Metastatic Ewing’s Sarcoma: Revisiting the “Evidence on the Fence”
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 173-181
DOI: DOI: 10.4103/ijmpo.ijmpo_24_17
Abstract
Metastatic Ewing's sarcoma is a challenging disease for oncology care providers with wide spectrum of disease at presentation, widely varying approach to the treatment and varied outcomes. The paucity of randomized evidence is a barrier in developing a consensus. This perspective provides the evidence ”for and against” the benefit of aggressive approach including local and systemic therapy in patients presenting with metastatic Ewing's sarcoma and provide general recommendations so as to help select patients who will benefit with definitive intent treatment and also, avoid aggressive approach in patients with dismal outcome.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Metastatic Ewing's sarcoma is a challenging disease for oncology care providers with wide spectrum of disease at presentation, widely varying approach to the treatment and varied outcomes. The paucity of randomized evidence is a barrier in developing a consensus. This perspective provides the evidence ”for and against” the benefit of aggressive approach including local and systemic therapy in patients presenting with metastatic Ewing's sarcoma and provide general recommendations so as to help select patients who will benefit with definitive intent treatment and also, avoid aggressive approach in patients with dismal outcome.
Introduction
Ewing's sarcoma is the second most common malignant bone tumor in adolescents and young adults, with high propensity to metastasize due to its aggressive behavior. It includes a family of tumors with common mesenchymal progenitor cell origin, characterized by similar histological and immunohistochemical features with unique nonrandom chromosomal translocation involving ESWR gene on chromosome 22. About 25%-patients present with upfront clinical metastatic disease, while 80%–90%-have subclinical microscopic widespread disease at baseline.[1] Hence, local therapy alone is ineffective and use of chemotherapy has led to remarkable improvement in survival.[2,3] However, outcome of patients with upfront metastasis is dismal with long-term cure in 20%–30%-of patients despite aggressive chemotherapy and local therapy.[4] Meticulous selection of patients with metastatic disease may help in salvaging good-risk patients with aggressive systemic and local therapy while help conserve resources and spare serious toxicity to patients with widespread poor-risk metastatic disease.
There are lack of consensus and absence of uniform treatment policy for patients with upfront metastatic Ewing's sarcoma. The primary reason is that most of the series and published trials are retrospective in nature with selection and reporting bias. Moreover, majority of studies have heterogeneous and diverse patient population with equally varied local therapy to primary, treatment for metastatic site as well as different combination and dose intensity of chemotherapy used. The objective of this review is to collate evidence for benefit of local therapy and systemic therapy in patients presenting with upfront metastatic Ewing's sarcoma and provide general recommendations so as to select patients who will benefit with definitive intent treatment and also avoid aggressive approach in patients with dismal outcome.
Method and Design
Published trials, case series, and studies were identified using PubMed search engine to assess Medline Express database (National Library of Medicine, Washington, DC, USA) and Cochrane Collaboration database for data until May 2016. The keywords used were ”Ewing's sarcoma,” ”pulmonary metastasis,” ”bone metastasis,” ”lung irradiation,” whole lung irradiation, ”pulmonary metastasectomy,” ”high dose chemotherapy,” ”autologous transplant Ewing's,” ”allogenic transplant.” Due to several studies combining local and systemic therapy intensification for metastatic Ewing sarcoma for optimizing outcomes, we segregated this review into:
- Local therapy for metastatic disease: (i) Pulmonary metastasis: Whole lung irradiation (WLI) and pulmonary metastasectomy (PM); (ii) Bone metastasis: Curative intent radiotherapy for bone metastasis
- Systemic chemotherapy: (i) Conventional dose; (ii) Dose-dense and dose-intense; (iii) High-dose chemotherapy and transplant.
Local therapy for metastatic disease: Pulmonary metastasis
Whole lung irradiation
Synchronous pulmonary metastasis at presentation can be seen in 12%–20%-of patients with Ewing's sarcoma.[4] Studies evaluating the role of WLI in patients with de novo pulmonary metastasis in Ewing's sarcoma are summarized in Table 1. Outcome of patients with pulmonary-only metastasis is better than those with bone metastasis, which in turn is better than patients who present with bone and pulmonary metastasis.[4,5,6,7] The impact of local therapy for pulmonary metastasis is substantial in terms of 3-fold improvement in survival, from 14% 3-year overall survival to 39%, as per one of the largest series reported by EURO-EWING 99 study.[5] Moreover, patients with unilateral lung metastasis do better than bilateral metastasis.[7]
Table 1
Studies showing outcomes with consolidative whole lung irradiation (WLI) in patients with de novo pulmonary metastasis
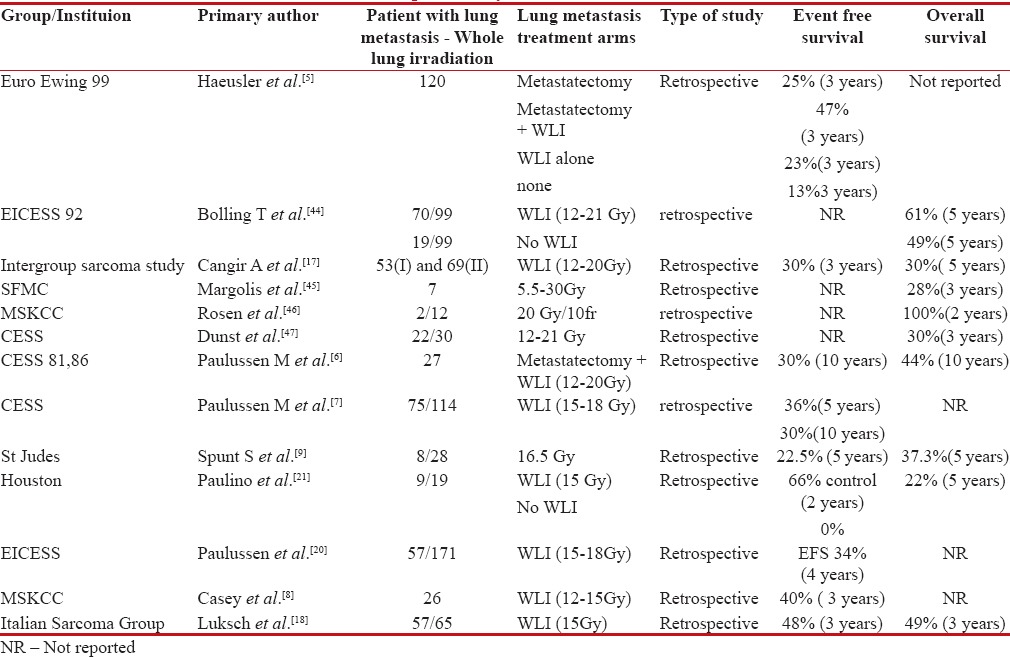
|
Retrospective evaluation of Centre for Economic and Social Studies study showed that addition of WLI after induction chemotherapy, irrespective of PM improved survival up to 30%. Doses between 12 and 15 Gy have acceptable toxicity and potential therapeutic effect.[8] In one of the largest retrospective studies reported, Paulussen et al. demonstrated improved benefit of WLI sustained after 10 years of follow-up with 30% event-free survival (EFS). Poor response to induction chemotherapy to primary, bilateral pulmonary metastasis and without WLI was significant negative prognostic factor in multivariate analysis.[6,7]
