippocampus were delineated as per standard guideline.
RT technique was modulated radiation (either intensity-modulated radiotherapy [IMRT] or volumetric modulated arc therapy [VMAT]). The prescribed total dose was 58–60 Gy in conventional fractionation (at 1.8–2 Gy per fraction), and the patients were treated from Monday through Friday.
Acute and late RT side effects were noted as per RTOG toxicity criterion, and quality of life (QOL) scales EORTC QOL Q C30 and BN 20 were administered at beginning of RT, on completion, and at each follow-up.
Temozolomide and supportive medications
Although edema was more prominent during RT, glucocorticoids were not given prophylactically and were administered if signs and symptoms of increased intracranial pressure were manifested. All patients had antiepileptic drugs with levetiracetam as sodium valproate and phenytoin are hepatic enzyme modulators.
Patients received concurrent TMZ at 75 mg/m2 throughout RT period starting from the 1st day and also continued over the weekends till the last day of RT. Along with TMZ prophylactic antiemetics, mostly 5HT3 inhibitors (granisetron or ondansetron) and proton pump inhibitors (pantoprazole) were given 30 min before receiving TMZ. TMZ was given 30–45 min before RT, and gap of 2 h between food and TMZ was maintained. TMZ was administered by a nurse in the radiation oncology day care so that the patient could be taken for radiation within the stipulated time of action of TMZ as radiosensitizer. Pneumocystis carinii pneumonia prophylaxis was not given routinely. Complete blood counts were done at least weekly (if not twice a week) while on RT. The acceptable blood parameters to continue RT plus TMZ were total leukocyte counts at least 4000/cmm, platelet counts >150,000/cmm, and hemoglobin level more or equals to 10 g/dl. Routine administration of growth factors was not considered, and if platelet counts dropped below 80,000/cmm, RT was stopped.
Follow-up
At completion of RT, TMZ was also stopped. Patients came for the first follow-up at 4 weeks from completion of RT with CEMRI of the brain. Adjuvant TMZ was started after verifying blood counts and MRI report at 150 mg/m2 for 5 days in a month and from the 2nd cycle at 200 mg/m2 up to 6 cycles at the same dosage if blood counts were adequate, interim MRI brain remained stable, and patient's Karnofsky performance status (KPS) was ≥70. Antiepileptics were continued throughout. CEMRI brain was repeated after three cycles of TMZ and also after completion of six cycles.
OS was calculated from the date of registration till the last follow-up, and disease-free survival (DFS) was calculated from the end of RT and TMZ till the last date of disease control. Radiological progression was validated through neuro-oncology meeting comprising of neurosurgeons, radiation oncologist, and neuroradiologists. Treatment options at progression were salvage surgery, re-RT, and challenge with low-dose TMZ or bevacizumab. At the time of analysis, patients who were not available for follow-up for more than 6 months were considered lost to follow-up.
Patient and disease characteristics such as age, gender, histopathological type of AG, surgery type and extent, RT dose and TMZ details, post-RT response at completion, 3rd, and 6th months, and other characteristics were considered for OS and DFS calculation. The statistical analysis was done using IBM SPSS version 18.0 (UNICOM system, IBM Corporation, Armonk, New York).
Results
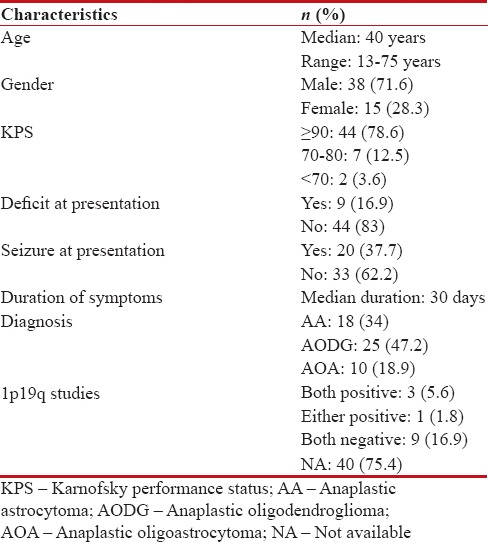
A total of 53 patients were analyzed. Out of the entire cohort, 71.6% were male and 28.4% were female. Median age was 40 years with majority (83%) had KPS ≥90. The histopathological varieties were equally distributed with AODG (47.2%) being most common. Very few patients could undergo molecular testing for 1p19q due to logistic reasons. The detailed demographics are being represented in Table 1.
