Managing metastatic renal cell carcinoma-challenges, pitfalls, and outcomes in the real world
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(04): 260-264
DOI: DOI: 10.4103/0971-5851.195738
Abstract
Introduction: Renal cell carcinoma (RCC) is the most common cancer of the kidney accounting for 85% of renal tumors. Metastatic RCC (mRCC) had a poor prognosis and with the introduction of tyrosine-kinase inhibitors, such as sunitinib, pazopanib the outcomes improved. There is only one study reported from India on the use of sunitinib in mRCC. We present our analysis of mRCC and use of sunitinib at our institute over 5 years. Materials and Methods: All patients with mRCC receiving sunitinib were analyzed with respect to patient characteristics, response, toxicity, and outcomes. Results: A total of 108 patients were seen during the study period. The male to female ratio was 9.8:1. The median age of patients at presentation was 58 years (range: 15–80 years). Of the 108 patients, 68.51% had metastatic disease at initial presentation. The most common sites of metastases were lung followed by bone. Of the 97 patients eligible for sunitinib, only 76 received at least one cycle of sunitinib, out of which only 48 received further cycles (range: 2–36). The median progression-free survival (PFS) and overall survival (OS) in our patients were 10.2 and 28.2 months, respectively. The most common adverse effect noticed in our population was mucositis followed by hand-foot syndrome. Conclusion: Sunitinib is an option for the treatment of mRCC and shows a good PFS in Indian patients. Median OS and PFS in this study are similar to other reported studies despite the presence of poor risk factors in the patient population. The pitfall in this study is significant attrition due to poor compliance to treatment and follow-up, which is a major factor in the clinic thereby compromising outcomes.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Renal cell carcinoma (RCC) is the most common cancer of the kidney accounting for 85% of renal tumors. Metastatic RCC (mRCC) had a poor prognosis and with the introduction of tyrosine-kinase inhibitors, such as sunitinib, pazopanib the outcomes improved. There is only one study reported from India on the use of sunitinib in mRCC. We present our analysis of mRCC and use of sunitinib at our institute over 5 years.
Materials and Methods:
All patients with mRCC receiving sunitinib were analyzed with respect to patient characteristics, response, toxicity, and outcomes.
Results:
A total of 108 patients were seen during the study period. The male to female ratio was 9.8:1. The median age of patients at presentation was 58 years (range: 15–80 years). Of the 108 patients, 68.51% had metastatic disease at initial presentation. The most common sites of metastases were lung followed by bone. Of the 97 patients eligible for sunitinib, only 76 received at least one cycle of sunitinib, out of which only 48 received further cycles (range: 2–36). The median progression-free survival (PFS) and overall survival (OS) in our patients were 10.2 and 28.2 months, respectively. The most common adverse effect noticed in our population was mucositis followed by hand-foot syndrome.
Conclusion:
Sunitinib is an option for the treatment of mRCC and shows a good PFS in Indian patients. Median OS and PFS in this study are similar to other reported studies despite the presence of poor risk factors in the patient population. The pitfall in this study is significant attrition due to poor compliance to treatment and follow-up, which is a major factor in the clinic thereby compromising outcomes.
INTRODUCTION
Renal cell carcinoma (RCC) is the most common cancer of the kidney, accounting for 85% of renal tumors.[1] According to the American Cancer Society estimates, RCC is the seventh most common cancer and is the tenth leading cause for cancer-specific deaths.
Data from India regarding the incidence and mortality of RCC are lacking, but RCC does not figure in the top ten cancers-incidence and mortality wise.
Up to 30% patients with RCC present with metastatic disease[2,3] and recurrence develops in approximately 40% of patients treated for a localized tumor.[2,4] Treatment for localized disease is surgery whereas metastatic disease is treated with systemic therapy. There has been a paradigm shift in the management of metastatic RCC (mRCC) with the emergence of tyrosine-kinase inhibitors (TKIs) and monoclonal antibodies as systemic therapy.
Despite these advancements, survival has largely remained unchanged, and prognosis for Stage IV disease continues to remain poor.
MATERIALS AND METHODS
All patients with a histopathological diagnosis of RCC in tertiary care university hospital in South India between January 2009 and December 2013, and a minimum follow-up of 12 months were included in this analysis. All patients with metastatic disease received sunitinib as the first line therapy, irrespective of histology and risk score. Sunitinib was provided free of cost through a patient assistance program. Analysis consisted of demographic profile, sites of metastases, starting dose of sunitinib, response, toxicity profile, progression-free survival (PFS), and overall survival (OS). The presence of comorbidities especially smoking, obesity, and chronic renal failure (chronic kidney disease [CKD]) were noted. Patients were started on sunitinib at a dose of 37.5 mg or 50 mg once a day based on Eastern Cooperative Oncology Group Performance Score. The schedules followed were 4 weeks on and 2 weeks off drug, or a 2-week on 1-week off schedule. Response assessment was based on clinical and radiological criteria. Response evaluation was done using response evaluation criteria in solid tumors version (RECIST) criteria. Response was classified as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD). Clinical progression and cancer-related deaths were considered as progression. Response evaluation was done after 2–4 cycles of sunitinib. Progression-free survival was calculated as the time between the start of therapy and the date of progression or death from any cause. OS was calculated as the time between start of therapy and the date of death due to any cause. Toxicity profile was calculated according to the common terminology criteria for adverse effects version 4.0. Graphpad prism 6 was used for statistical analysis. Survival was calculated using Kaplan–Meier method.
RESULTS
A total of 108 patients were seen during the study period. Ninety-eight patients (90.74%) were male. The male to female ratio was 9.8:1. The median age of patients at presentation was 58 years (range: 15–80 years). Risk factors such as smoking, obesity, and CKD were noted in 64 (59.25%), 36 (33.33%), and 9 (8.33%), respectively. Bilateral RCC was seen in only one patient. The most common presentations were hematuria in 64 patients (59.25%) followed by loin pain in 62 patients (57.4%). The clinical triad of hematuria, loin pain, and fever was present in 23 patients (21%) only [Table 1].
Table 1
Demographic and clinical characteristics of patients with renal cell carcinoma

