Metastatic perivascular epithelioid cell tumor responding to mammalian target of rapamycin inhibition
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 99-102
DOI: DOI: 10.4103/0971-5851.133733
Abstract
Perivascular epithelioid cell tumors (PEComa) are a family of rare mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells. Female genital tract and retroperitoneum are common sites of origin of PEComa-not otherwise specified. Diagnosis depends upon characteristic morphology and immunohistochemistry findings. Prognosis of unresectable or metastatic disease is poor. Responses to mammalian target of rapamycin (mTOR) inhibition are encouraging but mostly short-lived. We report a case of metastatic PEComa who responded to mTOR inhibition, albeit for a short duration. We also review the existing literature on mTOR inhibitors in PEComa.
Keywords
Immunohistochemistry - mammalian target of rapamycin inhibitors - perivascular epithelioid cell tumors - temsirolimusPublication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Perivascular epithelioid cell tumors (PEComa) are a family of rare mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells. Female genital tract and retroperitoneum are common sites of origin of PEComa-not otherwise specified. Diagnosis depends upon characteristic morphology and immunohistochemistry findings. Prognosis of unresectable or metastatic disease is poor. Responses to mammalian target of rapamycin (mTOR) inhibition are encouraging but mostly short-lived. We report a case of metastatic PEComa who responded to mTOR inhibition, albeit for a short duration. We also review the existing literature on mTOR inhibitors in PEComa.
INTRODUCTION
Perivascular epithelioid cell tumors (PEComa) are a family of rare mesenchymal tumors composed of histologically and immunohistochemically distinctive perivascular epithelioid cells.[1] We report a case of metastatic PEComa with favorable but short-lived response to mammalian target of rapamycin (mTOR) inhibition.
CASE REPORT
The present case report is about a 57-year-old female patient from Bhutan presented with abdominal pain for 3 months. She had total abdominal hysterectomy and left salpingo-oophorectomy 7 years back. Indications for the surgery and histopathology results were not available. Recent computed tomography (CT) scans of the abdomen and thorax showed a large pelvic mass, with enlarged retroperitoneal nodes and bilateral pleural nodules. Exploratory laparotomy undertaken in Bhutan for her recent complaints showed a large pelvic mass with dense adhesions. Sub-optimal debulking and omentectomy was performed. Histology was reported as probable dysgerminoma, with omentum positive for malignancy. At this point, she was referred to our center. Examination revealed Eastern Cooperative Oncology Group performance status 1, no palpable neck or groin nodes and a fixed pelvic mass palpable in right iliac fossa and hypogastric region. Magnetic resonance imaging of pelvis showed complex SOL of size 8.6 cm × 8.1 cm × 6.8 cm with solid and cystic components, occupying the pelvis [Figure 1a]. Bilateral involved external iliac nodes with multiple inguinal nodes were seen. CT scan of thorax and upper abdomen showed bilateral pleural nodules [Figure [Figure1b1b and andc].c]. Histopathology review revealed an infiltrating neoplasm arranged in lobules separated by thick and thin fibrovascular septae with lymphoplasmacytic infiltrate [Figure 2a]. Tumor cells showed abundant pale vacuolated or eosinophilic cytoplasm, round or oval nuclei with moderate nuclear atypia and variably prominent nucleoli [Figure 2b]. Large areas of coagulative necrosis, areas of hemorrhage and three mitotic figures per 10 high-power fields (HPF) were noted. Immunohistochemical stains showed the neoplastic cells to be positive for vimentin. Melan-A, HMB-45, smooth muscle actin (SMA) and epithelial membrane antigen showed patchy positivity [Figure [Figure2c2c and andd].d]. Tumor cells were negative for S100, ER, partial response, PLAP, AFP, inhibin, WT1, CD10, D2-40, CK7, CD15, desmin, h-caldesmon, CD117. Based on above findings, a diagnosis of malignant PEComa was established.