Table 1
Demographic profile of the cohort (n=53)
|
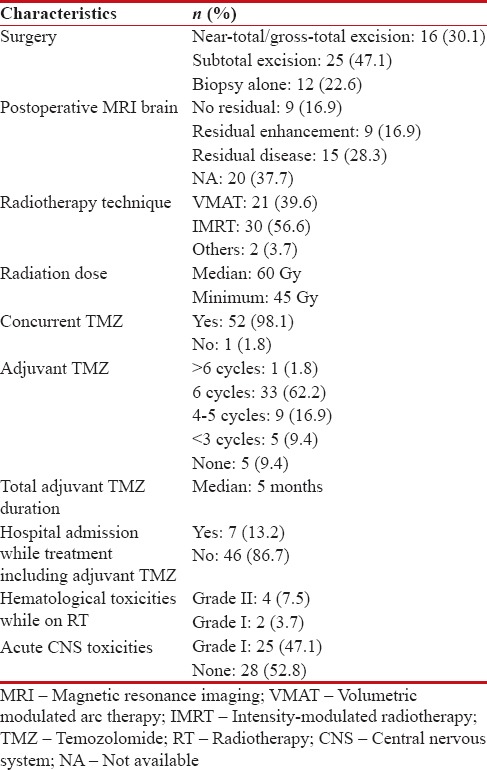
Treatment characteristics
Surgery
All the 53 patients were evaluated by neurosurgeon for surgical intervention. Twelve out of 53 patients had STS biopsy only keeping in mind the location of the primary tumor. About 65% of patients had postoperative MRI brain, and for the rest, we used the intraoperative MRI suite (Brain Lab) images for RT planning. Gross-total excision was done in about 50% of cases.
Radiotherapy
All patients received postoperative RT. The RT technique was IMRT in 56% of cases, and about 40% had VMAT technique. Forty-six out of 53 patients (87%) received RT dose between 5800 and 6000 cGy in 200 cGy per fractions. All the patients received concurrent TMZ with interruption only if acute hematological toxicities. About 60% of patients completed total 6 cycles of adjuvant TMZ. The treatment was mostly well tolerated with Grade I nonspecific central nerves system toxicities and Grade I hematological toxicities (mostly thrombocytopenia). Only four patients had treatment interruption due to thrombocytopenia. The treatment characteristics are presented in Table 2.
Table 2
Treatment characteristics
|
Survival
At the first follow-up posttreatment, 30.4% of cases had complete response (CR), at 3 months 40%, and at 6 months 43%. At 6 months, only 10% (6 patients) had progressive disease. About 32% of patients had stable to partial response status at 6 months. Hence, majority of the patients had stable to CR at treatment completion with adjuvant TMZ.
The median follow-up duration was 25 months, and till the last follow-up, 46 out of 53 patients were evaluable with 8 deaths and 55% having stable to CR.
The median DFS and OS were 24 and 25 months, respectively. At 2 years, the DFS and OS were 75% and 88%, respectively, and at 3 years, it was 65% and 78%, respectively [Figures [Figures11 and and22].