WLI in patients who are poor pulmonary responders to induction chemotherapy demonstrated similar benefit as compared to patients who achieved complete pulmonary response to induction chemotherapy, but without WLI.[9] However, the authors concluded that consolation radiotherapy to lung after good response to induction chemotherapy may provide similar benefits and should be explored further.[9]
With respect to evaluation of prognostic factors affecting outcomes in disseminated Ewing's, patient with marrow metastasis and bone metastasis along with primary disease more than 200 ml in volume conferred poor outcome (10% survival) as compared to patient with solitary pulmonary metastasis (50%).[10] They also proposed a three-tier prognostic risk scoring system which was subsequently validated with French Ewing's sarcoma data, hence demarcating outcomes between good-risk and poor-risk metastatic disease which will be helpful for selecting patients before aggressive therapy.[10]
The concern regarding pulmonary toxicity with WLI in survivors of pulmonary metastasis is uncalled for as <7 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582556/#ref11" rid="ref11" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635949932" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>11] Even with longer follow-up studies for patients receiving WLI, only about 10% patients develop significant pulmonary dysfunction, while more than 50% of patients have normal pulmonary function test.[11,12]
Pulmonary metastasectomy
Surgical resection of pulmonary metastasis has demonstrated survival benefit in certain small round blue tumors with synchronous and metachronous pulmonary metastasis such as osteosarcoma.[13] However, in Ewing's sarcoma, there is a paucity of data comparing PM versus lung irradiation in de novo lung metastasis. In one of the largest retrospective studies reported by MD Anderson, Texas, PM with or without WLI significantly improved survival, 80% versus 0% at 5 years. However, the caveat to this study is that majority of the patients chosen for PM had selection bias as smaller and lesser number of pulmonary metastasis was chosen along with those who had good pulmonary function to sustain metastasectomy. Nonetheless, this study did show potential for improved survival among patients who undergo PM.[14]
In another retrospective analysis of children undergoing PM for childhood small round cell malignancies including Ewing's sarcoma, the number of pulmonary metastasis and unilateral or bilateral metastasis did not have significant impact on outcome.[15] However, if the primary lesion is not controlled and/or the patient is developing progressive metastatic disease while undergoing chemotherapy, it is considered to have a poor outcome.15 On the contrary, another series reported from France suggested that the number of pulmonary metastasis was an independent predictor of survival with patients less than two pulmonary metastasis, unilateral metastasis, and complete excision showing long-term survival.[16] Large data from the Thames registry on bone sarcomas have shown that the 5-year survival rate among sarcoma patients who are selected to have PM is higher than that observed among unselected registry data for patients with any metastatic disease at diagnosis.[49]
Table 2 demonstrates studies addressing PM in de novo pulmonary metastasis of Ewing's sarcoma. In summary, most of the series have not shown an effect of number of pulmonary metastases on overall outcome, and hence, all patients presenting with only pulmonary metastases should be treated with definitive intent.[8,17] However, fewer PMs and patients with complete response (CR) to induction chemotherapy will have an improved survival.[11,18,19]
Table 2
Studies showing outcomes with pulmonary metastatectomy (PM) in patients with de novo pulmonary metastasis
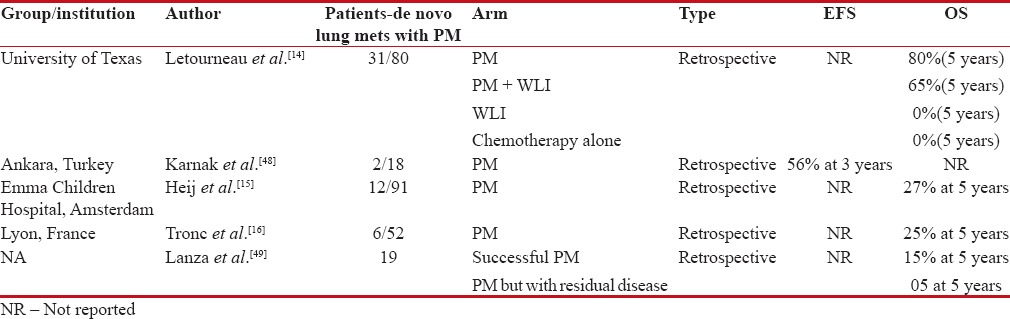
|
Local therapy for metastatic disease: Bone metastasis
Patients with bone metastasis fare poorly compared to patients presenting with pulmonary-only metastasis.[5,20] Moreover, solitary bone metastasis have superior outcomes compared to those with more than one bony metastasis.[10] In patients with oligometastatic disease, chemotherapy by itself fails to have good long-term control rate of primary and metastatic disease alone. Long-term survivors with metastatic disease are the ones who underwent local radiotherapy for bone metastasis along with WLI for pulmonary metastasis.[5,21] Patients undergoing local radiotherapy for bone metastasis had longer disease control rate at 3 years (94% vs. 32%).[21] However, demographic distribution of patients with bone metastasis demonstrated only 10% having solitary metastasis, while 25% had 2–5 lesions, 44% had more than five bone metastases, thus limiting the numbers to less than one-third who would have long-term outcomes with aggressive chemotherapy and local radiotherapy.[5]
Another study, reported by MSKCC, showed that use of radiotherapy in bone metastasis to a dose of 50 Gy leads to 91% local control rates at 3 years. It also concluded that patients with more than five bony metastases had unequivocally worse outcomes.[22] To summarize, patients with de novo solitary bone metastasis enjoy better long-term outcomes if local radiotherapy to a dose of 50 Gy is added to bone metastasis along with systemic chemotherapy; however, this benefit drastically declines with increasing number of metastasis correlating with aggressive systemic disease [Table 3].