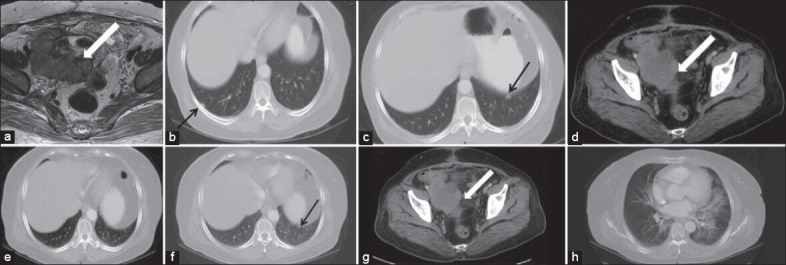
| Figure 1:(a) Magnetic resonance imaging pelvis showing intermediate signal SOL with cystic areas and necrosis (block arrows). (b and c) Contrast enhanced computed tomography (CECT) thorax shows bilateral pleural nodules (arrows). (d-f) CECT 8 weeks after therapy shows 10% reduction of the dimensions of the pelvic mass and disappearance of right sided pleural nodule while left pleural nodule persists. (g and h) CECT after another 8 weeks shows further reduction in the size of the pelvic mass while left lower lobe of lung and perihilar regions show interstitial pneumonitis
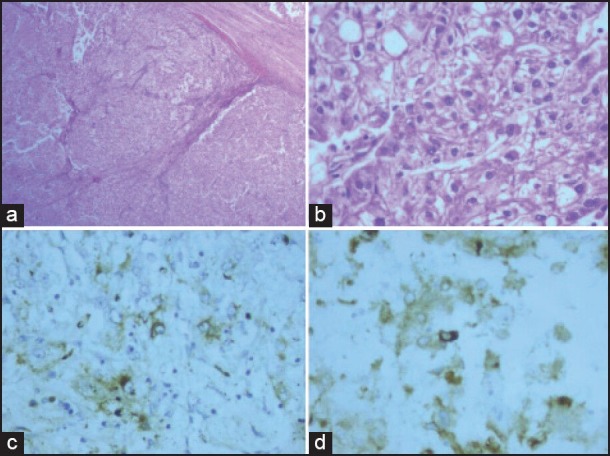
| Figure 2:Pelvic mass. (a) Section shows infiltrating neoplasm arranged in lobules separated by thick and thin fibrovascular septae with lymphoplasmacytic infiltrate (H and E, ×40). (b) Tumor cells with abundant pale vacuolated cytoplasm, with moderate nuclear atypia and variably prominent nucleoli (H and E, ×400). (c) Patchy positivity for HMB-45 (×400). (d) Patchy positivity for melan-A (×400)
She was started on weekly temsirolimus 25 mg intravenous infusion, which she tolerated well except oral mucositis (grade 1), edema, hypertriglyceridemia and hyperglycemia. These were well-controlled with appropriate therapy. Post week 8 CT thorax and abdomen revealed 10% reduction in size of pelvic mass and significant reduction in number and size of lung nodules [Figure [Figure1d1d–f]. After another 8 weeks of temsirolimus, another CT scan showed further 25% reduction in the pelvic mass with small left pleural nodules but showed new development of diffuse ground glass opacities along with interlobular septal thickening in both lungs, predominantly in the perihilar regions, likely to be interstitial pneumonitis [Figure [Figure1g1g and andh].h]. She did not have any cough or chest signs. She wanted to go back to her home country and hence started on oral sirolimus 2 mg daily. After 1 month, she had cough for which she stopped sirolimus. Within 3 months, she developed progression of disease, CT revealed large pelvic mass of 20 cm and bilateral numerous lung nodules. She died soon thereafter, 10 months from diagnosis.
DISCUSSION
The PEComa family of tumors comprises of angiomyolipoma (AML), clear cell sugar tumor of lung, lymphangiomyomatosis (LAM) of lung and PEComas arising from various other sites which are also termed as PEComa not otherwise specified.[1] PEComas of various anatomical sites have been reported of which uterus and retroperitoneum are the most common sites of origin.[2] Histologically, PEComas are characterized by proliferation of spindle and epithelioid cells in various proportions and exhibit a characteristic perivascular pattern of arrangement of tumor cells. PEComas have characteristic immunohistochemical findings.[3] In one review, 92% were HMB-45 positive, 85% were vimentin positive, 80% were SMA positive, 72% were melan-A positive, 36% were desmin positive, 33% were S-100 positive, 13% were cytokeratin positive and 5% were CD117 positive.[3]
Major differential diagnoses considered were endometrial stromal sarcoma, malignant melanoma or clear cell sarcoma and epithelioid smooth muscle tumors. Endometrial stromal sarcomas were excluded as they are HMB-45 negative. Malignant melanoma and clear cell sarcoma were ruled out as they usually show strong nuclear and cytoplasmic immunoreactivity for S-100 protein and diffuse immunoreactivity for other melanocytic markers. Epithelioid leiomyosarcoma lacks the distinctive vascular network seen in PEComas and usually shows areas of smooth muscle morphology. In our case, the histomorphology of the tumor as well as immunophenotypic findings were consistent with a PEComa. Dysgerminoma was excluded as the tumor cells were negative for placental alkaline phosphatase and CD117. There are no well-defined criteria for distinguishing benign from malignant PEComas. The study of Folpe et al. have classified soft-tissue and gynecologic PEComas as malignant when two or more of the following features were present: tumor size >5 cm, infiltrative growth, high nuclear grade and cellularity, coagulative tumor necrosis, vascular invasion and >1 mitotic figure/50 HPFs.[3] Our case was diagnosed as malignant PEComa based on these criteria.