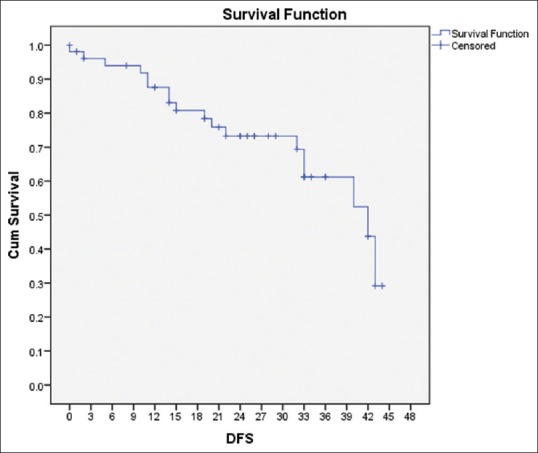
| Figure 1:Disease-free survival of the cohort
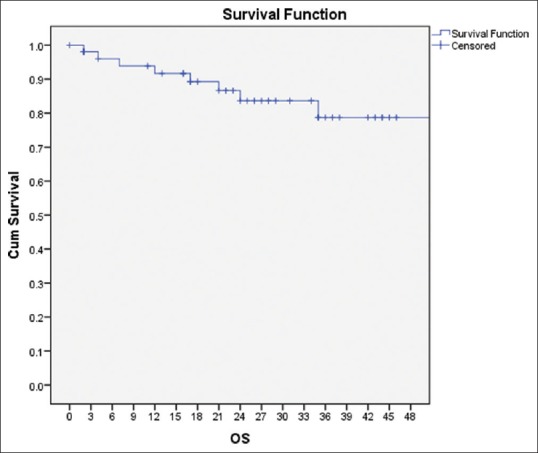
| Figure 2:Overall survival of the cohort
On univariate analysis for DFS, KPS at presentation >90 (P = 0.001) and response at 6 months (P = 0.02) were significant, and for OS, KPS at presentation (P = 0.004) was a significant factor [Figure 3]. GTR, no residual at postoperative MRI, up to six cycles of adjuvant TMZ, and CR at 6 months were favorable in terms of both DFS and OS [Figure 4]. Histopathological types were not significant for DFS and OS, and only three patients were 1p19q co-deletion positive. The details are in Table 3.
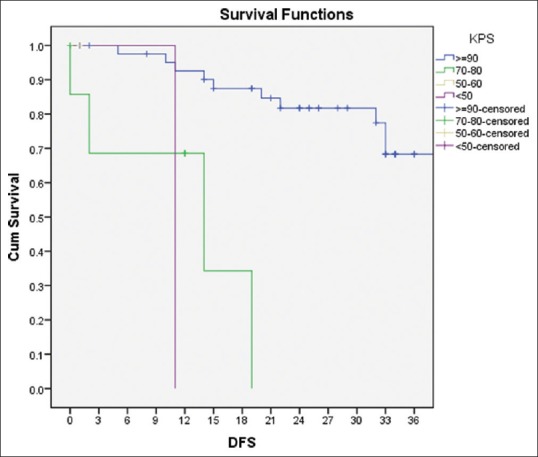
| Figure 3:Overall survival of the cohort
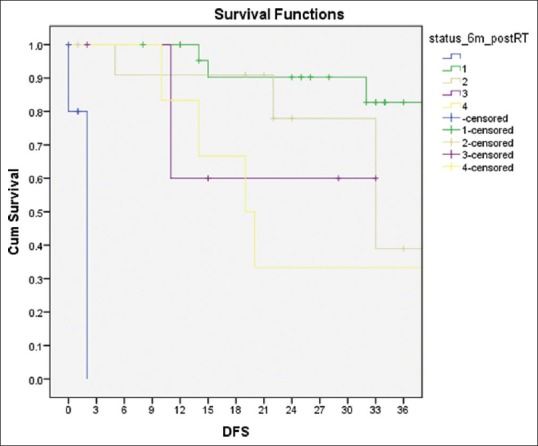
| Figure 4:Disease-free survival and status 6-month postradiotherapy + temozolomide
Table 3
Response and survival data

|
QOL scales suggested decline in mood, cognition, fatigue and toilet control initially, and improvement beyond 3 months. There were no significant late effects till the last follow-up.