Table 3
Studies showing benefit of local bone radiotherapy in patients with de novo metastatic Ewings
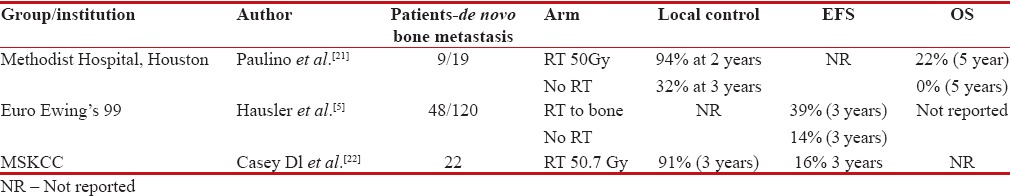
|
Systemic therapy
Conventional chemotherapy
Ewing's sarcoma is a systemic aggressive disease which is sensitive to chemotherapy. In advanced metastatic disease, conventional dose of chemotherapy produces excellent short-term response rates up to 88%.[23] However, despite aggressive local therapy of primary and pulmonary/bone metastasis, systemic relapse is the predominant pattern of failure, suggesting need for more intense systemic therapy.[24] Addition of ifosfamide and etoposide (IE), in initial studies, when added to standard-dose cyclophosphamide (C), adriamycin (D), vincristine (V), and actinomycin D (A) showed modest benefit.[24,25] Similar enthusiastic outcomes were seen in studies comparing patients treated before 1980 and after that incorporating IE.[26] Another French study similarly demonstrated higher responders to ifosfamide over standard voltage-dependent anion channel (VDAC) combination even in malignant high-risk Ewing's sarcoma.[27] However, addition of 5-fluorouracil over standard dose conventional VDAC in two series of Intergroup Ewing's sarcoma study (IESS) did not show any benefit over standard combination with 5-year survival around 30% in both arms.[7]
Despite initial encouraging results with addition of IE, subsequent larger studies showed that above combination improved outcomes exclusively in high-risk nonmetastatic Ewing's only, while in metastatic de novo disease, the outcomes were similar to conventional VDAC combination. Paulussen et al. further confirmed that addition of etoposide along with vincristine, adriamycin, ifosfamide, and actinomycin D improved outcomes only in high-risk nonmetastatic disease.[28] In one of the largest reported de novo metastatic Ewing's sarcoma data from India, despite aggressive local therapy for primary and metastatic disease after good response to induction chemotherapy, use of standard chemotherapy alone resulted in poor outcomes. Remarkably, there was lack of survival difference irrespective of lung only metastasis, bone only or bone with pulmonary metastasis, hence concluding all patients with metastatic disease should be considered for best supportive care only upfront [Table 4].[29]
Table 4
Studies showing outcomes with standard dose chemotherapy in patients with de novo metastatic Ewing's
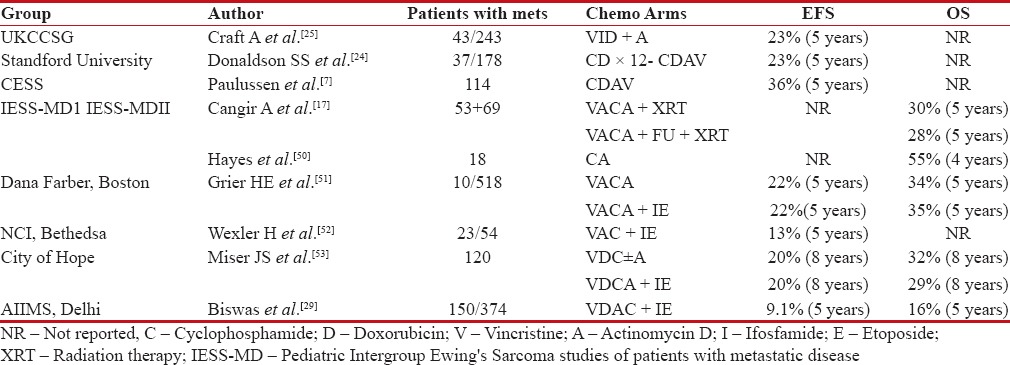
|
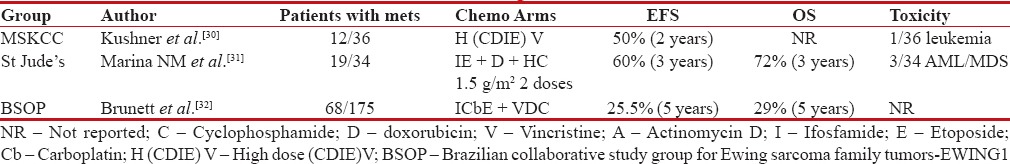
Dose dense and intense chemotherapy
Use of conventional dose chemotherapy VDAC + IE resulted in short-term good responses, but majority of patients succumb to early systemic relapses. Hence, increase in dose density and intensity was attempted in some studies to increase outcomes of patients with metastatic Ewing's. In MSKCC retrospective series, high-dose chemotherapy was used in 36 patients with poor-risk primitive neuroectodermal tumor, either due to high volume of primary >100 cm3 or metastasis to bone or marrow metastasis. Despite higher dose of adriamycin, cyclophosphamide, IE, patients had early distant relapses soon after achieving excellent early remission of bone and marrow metastasis.[30] In another feasibility study by St. Jude Children's Research Hospital, dose intensification during maintenance phase was attempted. Only 66% patients completed planned therapy, while 80%–85% had severe Grades 3 and 4 neutropenia and thrombocytopenia. Three out of 34 patients developed secondary myeloid malignancies. This study documented an impressive 72% 3 year survival.[31]
Brazilian collaborative group added carboplatin along with IE at induction and maintenance for patients with high-risk metastatic Ewing's, suggesting an improved survival up to 30% at 5 years, though toxicity was a major concern.[32] In another feasibility study of interval-compressed chemotherapy given every 14 days with granulocyte-colony stimulating factor support, Womer et al. showed modest toxicity and EFS similar to contemporary studies.[33] These above heterogeneous retrospective studies showed modest to poor outcomes with dose intensification with a significant increase in acute toxicities [Table 5].
Table 5
Studies showing outcomes with dose dense/dose intense chemotherapy in patients with de novo metastatic Ewing's
|
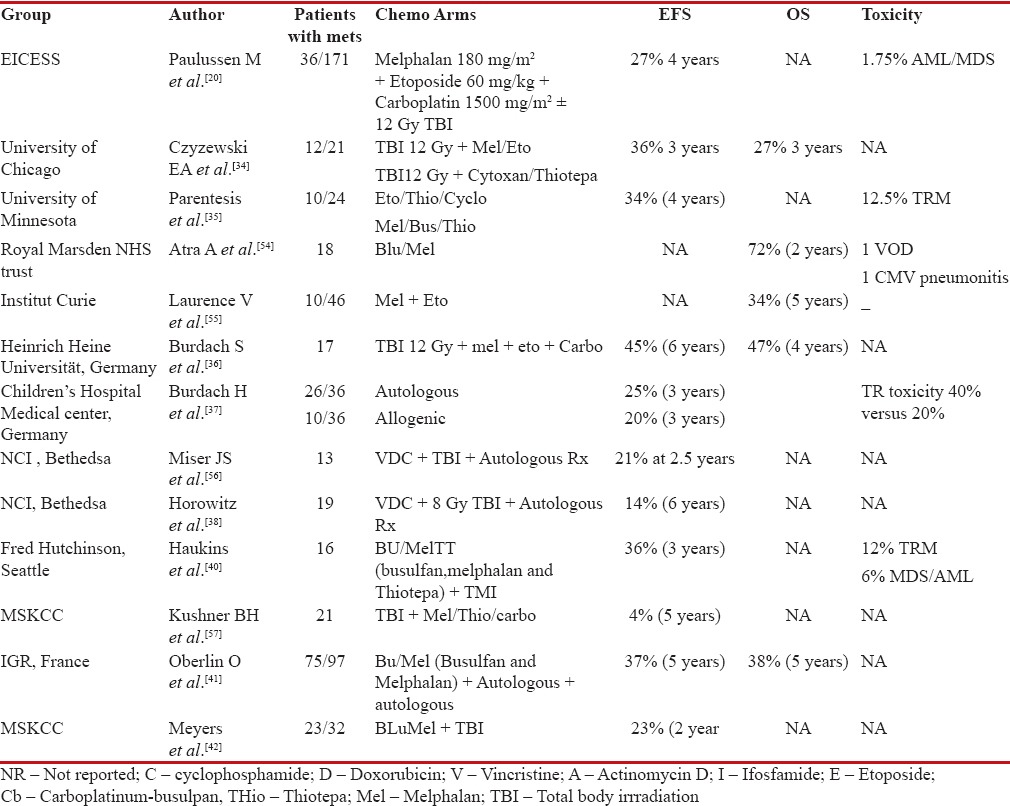
High-dose chemotherapy and transplant
Ewing's sarcoma being a chemosensitive disease with steep dose–response curve, high-dose chemotherapy with stem cell transplant was thought to be rationale approach in treating high-risk Ewing's with large primary tumor or de novo metastatic disease. Use of melphalan, etoposide, and high-dose carboplatin followed by autologous transplant in a small subset of patients with upfront pulmonary and skeletal metastasis resulted in 27%-EFS at 3 years.[20] In another study, use of total-body irradiation (TBI) with high-dose chemotherapy (melphalan, etoposide, or cytoxan-thiotepa) after induction chemotherapy in metastatic Ewing's leads to 27%-3-year overall survival. In this study, only response to induction chemotherapy was the significant factor affecting outcome in multivariate analysis. Local control was boosted with the use of consolidative radiotherapy in selected metastatic sites.[34]
The impact of good response to induction chemotherapy was further demonstrated in a subset of patients from the University of Minnesota. Use of high-dose chemotherapy, etoposide, thiotepa, and cyclophosphamide or melphalan, busulfan, and thiotepa conditioning followed by peripheral blood stem cell (PBSC) transplant leads to 4-year EFS of 34%. However, only patients who achieved complete remission after standard induction therapy had better long-term outcomes. Among those who were transplanted after partial response to induction therapy, 13/15 patients died because of disease progression or transplant-related complications.[35] Outcomes with 45%-relapse-free survival at 6 years were demonstrated by Burdach et al., in a retrospective study of multifocal Ewing's after induction chemotherapy followed by high-dose melphalan, etoposide, and carboplatin. However, all patients also received local therapy at primary and metastatic site.[36] Allogenic transplant did not improve survival compared to autologous transplant in multifocal Ewing's. On the contrary, treatment-related toxicity was twice higher in allogenic arm along with threat for higher secondary malignancies. Similarly, no benefit of graft versus tumor was demonstrated in allogenic transplant.[37]
