Maternal transmission of human papillomavirus in retinoblastoma: A possible route of transfer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(04): 210-215
DOI: DOI: 10.4103/0971-5851.107080
Abstract
Context: After establishing the presence of human papillomavirus (HPV) in retinoblastoma (RB), the probable role of the mother was investigated. Materials and Methods: A total of 21 sporadic RB cases and 15/21 corresponding mothers′ cervical brushings were collected. HPV testing was carried out using multiplex PCR (PGMY09/11 primers) followed by genotyping using line blot assay. Results: We found both high- (83%) and intermediate-risk (17%) HPV types in 12/21 (57%) RB samples and only high-risk (100%) types in 6/15 (40%) cervical brushing samples. The single genotype of HPV 16 was found in six cases and HPVs 82, 68 and 35 in one case each. Both HPVs 16 and 59 were found in two cases and HPV 16 and 73 in one case. Three samples of RB harboring HPV 16, HPVs 16 and 59, and HPVs 16 and 73 had HPV genotype 16 in the respective mothers′ cervical brushing samples. Conclusions: Maternal transfer of HPV in RB could be a possible route of transmission. However, a larger cohort and sampling of the mothers′ cervical brushings at various stages, i.e. before, during, and after pregnancy will give us insight to propound an alternate mechanism for the development of sporadic RB.
Publication History
Article published online:
20 July 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
After establishing the presence of human papillomavirus (HPV) in retinoblastoma (RB), the probable role of the mother was investigated.
Materials and Methods:
A total of 21 sporadic RB cases and 15/21 corresponding mothers′ cervical brushings were collected. HPV testing was carried out using multiplex PCR (PGMY09/11 primers) followed by genotyping using line blot assay.
Results:
We found both high- (83%) and intermediate-risk (17%) HPV types in 12/21 (57%) RB samples and only high-risk (100%) types in 6/15 (40%) cervical brushing samples. The single genotype of HPV 16 was found in six cases and HPVs 82, 68 and 35 in one case each. Both HPVs 16 and 59 were found in two cases and HPV 16 and 73 in one case. Three samples of RB harboring HPV 16, HPVs 16 and 59, and HPVs 16 and 73 had HPV genotype 16 in the respective mothers′ cervical brushing samples.
Conclusions:
Maternal transfer of HPV in RB could be a possible route of transmission. However, a larger cohort and sampling of the mothers′ cervical brushings at various stages, i.e. before, during, and after pregnancy will give us insight to propound an alternate mechanism for the development of sporadic RB.
INTRODUCTION
Human papillomaviruses (HPVs) are DNA viruses that can induce various malignancies in humans and are proven to be transmitted sexually and otherwise.[1] Today, the plurality of HPVs is well-established; more than 100 HPV types have been identified.[2] HPVs have gained increasing interest because of their potential role in the pathogenesis of various malignant tumors. Both low-risk and high-risk HPV types exist - the latter constituting a significant risk for cervical, anogenital, and head and neck cancers.[3] Reports have shown the association of HPV with retinoblastoma, although the results are contrasting.[4,5] However, recently we and others have shown the association of both low-risk and high-risk HPVs with retinoblastoma (RB) in children.[6–12] Reports on the mode of infection however remain unclear.
High-risk human papillomaviruses are spread by sexual activity, but the possibility of non-sexual transmission remains controversial.[13] There is growing evidence that vertical transmission of HPV from mothers to infants, horizontal transmission from other family members and those in close contact with the child, autoinoculation from one site to another, and possibly indirect transmission via fomites[14] can transmit infection. The potential mother-to-child HPV transmission route in the perinatal period has also been demonstrated.[15–19] There is evidence of vertical transmission, presumably occurring during passage of the fetus through an infected birth canal.[17] HPV DNA has been detected in peripheral blood mononuclear cells of pregnant women,[20] cord blood specimens of neonates,[20] oropharyngeal secretions of neonates,[21] amniotic fluid,[22–24] fetal membrane,[25] placental trophoblastic cells,[26] infants born by elective cesarean section delivery,[17,23,25,27] and in syncytiotrophoblastic cells of spontaneously aborted material.[28] With the emerging evidence from the literature on the vertical transmission of HPV from mother to child,[20] we hypothesized that vertical transmission of HPV from mother to child could be a possible route of infection of HPV to the infant eye, leading to retinoblastoma. Hence, we made an attempt to investigate the possible (alternative) route of infection to the child from the mother in cases of retinoblastoma.