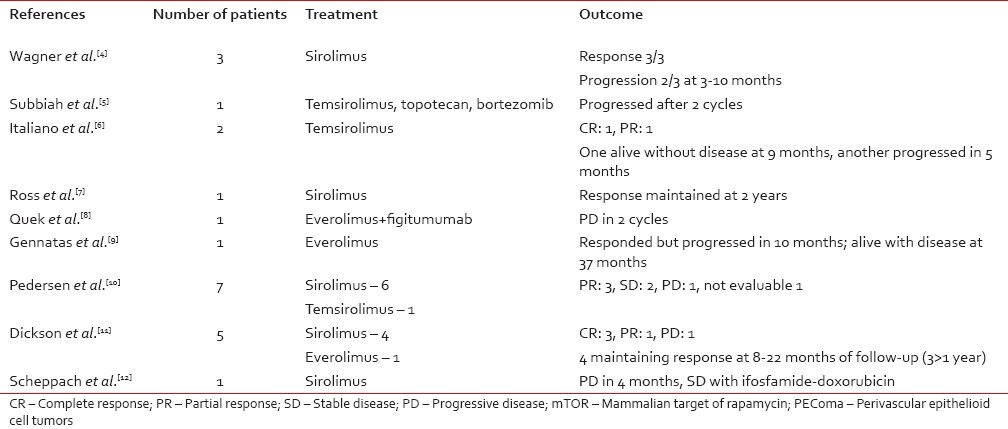
Though the site of origin was unclear, a pelvic mass with prior history of hysterectomy for unknown indication points toward a possible uterine origin. Treatment consists of surgery but there is no data on role of adjuvant therapy. In unresectable or metastatic disease, chemotherapy seldom produces response. LAM and AML were shown to have mTOR activation and amenable to treatment with mTOR inhibitors. Because PEComas share activation of the mTOR pathway with LAM and AML, mTOR inhibition has been tried in PEComas also. A review of literature for published and presented reports show that 14/21 patients responded and 2 had stable disease [Table 1].[4,5,6,7,8,9,10,11,12] Though response rates are probably high, they are mostly short-lived. We could find only five evaluable patients with response documented beyond 1 year. Toxicity of mTOR inhibitors may be prohibitive in some cases. In our patient also, temsirolimus produced good response with symptomatic improvement but sirolimus had to be stopped because of pneumonitis and the disease progressed soon after.
Table 1
Results of mTOR inhibitors in PEComa
|
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Folpe AL. Neoplasms with perivascular epithelioid cell differentiation (PEComas). In: Fletcher CD, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC Press; 2002. p. 221-2.
- Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: Risk stratification and treatment strategies. Sarcoma 2012;2012:541626.
- Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005;29:1558-75.
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: Targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010;28:835-40.
- Subbiah V, Trent JC, Kurzrock R. Resistance to mammalian target of rapamycin inhibitor therapy in perivascular epithelioid cell tumors. J Clin Oncol 2010;28:e415.
- Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, et al. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol 2010;21:1135-7.
- Ross C, Sharma S, Louca O, Scurr M, Hayes A, Judson I. A patient presenting with a perivascular epithelioid cell tumor in the broad ligament: A case report. J Med Case Rep 2011;5:383.
- Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: Phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res 2011;17:871-9.
- Gennatas C, Michalaki V, Kairi PV, Kondi-Paphiti A, Voros D. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J Surg Oncol 2012;10:181.
- Pedersen JV, Benson C, Tunariu N, Mitchell S, Fisher C, Thway K, et al. A retrospective study from the Royal Marsden Hospital (RMH) of patients with malignant perivascular epithelioid cell tumors (PEComa) receiving treatment with sirolimus (SI) or temsirolimus (TSI). J Clin Oncol 2012;30 Suppl: Abstr 10038.
- Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: Clinical and molecular correlates. Int J Cancer 2013;132:1711-7.
- Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol 2013;19:1657-60.

| Figure 1:(a) Magnetic resonance imaging pelvis showing intermediate signal SOL with cystic areas and necrosis (block arrows). (b and c) Contrast enhanced computed tomography (CECT) thorax shows bilateral pleural nodules (arrows). (d-f) CECT 8 weeks after therapy shows 10% reduction of the dimensions of the pelvic mass and disappearance of right sided pleural nodule while left pleural nodule persists. (g and h) CECT after another 8 weeks shows further reduction in the size of the pelvic mass while left lower lobe of lung and perihilar regions show interstitial pneumonitis

| Figure 2:Pelvic mass. (a) Section shows infiltrating neoplasm arranged in lobules separated by thick and thin fibrovascular septae with lymphoplasmacytic infiltrate (H and E, ×40). (b) Tumor cells with abundant pale vacuolated cytoplasm, with moderate nuclear atypia and variably prominent nucleoli (H and E, ×400). (c) Patchy positivity for HMB-45 (×400). (d) Patchy positivity for melan-A (×400)
References
- Folpe AL. Neoplasms with perivascular epithelioid cell differentiation (PEComas). In: Fletcher CD, Unni KK, Mertens F, editors. World Health Organization Classification of Tumors: Pathology and Genetics of Tumors of Soft Tissue and Bone. Lyon: IARC Press; 2002. p. 221-2.
- Bleeker JS, Quevedo JF, Folpe AL. "Malignant" perivascular epithelioid cell neoplasm: Risk stratification and treatment strategies. Sarcoma 2012;2012:541626.
- Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: A clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol 2005;29:1558-75.
- Wagner AJ, Malinowska-Kolodziej I, Morgan JA, Qin W, Fletcher CD, Vena N, et al. Clinical activity of mTOR inhibition with sirolimus in malignant perivascular epithelioid cell tumors: Targeting the pathogenic activation of mTORC1 in tumors. J Clin Oncol 2010;28:835-40.
- Subbiah V, Trent JC, Kurzrock R. Resistance to mammalian target of rapamycin inhibitor therapy in perivascular epithelioid cell tumors. J Clin Oncol 2010;28:e415.
- Italiano A, Delcambre C, Hostein I, Cazeau AL, Marty M, Avril A, et al. Treatment with the mTOR inhibitor temsirolimus in patients with malignant PEComa. Ann Oncol 2010;21:1135-7.
- Ross C, Sharma S, Louca O, Scurr M, Hayes A, Judson I. A patient presenting with a perivascular epithelioid cell tumor in the broad ligament: A case report. J Med Case Rep 2011;5:383.
- Quek R, Wang Q, Morgan JA, Shapiro GI, Butrynski JE, Ramaiya N, et al. Combination mTOR and IGF-1R inhibition: Phase I trial of everolimus and figitumumab in patients with advanced sarcomas and other solid tumors. Clin Cancer Res 2011;17:871-9.
- Gennatas C, Michalaki V, Kairi PV, Kondi-Paphiti A, Voros D. Successful treatment with the mTOR inhibitor everolimus in a patient with perivascular epithelioid cell tumor. World J Surg Oncol 2012;10:181.
- Pedersen JV, Benson C, Tunariu N, Mitchell S, Fisher C, Thway K, et al. A retrospective study from the Royal Marsden Hospital (RMH) of patients with malignant perivascular epithelioid cell tumors (PEComa) receiving treatment with sirolimus (SI) or temsirolimus (TSI). J Clin Oncol 2012;30 Suppl: Abstr 10038.
- Dickson MA, Schwartz GK, Antonescu CR, Kwiatkowski DJ, Malinowska IA. Extrarenal perivascular epithelioid cell tumors (PEComas) respond to mTOR inhibition: Clinical and molecular correlates. Int J Cancer 2013;132:1711-7.
- Scheppach W, Reissmann N, Sprinz T, Schippers E, Schoettker B, Mueller JG. PEComa of the colon resistant to sirolimus but responsive to doxorubicin/ifosfamide. World J Gastroenterol 2013;19:1657-60.


 PDF
PDF  Views
Views  Share
Share