Although few studies mentioned above demonstrated better outcomes with high-dose chemotherapy followed by transplant, another set of studies highlighted shortcomings of high-dose transplant with poor outcomes, high treatment-related complications, and secondary malignancies. Horowitz et al. showed lack of benefit of 8 Gy TBI and autologous transplant for metastatic Ewing's sarcoma, suggesting poor response to this subset of patients and need for further intensification.[38] Similarly, Bader et al. also showed poorer outcomes despite TBI and PBSC transplant.[39] Further intensification of therapy using dual intensive myeloablative therapy (busulfan, melphalan, and thiotepa) followed by total marrow irradiation in high-risk Ewing's sarcoma of family tumor led to 3-year EFS of 36%. However, this intensification was marred by inability to collect sufficient PBSC due to previous radiation therapy in 43%-of patients.[40] Even in MSKCC series, poor EFS was achieved despite TBI and melphalan/thiotepa conditioning in patients with de novo bone and bone marrow metastasis [Table 6]. On the contrary, use of such high-dose increased severe acute toxicities along with an increased risk of secondary malignancies.
Table 6
Studies showing outcomes with high dose chemotherapy and PBSC transplant in patients with de novo Ewing's sarcoma
|
Another alternative strategy is to use high-dose chemotherapy followed by hematopoietic stem cell transplant selectively in patients who achieve CR or very good partial response to standard induction chemotherapy. Data from Institut Gustave Roussy showed better overall survival of 38% at 5 years in patients who received BluMel conditioning followed by autologous transplant after achieving CR or very good partial response to induction chemotherapy. Benefit was exclusively for pulmonary metastasis (52% survival) or bone metastasis (36% survival at 5 years) only, while marrow involvement fared poorly (1% long-term survival).[41] Contrary to above result, similar study reported by MSKCC but with bone and marrow metastasis failed to show any benefit of consolidative autologous transplant.[42] This was further substantiated with European bone marrow transplant solid tumor registry, in which consolidative mega therapy when used in patients with bone or marrow metastasis after achieving response to induction chemotherapy led to poorer outcome.[43]
Thus, due to equivocal results in patients with advanced disease as shown in above retrospective heterogeneous series, high-dose chemotherapy and autologous stem cell transplant remain an investigational approach. The ongoing EURO-EWING 99 trial is comparing high-dose chemotherapy versus standard approach in a variety of clinical scenarios including metastatic disease at presentation.
Discussion
Ewing's sarcoma is an aggressive primary bone tumor with high propensity of systemic metastasis. One-fourth of patients are diagnosed with upfront de novo metastatic disease in which long-term outcomes are dismal.[1] This cohort of patients usually have heterogeneous disease extent at presentation ranging from solitary pulmonary or solitary bone metastasis to extensive multiorgan disseminated disease with outcomes as varied as 40%–< 10 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5582556/#ref4" rid="ref4" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_635949947" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>4] In view of above, this heterogeneous group of de novo metastatic Ewing's needs to be stratified into risk groups so as to decide which subset of patients will have superior long-term outcome with aggressive local therapy and systemic chemotherapy, while segregating others at baseline diagnosis who can be considered to best supportive care upfront. This would lead to sparing of acute toxicities of aggressive systemic chemotherapy in poor-risk group along with optimization of scarce resources, especially in low- and middle-income countries.
Majority of studies reporting outcome in upfront metastatic Ewing's have used varied combination of chemotherapy with equally diverse local metastatic site consolidative therapy with heterogeneous doses of chemotherapy and radiotherapy. Moreover, the case series published are predominantly retrospective in nature with heterogeneous patient population with considerable selection, reporting, and publication bias, hence limiting our ability to interpolate the evidence for routine clinical practice. There is urgent unmet medical need to segregate patients among de novo metastatic disease who would benefit with aggressive curative intent prolonged therapy versus those who would have early systemic relapse and hence poor survival.
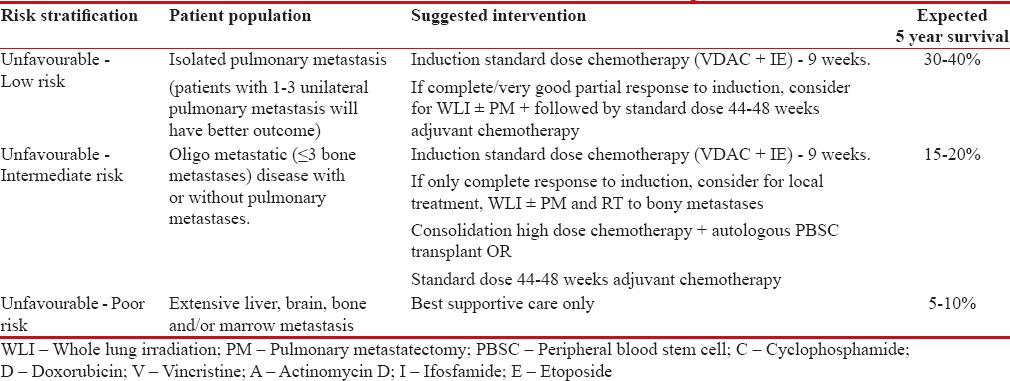
After careful evaluation of available literature, it is feasible to categorize these de novo metastatic Ewing's sarcoma into three unfavorable risk categories: low, intermediate, and poor [Table 7]. Patients with isolated/or only pulmonary metastases at presentation will come within the low-risk group and should be considered for standard dose curative induction chemotherapy (VDAC ± IE). Out of these, patients with unilateral 1–3 pulmonary nodules will have better outcome than the rest. Patients who achieve CR or very good partial response after 9 weeks of induction chemotherapy should proceed with consolidative local metastatic therapy of WLI, WLI (12–15 Gy) with or without PM (residual nodules after induction chemotherapy) for pulmonary metastasis. This has to be followed with consolidative standard dose chemotherapy (VDAC ± IE) to a total duration of 44–48 weeks. This relatively favorable cohort may have better outcomes with standard dose chemotherapy and aggressive local therapy, provided they have CR or near-CR to 9 weeks of induction therapy. Unfavorable intermediate-risk group comprising oligo-bone metastasis (≤3 bone metastases) has inferior outcomes as compared to unfavorable low-risk group. Patients with response at primary and metastatic sites with standard dose induction chemotherapy should proceed for further curative therapy in this cohort of patients. Patients with lung metastases should proceed with WLI with or without PM with consolidative radiotherapy to the sites of bone metastasis and local treatment of the primary disease. This cohort of patients may benefit with further consolidative high-dose chemotherapy with autologous PBSC transplant, if resources permit or they should be considered otherwise for completion of adjuvant standard dose chemotherapy till 44–48 weeks. Widely disseminated disease at presentation constitutes poor risk, unfavorable group; these patients with upfront marrow, liver, brain, and multiple bone metastasis (>3 bone metastases) may be considered for palliative intent systemic therapy; however, the outcomes are dismal. These patients may also be considered for only best supportive care, especially in resource-limited settings. There is a need to identify subset of patients among this group which may benefit with aggressive approach.