QOL scales suggested decline in mood, cognition, fatigue and toilet control initially, and improvement beyond 3 months. There were no significant late effects till the last follow-up.
References
- GLOBOCAN 2012 Ver. 1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. International Agency for Research on Cancer; 2013. Available from: http://www.globocan.iarc.fr. [Last accessed on 2014 Feb 19].
- Salazar OM, Rubin P, Feldstein ML, Pizzutiello R. High dose radiation therapy in the treatment of malignant gliomas: Final report. Int J Radiat Oncol Biol Phys 1979;5:1733-40.
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol 2003;30 6 Suppl 19:10-4.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016;131:803-20.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66.
- Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J Clin Oncol 2013;31:337-43.
- van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 2013;31:344-50.
- ;Wick W, Roth P, Hartmann C, Hau P, Nakamura M, Stockhammer F, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol 2016;18:1529-37.
- Speirs CK, Simpson JR, Robinson CG, DeWees TA, Tran DD, Linette G, et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int J Radiat Oncol Biol Phys 2015;91:268-76.
- Brandes AA, Tosoni A, Cavallo G, Reni M, Franceschi E, Bonaldi L, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: A prospective GICNO study. J Clin Oncol 2006;24:4746-53.
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys 1979;5:1725-31.
- Walker MD, Alexander E Jr., Hunt WE, MacCarty CS, Mahaley MS Jr., Mealey J Jr., et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333-43.
- Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomized studies of nitrosoureas in primary high grade malignant glioma. Br J Cancer 1987;56:89-90.
- Lonardi S, Tosoni A, Brandes AA. Adjuvant chemotherapy in the treatment of high grade gliomas. Cancer Treat Rev 2005;31:79-89.
- Lassman AB, Iwamoto FM, Cloughesy TF, Aldape KD, Rivera AL, Eichler AF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol 2011;13:649-59.
- Paulsson AK, McMullen KP, Peiffer AM, Hinson WH, Kearns WT, Johnson AJ, et al. Limited margins using modern radiotherapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol 2014;37:177-81.
- Wick W, Wiestler B, Platten M. Treatment of anaplastic glioma. Cancer Treat Res 2015;163:89-101.
- Lassman AB. Procarbazine, lomustine and vincristine or temozolomide: Which is the better regimen? CNS Oncol 2015;4:341-6.
- Lecavalier-Barsoum M, Quon H, Abdulkarim B. Adjuvant treatment of anaplastic oligodendrogliomas and oligoastrocytomas. Cochrane Database Syst Rev 2014;(5):CD007104.
- Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 2009;27:5874-80.
- Yu T, Kang HC, Lim DH, Kim IH, Chung WK, Suh CO, et al. Pattern of care of anaplastic oligodendroglioma and oligoastrocytoma in a Korean population: The Korean Radiation Oncology Group study 13-12. J Neurooncol 2015;121:531-9.
- Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev 2013;(4):CD007415.
- Phase III Trial of Anaplastic Glioma Without 1p/19q LOH (CATNON). Identifier: NCT00626990. Available from: htttp://www. ClinicalTrials.gov. [Last accessed 2017 Jul 26].
- Jaeckle K, Vogelbaum M, Ballman KV, et al. CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): Phase III Randomized Study of RT vs. RT+TMZ vs. TMZ for Newly Diagnosed 1p/19q-Codeleted Anaplastic Oligodendroglial Tumors. Analysis of Patients Treated on the Original Protocol Design. Neurology 2016;86 Suppl 16: Abstract PL02.005.
- Strowd RE, Abuali I, Ye X, Lu Y, Grossman SA. The role of temozolomide in the management of patients with newly diagnosed anaplastic astrocytoma: Acomparison of survival in the era prior to and following the availability of temozolomide. J Neurooncol 2016;127:165-71.
- Shonka NA, Theeler B, Cahill D, Yung A, Smith L, Lei X, et al. Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: A retrospective review within the era of temozolomide. J Neurooncol 2013;113:305-11.
