Metronomic chemotherapy in platinum-insensitive failures and/or early failures postmultimodality management in oral cancers
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(03): 161-165
DOI: DOI: 10.4103/0971-5851.166725
Abstract
Purpose: Oral cancer patients with platinum-resistant disease and or early failures have limited treatment options. This analysis was planned to study the efficacy of metronomic chemotherapy in this group of patients. Materials and Methods: This was a retrospective analysis of oral cancer patients who had squamous cell carcinoma and had an early failure and/or platinum-insensitive failure. Early failure was defined as a failure either within 1-month of adjuvant radiotherapy or within 6 months of chemoradiation (CTRT). A sample size of 100 patients was selected for this study. If ≥39 of 100 patients would have survived at 6 months with metronomic chemotherapy, then additional studies would be warranted. Results: The ECOG PS was 0-1 in 92 patients and 2 in 8 patients. The subsite of primary was buccal mucosa in 38 patients (38%), anterior two-third tongue (oral tongue) in 51 patients (51%), and alveolus in 11 patients (11%). The median estimated overall survival was 110 days (95% confidence interval [CI]: 85-134 days). The proportion of patients surviving at 6 months was 26.4% (95% CI: 17.9-35.6). Conclusion: Metronomic combination of methotrexate and celecoxib failed to meet its prespecified efficacy limit and should not be used in these patients as routine.
Keywords
Head and neck cancer - metronomic - oral cancer - palliative chemotherapy - platinum-insensitive - platinum refractory - platinum-resistantPublication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Purpose:
Oral cancer patients with platinum-resistant disease and or early failures have limited treatment options. This analysis was planned to study the efficacy of metronomic chemotherapy in this group of patients.
Materials and Methods:
This was a retrospective analysis of oral cancer patients who had squamous cell carcinoma and had an early failure and/or platinum-insensitive failure. Early failure was defined as a failure either within 1-month of adjuvant radiotherapy or within 6 months of chemoradiation (CTRT). A sample size of 100 patients was selected for this study. If ≥39 of 100 patients would have survived at 6 months with metronomic chemotherapy, then additional studies would be warranted.
Results:
The ECOG PS was 0-1 in 92 patients and 2 in 8 patients. The subsite of primary was buccal mucosa in 38 patients (38%), anterior two-third tongue (oral tongue) in 51 patients (51%), and alveolus in 11 patients (11%). The median estimated overall survival was 110 days (95% confidence interval [CI]: 85-134 days). The proportion of patients surviving at 6 months was 26.4% (95% CI: 17.9-35.6).
Conclusion:
Metronomic combination of methotrexate and celecoxib failed to meet its prespecified efficacy limit and should not be used in these patients as routine.
INTRODUCTION
Multimodality management either a combination of surgery followed by adjuvant radiation or radical chemoradiation is the standard of care in locally advanced oral cancers.[1,2] However, in spite of multimodality management, locally advanced oral cancers have high failure rates.[2,3] Unfortunately, majority of these failures occur in first 1-2 years posttreatment completion.[3,4] Palliative chemotherapy remains a treatment option in such scenarios.[5,6] However, patients who fail within 1-month of adjuvant radiotherapy (RT) or within 6 months of CTRT (within 6 months of last dose of platinum) have a very poor prognosis.[7,8,9] These biologically aggressive tumors are commonly excluded from the treatment defining studies.[10,11,12] Unfortunately, there is no dearth of such patients in our country. Cetuximab-based chemotherapy seems to be an option in these patients. However, < 1% of patients in the developing countries can afford it.[13]
Metronomic chemotherapy consisting of oral methotrexate and celecoxib has shown promising results in head and neck cancer patients.[14] We recently reported a randomized study which compared metronomic chemotherapy consisting of oral methotrexate and celecoxib with intravenous cisplatin in palliative head and neck cancer patients who had failed post 3 months of curative therapy.[8] Metronomic chemotherapy was found to be superior in terms of progression-free survival (PFS) and overall survival (OS). Hence, these patients with early failures are treated with a metronomic combination of celecoxib and oral weekly methotrexate on compassionate grounds when they cannot afford cetuximab at our center. This analysis was planned to study the efficacy of metronomic chemotherapy in this group of patients. We hypothesized that if 6 months survival would be 30% or more, then metronomic chemotherapy should be studied in a randomized Phase 2.
MATERIALS AND METHODS
This was a retrospective analysis. We maintain a prospective database of patients undergoing palliative chemotherapy in head and neck cancers. Cases were selected from this database subjected to following criteria.