Table 7
Risk stratification for de novo metastatic Ewing's sarcoma
|
Perspective
Metastatic Ewing's sarcoma is an essentially aggressive systemic disease presenting as a wide spectrum varying from isolated pulmonary metastasis to widely disseminated multiorgan disease with varied outcomes. The paucity of randomized evidence makes the management further challenging. However, the current evidence suggests that in low- and intermediate-risk groups, aggressive (local and systemic) approach improves outcome, wherein in poor-risk group with dismal outcome, aggressive approach can be avoided.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Nesbit Jr ME, Gehan EA, Burgert Jr EO, Vietti TJ, Cangir A, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: A long-term follow-up of the First Intergroup study. J Clinical Oncology 1990;8:1664-74.
- Jürgens H, Exner U, Gadner H, Harms D, Michaelis J, Sauer R, et al. Multidisciplinary treatment of primary Ewing's sarcoma of bone. A 6-year experience of a European cooperative trial. Cancer 1988;61:23-32.
- Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M. Childhood cancer survival trends in Europe: A EUROCARE Working Group study. J Clinical Oncology 2005;23:3742-51.
- Cotterill S, Ahrens S, Paulussen M, Jurgens H, Voute P, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: Aanalysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clinical Oncology 2000;18:3108-14.
- Haeusler J, Ranft A, Boelling T, Gosheger G, Braun-Munzinger G, Vieth V, et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 2010;116:443-50.
- Paulussen M, Braun-Munzinger G, Burdach S, Deneke S, Dunst J, Fellinger E, et al. Results of treatment of primary exclusively pulmonary metastatic Ewing sarcoma. A retrospective analysis of 41 patients. Klinische Padiatrie 1992;205:210-6.
- Paulussen M, Ahrens S, Craft A, Dunst J, Fröhlich B, Jabar S, et al. Ewing's tumors with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing's Sarcoma Studies patients. J Clinical Oncology 1998;16:3044-52.
- Casey DL, Alektiar KM, Gerber NK, Wolden SL. Whole lung irradiation for adults with pulmonary metastases from Ewing sarcoma. International Journal of Radiation Oncology* Biology* Physics 2014;89:1069-75.
- Spunt SL, McCarville MB, Kun LE, Poquette CA, Cain AM, Brandao L, et al. Selective use of whole-lung irradiation for patients with Ewing sarcoma family tumors and pulmonary metastases at the time of diagnosis. J Pediatric Hematology/Oncology 2001;23:93-8.
- Ladenstein R, Pötschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clinical Oncology 2010;28:3284-91.
- Attard-Montalto S, Kingston J, Eden O, Plowman P. Late follow-up of lung function after whole lung irradiation for Wilms' tumour. The British J Radiology 1992;65:1114-8.
- Weiner DJ, Maity A, Carlson CA, Ginsberg JP. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatric Blood and Cancer 2006;46:222-7.
- Harting MT, Blakely ML, Jaffe N, Cox CS, Hayes-Jordan A, Benjamin RS, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatric Surgery 2006;41:194-9.
- Letourneau PA, Shackett B, Xiao L, Trent J, Tsao KJ, Lally K, et al. Resection of pulmonary metastases in pediatric patients with Ewing sarcoma improves survival. J Pediatric Surgery 2011;46:332-5.
- Heij HA, Vos A, De Kraker J, Voute P. Prognostic factors in surgery for pulmonary metastases in children. Surgery 1994;115:687-93.
- Tronc F, Conter C, Marec-Berard P, Bossard N, Remontet L, Orsini A, et al. Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. European Journal of Cardio-Thoracic Surgery 2008;34:1240-6.
- Cangir A, Vietti TJ, Gehan EA, Burgert EO, Thomas P, Tefft M, et al. Ewing's sarcoma metastatic at diagnosis results and comparisons of two intergroup Ewing's sarcoma studies. Cancer 1990;66:887-93.
- Luksch R, Tienghi A, Hall KS, Fagioli F, Picci P, Barbieri E, et al. Primary metastatic Ewing's family tumors: Results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Annals of Oncology 2012;23:2970-6.
- Treasure T, Fiorentino F, Scarci M, Møller H, Utley M. Pulmonary metastasectomy for sarcoma: A systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736.
- Paulussen M, Ahrens S, Burdach S, Craft A, Dockhorn-Dworniczak B, Dunst J, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. Annals of Oncology 1998;9:275-81.
- Paulino AC, Mai WY, Teh BS. Radiotherapy in metastatic Ewing sarcoma. American J Clinical Oncology 2013;36:283-6.
- Casey DL, Wexler LH, Meyers PA, Magnan H, Chou AJ, Wolden SL. Radiation for bone metastases in Ewing sarcoma and rhabdomyosarcoma. Pediatric Blood and Cancer 2015;62:445-9.
- Craft A, Cotterill S, Malcolm A, Spooner D, Grimer R, Souhami R, et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: The Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. Journal of Clinical Oncology 1998;16:3628-33.
- Donaldson SS, Torrey M, Link MP, Glicksman A, Gilula L, Laurie F, et al. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG# 8346. International Journal of Radiation Oncology* Biology* Physics 1998;42:125-35.
- Craft A, Cotterill S, Bullimore J. Long-term results from the first UKCCSG Ewing's tumour study (ET-1). European J Cancer 1997;33:1061-9.
- Sandoval C, Meyer WH, Parham DM, Kun LE, Hustu HO, Luo X, et al. Outcome in 43 children presenting with metastatic Ewing sarcoma: The St. Jude Children's Research Hospital experience, 1962 to 1992. Pediatric Blood and Cancer 1996;26:180-5.
- Demeocq F, Oberlin O, Benz-Lemoine E, Boilletot A, Gentet J, Zucker J, et al. Initial chemotherapy including ifosfamide in the management of Ewing's sarcoma: Preliminary results A protocol of the French Pediatric Oncology Society (SFOP). Cancer Chemotherapy and Pharmacology 1989;24:S45-S7.
- Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst Jr, et al. Results of the EICESS-92 Study: Two randomized trials of Ewing's sarcoma treatment—cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clinical Oncology 2008;26:4385-93.