MATERIALS AND METHODS
Population studied
A total of 21 prospective cases of sporadic RB were collected during the years 2007-2010 from the Kidwai Memorial Institute of Oncology (KMIO), Bangalore. The cervical specimens of the respective mothers were also collected using cervico-vaginal brushings by an expert gynaecologist from the Department of Gynaecologic Oncology, KMIO. Archived formalin-fixed paraffin-embedded (FFPE) blocks of non-neoplastic eyes (n=5) obtained from foetal autopsy specimens and prospectively collected non-neoplastic eyes (n=15) from an eye bank (Lions Eye Bank, Bangalore) were used as controls. This study was performed with the approval of the Medical Ethics Committee of KMIO, Bangalore, India. Written informed consent was obtained from the mothers (n=15) of the children with RB after explaining fully the importance of the study and the mode of collection of the specimen. Six mothers were unable/not willing to give consent to collect the cervical brushings specimens and hence were excluded from the study.
Sampling methods
Retinoblastoma samples
Fresh RB tumor samples were obtained from children who were clinically diagnosed as RB and underwent enucleation or orbital exenteration as one of the treatment procedures for RB which was subsequently confirmed by histopathological examination. The samples were collected directly from the operation theater by excising the globes into two halves using sterile scalpel blades. The tumor was collected from the posterior part of the globe and frozen in RNA later solution at -20°C until processed for DNA extraction. The remaining part of the globe was kept in 10% buffered formalin for histopathological evaluation.
Maternal genital samples
Cervical brushings from the respective mothers were obtained by an experienced gynecologist when the children with RB were recruited for the study. The samples were placed immediately in a TE solution (10 mM Tris HCl, pH 7.5; 1 mM EDTA), and stored at -20°C until the DNA was extracted.
DNA Extraction from fresh frozen tissues of RB (n=21)
DNA extraction from fresh tumor tissues was done using commercial kits (DNeasy tissue DNA extraction columns - QIAGEN, Hilden, Germany). Briefly, fresh tumor tissue was digested in ATL buffer with proteinase K (200 μg/ml) for 1 hour (hr) at 55°C followed by further lysis in AL buffer for 10 minutes (min) at 70°C. The lysate was incubated in 100% ethanol for 10 min and added to the columns for purification. The purified DNA was then eluted in 50 μl sterile water. The quality of extracted DNA as checked by β-globin PCR was good (all the samples were positive for the β-globin gene).
DNA extraction from cervical cells (n=15)
DNA was extracted from cells obtained by cervical brushing as per the procedure of Gravitt et al.[29] Briefly, cells were digested in Proteinase K (100 mM Tris, 0.1% sodium dodecyl sulfate, Proteinase K 200 μg/ml) at 55°C for 1 hr. Excess Proteinase K was inactivated by heating at 95°C for 10 min. The lysate was centrifuged and the supernatant was used for amplification of HPV DNA.
Multiplex PCR
DNA extracted from fresh tissues of RB and from cervical brushings was amplified for HPV by the in-house multiplex PCR comprising primers toward the β-globin gene (PCO4 and GH2O) and the HPV L1 consensus region (PGMY09/11). The parameters for denaturation, annealing, and elongation of the strands were as described earlier.[11] Plasmid constructs of HPV obtained from across the globe and DNA extracted from squamous cell carcinoma (SCC) of the cervix served as positive controls for the study. Negative controls for RB were DNA extracted from (a) archived FFPE blocks of non-neoplastic eyes (n=5) obtained from fetal autopsy specimens and (b) prospectively collected non-neoplastic eyes (n=15) from an eye bank. The PCR reaction mixture containing all the reagents except the template DNA served as the negative control for the cervical brushing samples. Amplified products were detected by running the amplicons in 1.5% agarose gel in 1× TAE (Tris acetic acid EDTA) with ethidium bromide and visualized using a UV transilluminator.
Genotyping of HPV in RB and cervical brushing specimens
Samples which were positive for HPV DNA by the in-house multiplex PCR were genotyped using a HPV linear array kit following the manufacturer′s instructions (Roche Molecular Systems, Branchburg, USA). Briefly, this comprised of two steps: (a) multiplex HPV PCR using the reagents provided in the kit; (b) genotyping of both low- and high-risk HPV types. Appropriate positive and negative controls were included (provided by the manufacturer).
RESULTS
Patients′ characteristics
The median age of diagnosis of the children was 2 years (range 11 months to 3.5 years) with leukokoria being the first symptom of the disease in all the cases. While 19 cases had unilateral disease, there were two cases with bilateral tumors. Out of the 21 cases, only one case had a family history of RB. Consanguineous marriage of parents was seen in six cases and all the women were of the median age of 18 years (range 17-23 years) at the time of marriage. No barrier methods of contraception had been used. All the women belonged to the lower socio-economic stratum.
Multiplex PCR for HPV
Twenty one RB samples and 15 cervical brushings samples had adequate good quality DNA for multiplex PCR as checked by β-globin PCR. HPV was detected in 12/21 (57.14%) cases of RB and 6/15 (40%) cervical brushing specimens [Figure 1].