- Rogne SG, Konglund A, Scheie D, Helseth E, Meling TR. Anaplastic astrocytomas: Survival and prognostic factors in a surgical series. Acta Neurochir (Wien) 2014;156:1053-61.
- Compostella A, Tosoni A, Blatt V, Franceschi E, Brandes AA. Prognostic factors for anaplastic astrocytomas. J Neurooncol 2007;81:295-303.
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997-1003.
- Kizilbash SH, Giannini C, Voss JS, Decker PA, Jenkins RB, Hardie J, et al. The impact of concurrent temozolomide with adjuvant radiation and IDH mutation status among patients with anaplastic astrocytoma. J Neurooncol 2014;120:85-93.
- Rajmohan KS, Sugur HS, Shwetha SD, Ramesh A, Thennarasu K, Pandey P, et al. Prognostic significance of histomolecular subgroups of adult anaplastic (WHO Grade III) gliomas: Applying the 'integrated' diagnosis approach. J Clin Pathol 2016;69:686-94.
- Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372:2499-508.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765-73.

| Figure 1:Disease-free survival of the cohort

| Figure 2:Overall survival of the cohort

| Figure 3:Overall survival of the cohort

| Figure 4:Disease-free survival and status 6-month postradiotherapy + temozolomide
References
- GLOBOCAN 2012 Ver. 1.0, Cancer Incidence and Mortality Worldwide: IARC Cancer Base No. 11. International Agency for Research on Cancer; 2013. Available from: http://www.globocan.iarc.fr. [Last accessed on 2014 Feb 19].
- Salazar OM, Rubin P, Feldstein ML, Pizzutiello R. High dose radiation therapy in the treatment of malignant gliomas: Final report. Int J Radiat Oncol Biol Phys 1979;5:1733-40.
- Buckner JC. Factors influencing survival in high-grade gliomas. Semin Oncol 2003;30 6 Suppl 19:10-4.
- Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, et al. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 2007;114:97-109.
- Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol 2016;131:803-20.
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987-96.
- Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459-66.
- Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, et al. Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: Long-term results of RTOG 9402. J Clin Oncol 2013;31:337-43.
- van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 2013;31:344-50.
- ;Wick W, Roth P, Hartmann C, Hau P, Nakamura M, Stockhammer F, et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol 2016;18:1529-37.
- Speirs CK, Simpson JR, Robinson CG, DeWees TA, Tran DD, Linette G, et al. Impact of 1p/19q codeletion and histology on outcomes of anaplastic gliomas treated with radiation therapy and temozolomide. Int J Radiat Oncol Biol Phys 2015;91:268-76.
- Brandes AA, Tosoni A, Cavallo G, Reni M, Franceschi E, Bonaldi L, et al. Correlations between O6-methylguanine DNA methyltransferase promoter methylation status, 1p and 19q deletions, and response to temozolomide in anaplastic and recurrent oligodendroglioma: A prospective GICNO study. J Clin Oncol 2006;24:4746-53.
- Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys 1979;5:1725-31.
- Walker MD, Alexander E Jr., Hunt WE, MacCarty CS, Mahaley MS Jr., Mealey J Jr., et al. Evaluation of BCNU and/or radiotherapy in the treatment of anaplastic gliomas. A cooperative clinical trial. J Neurosurg 1978;49:333-43.
- Stenning SP, Freedman LS, Bleehen NM. An overview of published results from randomized studies of nitrosoureas in primary high grade malignant glioma. Br J Cancer 1987;56:89-90.
- Lonardi S, Tosoni A, Brandes AA. Adjuvant chemotherapy in the treatment of high grade gliomas. Cancer Treat Rev 2005;31:79-89.
- Lassman AB, Iwamoto FM, Cloughesy TF, Aldape KD, Rivera AL, Eichler AF, et al. International retrospective study of over 1000 adults with anaplastic oligodendroglial tumors. Neuro Oncol 2011;13:649-59.