- Biswas B, Rastogi S, Khan S, Shukla N, Deo S, Agarwala S, et al. Hypoalbuminaemia is an independent predictor of poor outcome in metastatic Ewing's sarcoma family of tumours: A single institutional experience of 150 cases treated with uniform chemotherapy protocol. Clinical Oncology 2014;26:722-9.
- Kushner BH, Meyers PA, Gerald WL, Healey JH, La Quaglia MP, Boland P, et al. Very-high-dose short-term chemotherapy for poor-risk peripheral primitive neuroectodermal tumors, including Ewing's sarcoma, in children and young adults. J Clinical Oncology 1995;13:2796-804.
- Marina NM, Pappo AS, Parham DM, Cain AM, Rao BN, Poquette CA, et al. Chemotherapy dose-intensification for pediatric patients with Ewing's family of tumors and desmoplastic small round-cell tumors: A feasibility study at St. Jude Children's Research Hospital. J Clinical Oncology 1999;17:180-90.
- Brunetto AL, Castillo LA, Petrilli AS, Macedo CD, Boldrini E, Costa C, et al. Carboplatin in the treatment of Ewing sarcoma: Results of the first Brazilian Collaborative Study Group for Ewing Sarcoma Family Tumors–EWING1. Pediatric Blood and Cancer 2015;62:1747-53.
- Womer R, Daller R, Fenton JG, Miser J. Granulocyte colony stimulating factor permits dose intensification by interval compression in the treatment of Ewing's sarcomas and soft tissue sarcomas in children. European J Cancer 2000;36:87-94.
- Czyzewski EAD, Goldman S, Mundt AJ, Nachman J, Rubin C, Hallahan DE. Radiation therapy for consolidation of metastatic or recurrent sarcomas in children treated with intensive chemotherapy and stem cell rescue. A feasibility study. International Journal of Radiation Oncology* Biology* Physics 1999;44:569-77.
- Perentesis J, Katsanis E, DeFor T, Neglia J, Ramsay N. Autologous stem cell transplantation for high-risk pediatric solid tumors. Bone Marrow Transplantation 1999;24:609-15.
- Burdach S, Jürgens H, Peters C, Nürnberger W, Mauz-Körholz C, Körholz D, et al. Myeloablative radiochemotherapy and hematopoietic stem-cell rescue in poor-prognosis Ewing's sarcoma. J Clinical Oncology 1993;11:1482-8.
- Burdach S, Van Kaick B, Laws H, Ahrens S, Haase R, Körholz D, et al. Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors: An update after long-term follow-up from two centers of the European Intergroup Study EICESS. Annals of Oncology 2000;11:1451-62.
- Horowitz ME, Kinsella TJ, Wexler LH, Belasco J, Triche T, Tsokos M, et al. Total-body irradiation and autologous bone marrow transplant in the treatment of high-risk Ewing's sarcoma and rhabdomyosarcoma. J Clinical Oncology 1993;11:1911-8.
- Bader JL, Horowitz ME, Dewan R, Watkins E, Triche TJ, Tsokos M, et al. Intensive combined modality therapy of small round cell and undifferentiated sarcomas in children and young adults: local control and patterns of failure. Radiotherapy and Oncology 1989;16:189-201.
- Hawkins D, Barnett T, Bensinger W, Gooley T, Sanders J. Busulfan, melphalan, and thiotepa with or without total marrow irradiation with hematopoietic stem cell rescue for poor-risk Ewing-sarcoma-family tumors. Pediatric Blood and Cancer 2000;34:328-37.
- Oberlin O, Rey A, Desfachelles AS, Philip T, Plantaz D, Schmitt C, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: A study by the Societe Francaise des Cancers de l'Enfant. J Clinical Oncology 2006;24:3997-4002.
- Meyers PA, Krailo MD, Ladanyi M, Chan K-W, Sailer SL, Dickman PS, et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. J Clinical Oncology 2001;19:2812-20.
- Ladenstein R, Lasset C, Pinkerton R, Zucker J, Peters C, Burdach S, et al. Impact of megatherapy in children with high-risk Ewing's tumours in complete remission: A report from the EBMT Solid Tumour Registry. Bone Marrow Transplantation 1995;15:697-705.
- Bölling T, Schuck A, Paulussen M, Dirksen U, Ranft A, Könemann S, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Strahlentherapie und Onkologie 2008;184:193-7.
- Margolis LW, Phillips TL. Whole-lung irradiation for metastatic tumor. Radiology 1969;93:1173-9.
- Rosen G, Wollner N, Tan C, Wu S, Hajdu S, Cham W, et al. Disease-free survival in children with Ewing's sarcoma treated with radiation therapy and adjuvant four-drug sequential chemotherapy. Cancer 1974;33:384-93.
- Dunst J, Paulussen M, Jürgens H. Lung irradiation for Ewing's sarcoma with pulmonary metastases at diagnosis: results of the CESS-studies. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft[et al] 1993;169:621-3.
- Karnak I, Şenocak ME, Kutluk T, Tanyel FC, Büyükpamukçu N. Pulmonary metastases in children: An analysis of surgical spectrum. European J Pediatric Surgery 2002;12:151-8.
- Lanza L, Miser J, Pass H, Roth J. The role of resection in the treatment of pulmonary metastases from Ewing's sarcoma. The J Thoracic and Cardiovascular Surgery 1987;94:181-7.
- Hayes F, Thompson E, Parvey L, Rao B, Kun L, Parham D, et al. Metastatic Ewing's sarcoma: Remission induction and survival. Journal of Clinical Oncology 1987;5:1199-204.
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. New England J Medicine 2003;348:694-701.
- Wexler LH, DeLaney TF, Tsokos M, Avila N, Steinberg SM, Weaver-McClure L, et al. Ifosfamide and etoposide plus vincristine, doxorubicin, and cyclophosphamide for newly diagnosed Ewing's sarcoma family of tumors. Cancer 1996;78:901-11.
- Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide—a Children's Cancer Group and Pediatric Oncology Group study. J Clinical Oncology 2004;22:2873-6.
- Atra A, Whelan J, Calvagna V, Shankar A, Ashley S, Shepherd V, et al. High-dose busulphan/melphalan with autologous stem cell rescue in Ewing's sarcoma. Bone Marrow Transplantation 1997;20:843-6.
- Laurence V, Pierga J-Y, Barthier S, Babinet A, Alapetite C, Palangié T, et al. Long-term follow up of high-dose chemotherapy with autologous stem cell rescue in adults with Ewing tumor. American J Clinical Oncology 2005;28:301-9.
- Miser JS, Kinsella T, Triche T, Tsokos M, Forquer R, Wesley R, et al. Preliminary results of treatment of Ewing's sarcoma of bone in children and young adults: Six months of intensive combined modality therapy without maintenance. J Clinical Oncology 1988;6:484-90.
- Kushner BH, Meyers PA. How effective is dose-intensive/myeloablative therapy against Ewing's sarcoma/primitive neuroectodermal tumor metastatic to bone or bone marrow? The Memorial Sloan-Kettering experience and a literature review. J Clinical Oncology 2001;19:870-80.
References
- Nesbit Jr ME, Gehan EA, Burgert Jr EO, Vietti TJ, Cangir A, Tefft M, et al. Multimodal therapy for the management of primary, nonmetastatic Ewing's sarcoma of bone: A long-term follow-up of the First Intergroup study. J Clinical Oncology 1990;8:1664-74.