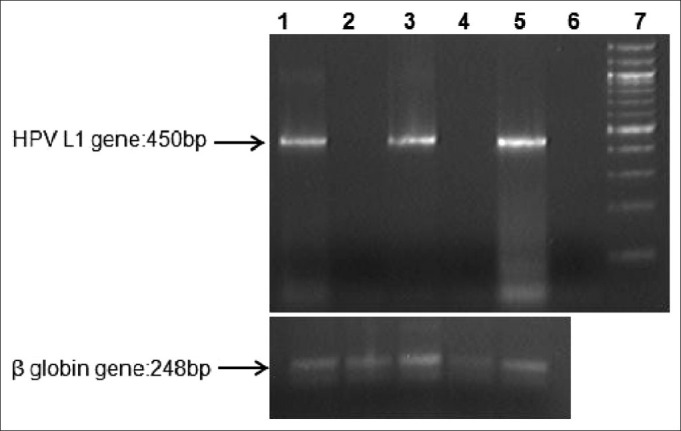
| Fig. 1 A representative gel image of Multiplex PCR amplification of the HPV L1 region. Lane 1: positive control: HPV positive tissue from cervical cancer; Lane 2: HPV-negative RB tissue; Lane 3: HPVpositive RB tissue; Lane 4: HPV-negative cervical brushing; Lane 5: HPV-positive cervical brushing; Lane 6: Negative control with all the PCR constituents except DNA; Lane 7: 100bp ladder
Genotyping
HPV-type speciation was performed by hybridization to a linear probe array containing 37 HPV types and β-globin (Roche Molecular Systems, Branchburg, USA). Samples that were positive for β-globin were considered adequate for analysis and scored as negative or positive for HPV DNA. We found both high- (83%) and intermediate-risk (17%) HPV types in 12/21 (57%) RB samples and only high-risk (100%) types in 6/15 (40%) cervical brushing samples. HPV16 singly (six cases) or in combination (three cases) with HPV 59 or HPV 73 was the predominant genotype in RB. The other genotypes detected singly were HPVs 35, 68, and 82. HPV 16 was detected in 4/6 mothers and two cases showed HPV 18 and 35, respectively [Table 1]. Correlation of HPV genotypes between samples of children and mothers (possible in 15 cases) showed commonality in three cases - HPV 16 in the mother and HPV 16, HPVs 16 and 59, and HPVs 16 and 73 in the respective children [Figure 2]. Three mothers showed HPV infection (genotypes 16, 18, and 35) while their children′s RB tumors were HPV negative. Eight mothers were HPV negative while the children′s RB tumors were HPV positive; in one case, both mother and child were HPV negative.
Table 1
HPV genotypes in children and mothers

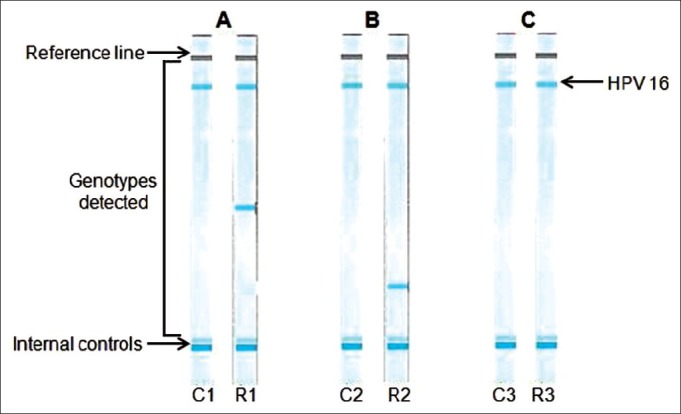
| Fig. 2 A representative line blot assay for HPV genotyping in the three matching cases. (A) C1-HPV 16 positive cervical brushing; R1-HPV 16, 59 positive RB tissue. (B) C2- HPV 16 positive cervical brushing; R2-HPV 16, 73 positive RB tissue. (C) C3-HPV 16 positive cervical brushing; R3-HPV 16 positive RB tissue. C: cervical brushing sample; R: retinoblastoma tumor
DISCUSSION
A role for HPV in the etiopathogenesis of RB could explain the overlap in risk factors for RB and cervical carcinoma. Cervical cancer is the major cause of mortality among women of child-bearing age,[30] and the prevalence of asymptomatic HPV infection in Indian women is higher when compared with other developed and developing nations.[31] A low level of education among women and a lack of use of barrier methods of contraception are both associated with an increased risk of cervical HPV infection,[32] as well as with a significantly increased risk of having a child with RB.[33] Poverty among urban Indian women has been associated with increased incidence of persistent (asymptomatic) cervical HPV infection and invasive cervical carcinoma. Poverty has also been associated with the incidence of RB in other developing nations as well,[34] and in the worldwide geographic distribution of RB.[35] Additionally, the association of an increasing incidence of RB in countries severely affected by the HIV epidemic is also consistent with the possible role of HPV since women with HIV have higher rates of persistent HPV infection.[36] Thus, the presence of HPV DNA in RB tissues is consistent with the overlap in the epidemiologically determined risk factors for both cervical cancer and RB. In this study, we have generated data on the association of the possible role of HPV in RB as an alternative mechanism for the development of sporadic RB.