Selection criteria
- Biopsy proven squamous cell carcinoma.
- Patients with site of tumor in oral cavity.
- Treated between August 2013 and August 2014.
- Early failure postmultimodality treatment. Early failure was defined as failure (loco-regional, distant, or both) either within 1-month of adjuvant RT or within 6 months of CTRT (adjuvant or radical).[15]
- Recipients of metronomic chemotherapy.
- ECOG PS 0-2.
Treatment and assessments
All of these patients had been seen in the multidisciplinary clinic consisting of surgical oncologist, radiation oncologist, medical oncologist, and radiologist. These patients were deemed unfit for surgery in the view of extensive disease and very short disease-free interval (DFI). These patients were not considered for palliative radiation too, as either they had received radiation before (n = 84), or the disease was extensive. Hence, these patients were referred for palliative chemotherapy. All of these patients were counseled regarding the poor prognosis and option of cetuximab-based chemotherapy was given. As these patients had logistics issues (financial) in procuring cetuximab, they were offered oral metronomic chemotherapy on compassionate grounds. Written informed consent was obtained before starting metronomic chemotherapy. These patients had received oral weekly methotrexate 15 mg/m2 along with oral celecoxib 200 mg PO twice daily. The chemotherapy was continued till either progression or until intolerable side effects. All of these patients were followed up till death.
Statistical analysis
Sample size calculation and decision considerations
The study sample size calculation was done with a Type 1 error of 5% and Type 2 error of 20%. Hence, this study had 80% power and to detect a lower critical limit of survival probability at 6 months of 0.3, that is, 30%. It was assumed that the selected samples’ survival distribution will follow the nonparametric exponential survival distribution. A total of 80 samples are found suitable to carry out this retrospective analysis. On the collection of data, we had 100 patients during the stipulated time interval. The number of patients was more than the required sample size for such analysis; however, as a higher sample size would improve the power of the study, we decided to include all the 100 patients.
Analysis
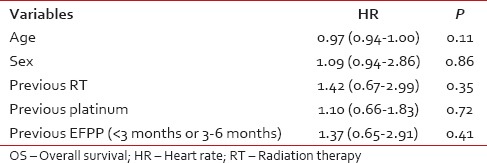
The categorical variables were expressed in frequencies and percentages. The continuous variables in median and interquartile range. The OS was calculated from the date of start of chemotherapy till the date of death. In case, if the patient had not died at the last follow-up date, then the respective patient was censored while estimating the OS. The OS was estimated by the Kaplan-Meier method. Known prognostic factors (age, gender, previous RT, previous platinum exposure, and previous event free period) would be tested for their influence on OS. Multivariate COX regression analysis was done for this purpose.
RESULTS
Baseline details at recurrence
The median age of the cohort was 45 years (IQR: 40-51.3 years). There were 93 males (93%). The ECOG PS was 0-1 in 92 patients and 2 in 8 patients. The distribution of primary site was oral cancer in 100 patients. The subsite of primary was buccal mucosa in 38 patients (38%), anterior two-third tongue (oral tongue) in 51 patients (51%), and alveolus in 11 patients (11%). Medical comorbidities and hypertension was seen in 14 patients, diabetes mellitus in 10 patients, and ischemic heart disease in 01 patient. Habits included oral tobacco consumption in 68 patients (68%), smoking in 28 patients (28%), and alcohol in 27 patients (27%). The baseline median hemoglobin and serum albumin were 11.9 g/dl (IQR: 10.7-13.0) and 3.9 g/dl (3.6-4.3), respectively.
Previous treatment details
The details about the previous treatment are shown in Figure 1. All patients had received previous treatment with curative intent. Forty-seven patients had underwent surgery followed by adjuvant chemoradiation, 29 patients had undergone surgery followed by adjuvant radiation while rest of the 24 patients were planned for neoadjuvant chemotherapy followed by local therapy. In these 24 patients, 16 patients had progressed after NACT; these patients had not received any local treatment. Other 8 patients underwent surgery followed by adjuvant chemoradiation post-NACT. Hence, overall previous radiation and chemotherapy were received by 84 and 71 patients, respectively. The median dose of previous radiation was 60 Gy. The details about previous chemotherapy are shown in Figure 1.