- Paulsson AK, McMullen KP, Peiffer AM, Hinson WH, Kearns WT, Johnson AJ, et al. Limited margins using modern radiotherapy techniques does not increase marginal failure rate of glioblastoma. Am J Clin Oncol 2014;37:177-81.
- Wick W, Wiestler B, Platten M. Treatment of anaplastic glioma. Cancer Treat Res 2015;163:89-101.
- Lassman AB. Procarbazine, lomustine and vincristine or temozolomide: Which is the better regimen? CNS Oncol 2015;4:341-6.
- Lecavalier-Barsoum M, Quon H, Abdulkarim B. Adjuvant treatment of anaplastic oligodendrogliomas and oligoastrocytomas. Cochrane Database Syst Rev 2014;(5):CD007104.
- Wick W, Hartmann C, Engel C, Stoffels M, Felsberg J, Stockhammer F, et al. NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol 2009;27:5874-80.
- Yu T, Kang HC, Lim DH, Kim IH, Chung WK, Suh CO, et al. Pattern of care of anaplastic oligodendroglioma and oligoastrocytoma in a Korean population: The Korean Radiation Oncology Group study 13-12. J Neurooncol 2015;121:531-9.
- Hart MG, Garside R, Rogers G, Stein K, Grant R. Temozolomide for high grade glioma. Cochrane Database Syst Rev 2013;(4):CD007415.
- Phase III Trial of Anaplastic Glioma Without 1p/19q LOH (CATNON). Identifier: NCT00626990. Available from: htttp://www. ClinicalTrials.gov. [Last accessed 2017 Jul 26].
- Jaeckle K, Vogelbaum M, Ballman KV, et al. CODEL (Alliance-N0577; EORTC-26081/22086; NRG-1071; NCIC-CEC-2): Phase III Randomized Study of RT vs. RT+TMZ vs. TMZ for Newly Diagnosed 1p/19q-Codeleted Anaplastic Oligodendroglial Tumors. Analysis of Patients Treated on the Original Protocol Design. Neurology 2016;86 Suppl 16: Abstract PL02.005.
- Strowd RE, Abuali I, Ye X, Lu Y, Grossman SA. The role of temozolomide in the management of patients with newly diagnosed anaplastic astrocytoma: Acomparison of survival in the era prior to and following the availability of temozolomide. J Neurooncol 2016;127:165-71.
- Shonka NA, Theeler B, Cahill D, Yung A, Smith L, Lei X, et al. Outcomes for patients with anaplastic astrocytoma treated with chemoradiation, radiation therapy alone or radiation therapy followed by chemotherapy: A retrospective review within the era of temozolomide. J Neurooncol 2013;113:305-11.
- Rogne SG, Konglund A, Scheie D, Helseth E, Meling TR. Anaplastic astrocytomas: Survival and prognostic factors in a surgical series. Acta Neurochir (Wien) 2014;156:1053-61.
- Compostella A, Tosoni A, Blatt V, Franceschi E, Brandes AA. Prognostic factors for anaplastic astrocytomas. J Neurooncol 2007;81:295-303.
- Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med 2005;352:997-1003.
- Kizilbash SH, Giannini C, Voss JS, Decker PA, Jenkins RB, Hardie J, et al. The impact of concurrent temozolomide with adjuvant radiation and IDH mutation status among patients with anaplastic astrocytoma. J Neurooncol 2014;120:85-93.
- Rajmohan KS, Sugur HS, Shwetha SD, Ramesh A, Thennarasu K, Pandey P, et al. Prognostic significance of histomolecular subgroups of adult anaplastic (WHO Grade III) gliomas: Applying the 'integrated' diagnosis approach. J Clin Pathol 2016;69:686-94.
- Eckel-Passow JE, Lachance DH, Molinaro AM, Walsh KM, Decker PA, Sicotte H, et al. Glioma groups based on 1p/19q, IDH, and TERT promoter mutations in tumors. N Engl J Med 2015;372:2499-508.
- Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med 2009;360:765-73.


 PDF
PDF  Views
Views  Share
Share