- Jürgens H, Exner U, Gadner H, Harms D, Michaelis J, Sauer R, et al. Multidisciplinary treatment of primary Ewing's sarcoma of bone. A 6-year experience of a European cooperative trial. Cancer 1988;61:23-32.
- Gatta G, Capocaccia R, Stiller C, Kaatsch P, Berrino F, Terenziani M. Childhood cancer survival trends in Europe: A EUROCARE Working Group study. J Clinical Oncology 2005;23:3742-51.
- Cotterill S, Ahrens S, Paulussen M, Jurgens H, Voute P, Gadner H, et al. Prognostic factors in Ewing's tumor of bone: Aanalysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clinical Oncology 2000;18:3108-14.
- Haeusler J, Ranft A, Boelling T, Gosheger G, Braun-Munzinger G, Vieth V, et al. The value of local treatment in patients with primary, disseminated, multifocal Ewing sarcoma (PDMES). Cancer 2010;116:443-50.
- Paulussen M, Braun-Munzinger G, Burdach S, Deneke S, Dunst J, Fellinger E, et al. Results of treatment of primary exclusively pulmonary metastatic Ewing sarcoma. A retrospective analysis of 41 patients. Klinische Padiatrie 1992;205:210-6.
- Paulussen M, Ahrens S, Craft A, Dunst J, Fröhlich B, Jabar S, et al. Ewing's tumors with primary lung metastases: survival analysis of 114 (European Intergroup) Cooperative Ewing's Sarcoma Studies patients. J Clinical Oncology 1998;16:3044-52.
- Casey DL, Alektiar KM, Gerber NK, Wolden SL. Whole lung irradiation for adults with pulmonary metastases from Ewing sarcoma. International Journal of Radiation Oncology* Biology* Physics 2014;89:1069-75.
- Spunt SL, McCarville MB, Kun LE, Poquette CA, Cain AM, Brandao L, et al. Selective use of whole-lung irradiation for patients with Ewing sarcoma family tumors and pulmonary metastases at the time of diagnosis. J Pediatric Hematology/Oncology 2001;23:93-8.
- Ladenstein R, Pötschger U, Le Deley MC, Whelan J, Paulussen M, Oberlin O, et al. Primary disseminated multifocal Ewing sarcoma: Results of the Euro-EWING 99 trial. J Clinical Oncology 2010;28:3284-91.
- Attard-Montalto S, Kingston J, Eden O, Plowman P. Late follow-up of lung function after whole lung irradiation for Wilms' tumour. The British J Radiology 1992;65:1114-8.
- Weiner DJ, Maity A, Carlson CA, Ginsberg JP. Pulmonary function abnormalities in children treated with whole lung irradiation. Pediatric Blood and Cancer 2006;46:222-7.
- Harting MT, Blakely ML, Jaffe N, Cox CS, Hayes-Jordan A, Benjamin RS, et al. Long-term survival after aggressive resection of pulmonary metastases among children and adolescents with osteosarcoma. J Pediatric Surgery 2006;41:194-9.
- Letourneau PA, Shackett B, Xiao L, Trent J, Tsao KJ, Lally K, et al. Resection of pulmonary metastases in pediatric patients with Ewing sarcoma improves survival. J Pediatric Surgery 2011;46:332-5.
- Heij HA, Vos A, De Kraker J, Voute P. Prognostic factors in surgery for pulmonary metastases in children. Surgery 1994;115:687-93.
- Tronc F, Conter C, Marec-Berard P, Bossard N, Remontet L, Orsini A, et al. Prognostic factors and long-term results of pulmonary metastasectomy for pediatric histologies. European Journal of Cardio-Thoracic Surgery 2008;34:1240-6.
- Cangir A, Vietti TJ, Gehan EA, Burgert EO, Thomas P, Tefft M, et al. Ewing's sarcoma metastatic at diagnosis results and comparisons of two intergroup Ewing's sarcoma studies. Cancer 1990;66:887-93.
- Luksch R, Tienghi A, Hall KS, Fagioli F, Picci P, Barbieri E, et al. Primary metastatic Ewing's family tumors: Results of the Italian Sarcoma Group and Scandinavian Sarcoma Group ISG/SSG IV Study including myeloablative chemotherapy and total-lung irradiation. Annals of Oncology 2012;23:2970-6.
- Treasure T, Fiorentino F, Scarci M, Møller H, Utley M. Pulmonary metastasectomy for sarcoma: A systematic review of reported outcomes in the context of Thames Cancer Registry data. BMJ Open 2012;2:e001736.
- Paulussen M, Ahrens S, Burdach S, Craft A, Dockhorn-Dworniczak B, Dunst J, et al. Primary metastatic (stage IV) Ewing tumor: survival analysis of 171 patients from the EICESS studies. Annals of Oncology 1998;9:275-81.
- Paulino AC, Mai WY, Teh BS. Radiotherapy in metastatic Ewing sarcoma. American J Clinical Oncology 2013;36:283-6.
- Casey DL, Wexler LH, Meyers PA, Magnan H, Chou AJ, Wolden SL. Radiation for bone metastases in Ewing sarcoma and rhabdomyosarcoma. Pediatric Blood and Cancer 2015;62:445-9.
- Craft A, Cotterill S, Malcolm A, Spooner D, Grimer R, Souhami R, et al. Ifosfamide-containing chemotherapy in Ewing's sarcoma: The Second United Kingdom Children's Cancer Study Group and the Medical Research Council Ewing's Tumor Study. Journal of Clinical Oncology 1998;16:3628-33.
- Donaldson SS, Torrey M, Link MP, Glicksman A, Gilula L, Laurie F, et al. A multidisciplinary study investigating radiotherapy in Ewing's sarcoma: end results of POG# 8346. International Journal of Radiation Oncology* Biology* Physics 1998;42:125-35.
- Craft A, Cotterill S, Bullimore J. Long-term results from the first UKCCSG Ewing's tumour study (ET-1). European J Cancer 1997;33:1061-9.
- Sandoval C, Meyer WH, Parham DM, Kun LE, Hustu HO, Luo X, et al. Outcome in 43 children presenting with metastatic Ewing sarcoma: The St. Jude Children's Research Hospital experience, 1962 to 1992. Pediatric Blood and Cancer 1996;26:180-5.
- Demeocq F, Oberlin O, Benz-Lemoine E, Boilletot A, Gentet J, Zucker J, et al. Initial chemotherapy including ifosfamide in the management of Ewing's sarcoma: Preliminary results A protocol of the French Pediatric Oncology Society (SFOP). Cancer Chemotherapy and Pharmacology 1989;24:S45-S7.
- Paulussen M, Craft AW, Lewis I, Hackshaw A, Douglas C, Dunst Jr, et al. Results of the EICESS-92 Study: Two randomized trials of Ewing's sarcoma treatment—cyclophosphamide compared with ifosfamide in standard-risk patients and assessment of benefit of etoposide added to standard treatment in high-risk patients. J Clinical Oncology 2008;26:4385-93.
- Biswas B, Rastogi S, Khan S, Shukla N, Deo S, Agarwala S, et al. Hypoalbuminaemia is an independent predictor of poor outcome in metastatic Ewing's sarcoma family of tumours: A single institutional experience of 150 cases treated with uniform chemotherapy protocol. Clinical Oncology 2014;26:722-9.
- Kushner BH, Meyers PA, Gerald WL, Healey JH, La Quaglia MP, Boland P, et al. Very-high-dose short-term chemotherapy for poor-risk peripheral primitive neuroectodermal tumors, including Ewing's sarcoma, in children and young adults. J Clinical Oncology 1995;13:2796-804.