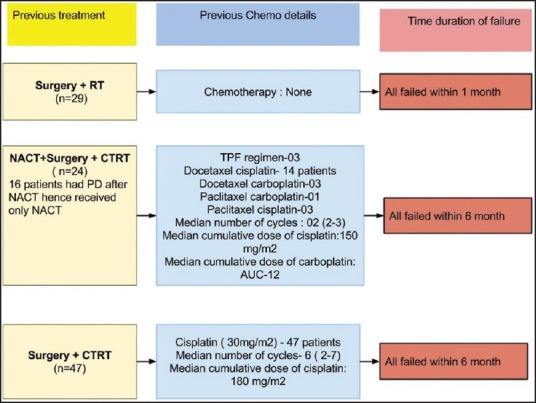
| Fig. 1 Previous treatment details
The sites of failure were loco-regional in 95 patients (95%) while 5 patients (5%) had distant metastasis. The median event free survival (EFS) post last treatment was 2 months (IQR: 2-4 months). The stage at recurrence is shown in Table 1.
Table 1
Details about recurrent stage

Survival
The median estimated OS was 110 days (95% confidence interval [CI]: 85-134 days) [Figure 2]. The proportion of patients surviving at 6 months was 26.4% (95% CI: 17.9-35.6). The median estimated OS was 75 days (95% CI: 41-117 days) in patients with event free period below 3 months while it was 132 days (95% CI: 91-149 days) (P = 0.41) [Figure 3]. Among the factors tested (age, sex, and previous treatment details) none of them would prognosticate for OS [Table 2].
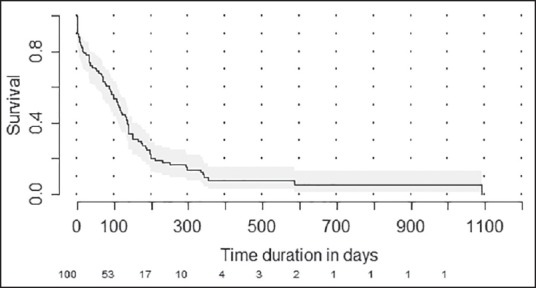
| Fig. 2 Kaplan-Meier plot showing estimated overall survival for the whole cohort. Numbers at risk are shown at the bottom of the graph
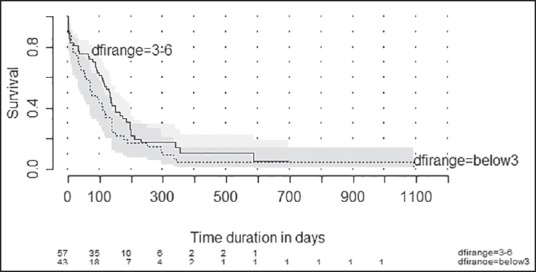
| Fig. 3 Kaplan-Meier plot showing estimated overall survival according to disease-free interval. Disease-free interval range = Below3 - Failure within 3 months of completing multimodality treatment. Disease-free interval range = 3-6: Failure after 3 months but within 6 months of completing multimodality treatment numbers at risk are shown at the bottom of the graph
Table 2
Influence of different factors on OS
DISCUSSION
Recurrent head and neck cancers have limited treatment options.[5] Salvage surgery provides the best chance of cure. Further, the results of salvage surgery are impressive if the DFI is >2 years and stage of recurrent tumor is Stage I or II.[5] Impressive 2-year DFS rates of 70% have been reported in such patients.[16] However, only 16% of recurrent patients are suitable for salvage surgery,[17] either the DFI is short or the disease is extensive. In our study, we have addressed such patients only. Hence, it's not a surprise that none of these patients was considered for salvage surgery by the multidisciplinary clinic. Re-irradiation is the next option available in recurrent head and neck cancers. However, again in the context of early failures as in the present study, re-irradiation is not a feasible option. Reirradiation is associated with 2 years OS 30-40% in different series; however, the series are limited to patients who had DFI of more than 1-year from previous treatment and the tumor volumes were small (< 27 cm3). [5] The cohort of patients in this study had extensive disease and the maximum DFI was 6 months hence palliative chemotherapy was offered.