Human papillomavirus infection is one of the most frequent sexually transmitted diseases.[37–39] Reports to date on the association of HPV with RB have been contradictory. Studies in the Mexican and South American populations have reported high-risk HPV types in 28% and 82% of their patients with sporadic non-familial RB, respectively.[6–8,40] We[11] and others[12] have recently shown that a proportion of RB tumors in the Southeast Asian Indian population harbor HPV - high-risk and low-risk genotypes. The route of infection is not defined as yet; however, it has been observed that non-sexual transmission of HPV may occur directly by contact with the skin or mucosae (between people or by self-inoculation), or indirectly through contaminated objects, or during the perinatal period.[41] There is now sufficient evidence for maternal transmission from at least 30% HPV positive mothers to their infants, resulting in persistent infection in children.[42,43]
Since the incidence of cervical cancer in India is high and no reliable information is available regarding the transmission of HPV from mothers to infants (and thereby its role in causation of RBs), we attempted to correlate the presence and type of HPV in RB tissues with those in the cervical brushing samples collected from the respective mothers. In this study, we found the association of HPV with RB, further arguing the possible role of HPV transfer from mother to child. HPV DNA was detected in 12/21 (57.14%) cases of RB and 6/15 (40%) cases of cervical brushing specimen by multiplex PCR. Genotyping showed both high- (83%) and intermediate-risk (17%) HPV types in RB samples and only high-risk (100%) types in cervical brushing samples. Three samples of RB harboring HPV 16, HPV 16 and 59, and HPV 16 and 73 correlated with HPV genotype 16 in the respective mothers′ cervical brushing samples. Statistical analysis was not possible due to the small sample size.
The sample size in this study was small due to inability to screen all mothers either due to lack of consent or their inability to revisit the hospital for the gynecological examination. It would have also been ideal albeit impossible to have obtained cervical brushings from the mothers at the time of birth, to accurately gauge the HPV status at that time. Clearly, it is possible for the mothers to have acquired the HPV infection postpartum, as evidenced in the finding that three mothers with HPV 18, 35 and 16 in the cervix had children whose RB tumors were HPV negative. Equally possible is the clearing of prior HPV infection in the time (median 2 years) between birth (of a child with RB) and collection of cervical brushings for HPV testing, which could account for the eight cases of HPV-positive RBs with HPV-negative mothers.
Nevertheless, data from this study depicts a possible role for the transfer of HPV from mother to child. This information could prove useful when the study is conducted on a large cohort. These results lay out a platform to address alternative strategies to counter this childhood cancer, in a country like India. The promise shown by the cervical tumor vaccines available in the market and the knowledge that at least some RB tumors are acquired through maternal transfer suggest that it is worth taking the vaccine strategies for HPV prevention seriously since it would help to evade malignancies associated with HPV in both mothers and children.
In conclusion, these results show possible evidence for the vertical transmission of high-risk HPVs which probably results in widespread infection among children. The consequences of such infections remain to be elucidated.
ACKNOWLEDGMENTS
We would like to thank Dr. Sujatha B. L. Rathod, Regional Institute of Ophthalmology, Minto Eye Hospital, Bangalore, for her assistance and Dr. Ashwin Mallipatna, Narayana Nethralaya, Bangalore, for kindly providing samples.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 A representative gel image of Multiplex PCR amplification of the HPV L1 region. Lane 1: positive control: HPV positive tissue from cervical cancer; Lane 2: HPV-negative RB tissue; Lane 3: HPVpositive RB tissue; Lane 4: HPV-negative cervical brushing; Lane 5: HPV-positive cervical brushing; Lane 6: Negative control with all the PCR constituents except DNA; Lane 7: 100bp ladder

| Fig. 2 A representative line blot assay for HPV genotyping in the three matching cases. (A) C1-HPV 16 positive cervical brushing; R1-HPV 16, 59 positive RB tissue. (B) C2- HPV 16 positive cervical brushing; R2-HPV 16, 73 positive RB tissue. (C) C3-HPV 16 positive cervical brushing; R3-HPV 16 positive RB tissue. C: cervical brushing sample; R: retinoblastoma tumor


 PDF
PDF  Views
Views  Share
Share