- Marina NM, Pappo AS, Parham DM, Cain AM, Rao BN, Poquette CA, et al. Chemotherapy dose-intensification for pediatric patients with Ewing's family of tumors and desmoplastic small round-cell tumors: A feasibility study at St. Jude Children's Research Hospital. J Clinical Oncology 1999;17:180-90.
- Brunetto AL, Castillo LA, Petrilli AS, Macedo CD, Boldrini E, Costa C, et al. Carboplatin in the treatment of Ewing sarcoma: Results of the first Brazilian Collaborative Study Group for Ewing Sarcoma Family Tumors–EWING1. Pediatric Blood and Cancer 2015;62:1747-53.
- Womer R, Daller R, Fenton JG, Miser J. Granulocyte colony stimulating factor permits dose intensification by interval compression in the treatment of Ewing's sarcomas and soft tissue sarcomas in children. European J Cancer 2000;36:87-94.
- Czyzewski EAD, Goldman S, Mundt AJ, Nachman J, Rubin C, Hallahan DE. Radiation therapy for consolidation of metastatic or recurrent sarcomas in children treated with intensive chemotherapy and stem cell rescue. A feasibility study. International Journal of Radiation Oncology* Biology* Physics 1999;44:569-77.
- Perentesis J, Katsanis E, DeFor T, Neglia J, Ramsay N. Autologous stem cell transplantation for high-risk pediatric solid tumors. Bone Marrow Transplantation 1999;24:609-15.
- Burdach S, Jürgens H, Peters C, Nürnberger W, Mauz-Körholz C, Körholz D, et al. Myeloablative radiochemotherapy and hematopoietic stem-cell rescue in poor-prognosis Ewing's sarcoma. J Clinical Oncology 1993;11:1482-8.
- Burdach S, Van Kaick B, Laws H, Ahrens S, Haase R, Körholz D, et al. Allogeneic and autologous stem-cell transplantation in advanced Ewing tumors: An update after long-term follow-up from two centers of the European Intergroup Study EICESS. Annals of Oncology 2000;11:1451-62.
- Horowitz ME, Kinsella TJ, Wexler LH, Belasco J, Triche T, Tsokos M, et al. Total-body irradiation and autologous bone marrow transplant in the treatment of high-risk Ewing's sarcoma and rhabdomyosarcoma. J Clinical Oncology 1993;11:1911-8.
- Bader JL, Horowitz ME, Dewan R, Watkins E, Triche TJ, Tsokos M, et al. Intensive combined modality therapy of small round cell and undifferentiated sarcomas in children and young adults: local control and patterns of failure. Radiotherapy and Oncology 1989;16:189-201.
- Hawkins D, Barnett T, Bensinger W, Gooley T, Sanders J. Busulfan, melphalan, and thiotepa with or without total marrow irradiation with hematopoietic stem cell rescue for poor-risk Ewing-sarcoma-family tumors. Pediatric Blood and Cancer 2000;34:328-37.
- Oberlin O, Rey A, Desfachelles AS, Philip T, Plantaz D, Schmitt C, et al. Impact of high-dose busulfan plus melphalan as consolidation in metastatic Ewing tumors: A study by the Societe Francaise des Cancers de l'Enfant. J Clinical Oncology 2006;24:3997-4002.
- Meyers PA, Krailo MD, Ladanyi M, Chan K-W, Sailer SL, Dickman PS, et al. High-dose melphalan, etoposide, total-body irradiation, and autologous stem-cell reconstitution as consolidation therapy for high-risk Ewing's sarcoma does not improve prognosis. J Clinical Oncology 2001;19:2812-20.
- Ladenstein R, Lasset C, Pinkerton R, Zucker J, Peters C, Burdach S, et al. Impact of megatherapy in children with high-risk Ewing's tumours in complete remission: A report from the EBMT Solid Tumour Registry. Bone Marrow Transplantation 1995;15:697-705.
- Bölling T, Schuck A, Paulussen M, Dirksen U, Ranft A, Könemann S, et al. Whole lung irradiation in patients with exclusively pulmonary metastases of Ewing tumors. Strahlentherapie und Onkologie 2008;184:193-7.
- Margolis LW, Phillips TL. Whole-lung irradiation for metastatic tumor. Radiology 1969;93:1173-9.
- Rosen G, Wollner N, Tan C, Wu S, Hajdu S, Cham W, et al. Disease-free survival in children with Ewing's sarcoma treated with radiation therapy and adjuvant four-drug sequential chemotherapy. Cancer 1974;33:384-93.
- Dunst J, Paulussen M, Jürgens H. Lung irradiation for Ewing's sarcoma with pulmonary metastases at diagnosis: results of the CESS-studies. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft[et al] 1993;169:621-3.
- Karnak I, Şenocak ME, Kutluk T, Tanyel FC, Büyükpamukçu N. Pulmonary metastases in children: An analysis of surgical spectrum. European J Pediatric Surgery 2002;12:151-8.
- Lanza L, Miser J, Pass H, Roth J. The role of resection in the treatment of pulmonary metastases from Ewing's sarcoma. The J Thoracic and Cardiovascular Surgery 1987;94:181-7.
- Hayes F, Thompson E, Parvey L, Rao B, Kun L, Parham D, et al. Metastatic Ewing's sarcoma: Remission induction and survival. Journal of Clinical Oncology 1987;5:1199-204.
- Grier HE, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Addition of ifosfamide and etoposide to standard chemotherapy for Ewing's sarcoma and primitive neuroectodermal tumor of bone. New England J Medicine 2003;348:694-701.
- Wexler LH, DeLaney TF, Tsokos M, Avila N, Steinberg SM, Weaver-McClure L, et al. Ifosfamide and etoposide plus vincristine, doxorubicin, and cyclophosphamide for newly diagnosed Ewing's sarcoma family of tumors. Cancer 1996;78:901-11.
- Miser JS, Krailo MD, Tarbell NJ, Link MP, Fryer CJ, Pritchard DJ, et al. Treatment of metastatic Ewing's sarcoma or primitive neuroectodermal tumor of bone: Evaluation of combination ifosfamide and etoposide—a Children's Cancer Group and Pediatric Oncology Group study. J Clinical Oncology 2004;22:2873-6.
- Atra A, Whelan J, Calvagna V, Shankar A, Ashley S, Shepherd V, et al. High-dose busulphan/melphalan with autologous stem cell rescue in Ewing's sarcoma. Bone Marrow Transplantation 1997;20:843-6.
- Laurence V, Pierga J-Y, Barthier S, Babinet A, Alapetite C, Palangié T, et al. Long-term follow up of high-dose chemotherapy with autologous stem cell rescue in adults with Ewing tumor. American J Clinical Oncology 2005;28:301-9.
- Miser JS, Kinsella T, Triche T, Tsokos M, Forquer R, Wesley R, et al. Preliminary results of treatment of Ewing's sarcoma of bone in children and young adults: Six months of intensive combined modality therapy without maintenance. J Clinical Oncology 1988;6:484-90.
- Kushner BH, Meyers PA. How effective is dose-intensive/myeloablative therapy against Ewing's sarcoma/primitive neuroectodermal tumor metastatic to bone or bone marrow? The Memorial Sloan-Kettering experience and a literature review. J Clinical Oncology 2001;19:870-80.


 PDF
PDF  Views
Views  Share
Share