Palliative chemotherapy in head and neck cancers has evolved over time. It started with a small study demonstrating a survival benefit of chemotherapy over patients treated with only supportive measures.[18] Over next 30-40 years, we have improved survivals modestly by adding targeted therapy to chemotherapy.[12] However, this benefit of targeted therapy, cetuximab was seen in a randomized study done in a selected cohort of patients. Likely, platinum-insensitive patients were excluded from this study and are routinely excluded from the treatment defining palliative chemotherapy studies.[10,11,12] Platinum-insensitive or resistant or refractory patients have never been systematically addressed.[19] The definition of this entity has also been variable. In general, patients who receive either cisplatin cumulative dose more than equal to 60 mg/m2 or carboplatin cumulative dose more than or equal to AUC-4,2 or more cycles of treatment with < 4 weeks among them and failure within 6 months of the last dose of platinum are considered as platinum-resistant.[15] Figure 1 depicts that 71 patients in our cohort who all had received platinum had failed within 6 months and cumulative doses were far beyond these recommendations. We have also included 29 patients who had failed within 1-month of surgery and adjuvant RT. These patients were included as these patients have a biological aggressive disease and even these patients are excluded from chemotherapy studies and hence are never addressed.[10,12]
The treatment options in the platinum-resistant and/or early failure patients studied are limited. Cetuximab as a single agent seems an appropriate option in this setting. In a study done by Vermorken et al., 117 patients who had progressed on platinum were treated with single-agent cetuximab. The disease control rate was 26%. The median time to progression and OS were 50 days and 178 days, respectively. This regimen had limited side effects and was well tolerated.[19] Similar results with a median PFS of 85 days and OS of 183 days with single agent cetuximab were reported in platinum refractory patients by Baselga et al.[7] Combination of cetuximab + cisplatin has shown similar survivals to single agent cetuximab in patients who had progressed within 90 days (3 months) on cisplatin-paclitaxel or cisplatin 5 FU combination.[20] Another combination tried in platinum-insensitive patients is weekly paclitaxel and cetuximab. This regimen in retrospective studies has consistently shown to have median OS around 10.0 months.[21,22] However, the cost of cetuximab precludes its use in most of the low and middle-income countries as a routine standard of care; hence, the search for cheaper alternatives.[13] The mechanism of the action of metronomic is by antiangiogenesis and its effects the endothelial cell of the vasculature supplying the tumor.[23] The endothelial cell is a genotypically stable cell hence it should be unaffected by somatic mutations in the tumor which leads to platinum-resistance.[24] Hence, metronomic chemotherapy theoretically should be equally effective in early failures or platinum-resistant patients. Metronomic chemotherapy combination of methotrexate and celecoxib, unfortunately, failed to meet its expectation in our study. The threshold considered by us for its further studies was 30% OS at 6 months, while the proportion of patients surviving was below this, 26.4% at 6 months.
The impact of time to failure post upfront multimodality treatment on OS with palliative chemotherapy was seen in this study. Patients with time to failure below 3 months had lower OS than patients who had failure between 3 and 6 months posttreatment completion. Previous treatment with radiation, poor PS, previous history of weight loss, nonoropharyngeal location, and poor differentiation are the known poor prognostic features in the patients treated with palliative chemotherapy in head and neck cancers. Patients having two or more of these features (poor risk status) have a survival of around 6 months with first-line platinum chemotherapy.[6] In our study, majority of patients (84 patients, 84%) had previous radiation and all had oral cancer primaries. In addition, nearly three-fourth of the patients had exposure to platinum and had failed within 6 months.
As this metronomic combination has failed to reach the prespecified proportion, we need to consider other options in the patients who cannot afford cetuximab. The mechanism of resistance to methotrexate in head and neck cancers is driven by ABCG2 protein.[25] This protein is overexpressed in 60% of head and neck cancers.[26] Erlotinib is a known potent inhibitor of this protein.[27] Addition of erlotinib to this combination of methotrexate and celecoxib seems a rational approach. Second-line treatment with erlotinib after first-line failure provides response rates of around 15-20% in our experience.[8] At this end, we now planned to study the combination of triple drug metronomic (methotrexate, celecoxib, and erlotinib) in this poor risk cohort.
CONCLUSION
Metronomic chemotherapy combination of methotrexate and celecoxib failed to meet its prespecified efficacy limit and hence should not be used in such patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Previous treatment details

| Fig. 2 Kaplan-Meier plot showing estimated overall survival for the whole cohort. Numbers at risk are shown at the bottom of the graph

| Fig. 3 Kaplan-Meier plot showing estimated overall survival according to disease-free interval. Disease-free interval range = Below3 - Failure within 3 months of completing multimodality treatment. Disease-free interval range = 3-6: Failure after 3 months but within 6 months of completing multimodality treatment numbers at risk are shown at the bottom of the graph


 PDF
PDF  Views
Views  Share
Share