In this study, most of the patients were included in intermediate and poor risk except 3.7% of the study group, which was depicted in Table 2.
Table 2
Heng risk stratification

The histopathological variants and sites of metastases are mentioned in Tables Tables33 and and4,4, respectively.
Table 3
Histopathology

Table 4
Sites of metastasis

Clear cell carcinoma was the most common histologic variant, reported in 84.25% of patients.
Of the 108 patients, 34 (31.48%) had localized disease and the remaining 74 (68.51%) had metastatic disease at initial presentation [Table 5]. The most common sites of metastases were lung in 47 patients (48.45%), followed by bone in 23 patients (23.71%).
Table 5
Targeted therapy
Surgery was performed in sixty patients (55.5%). Of the 60 patients, 34 (56.66%) had radical nephrectomy. Of the 34 patients who had radical nephrectomy for localized disease, 23 (67.64%) became metastatic over a median period of 13.65 months. Palliative nephrectomy was done in 26 patients with persistent hematuria or intractable pain in which 21 (80.76%) patients had disease progression.
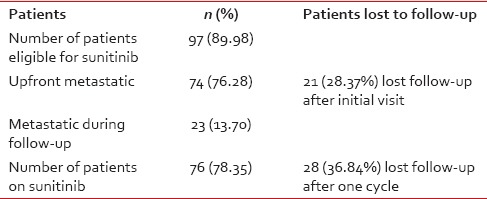
There was a significant attrition observed in this study. The number of patients eligible for sunitinib at first visit was 97 (89.98%). Out of them, 74 (76.28%) patients had metastasis upfront and 23 (13.70%) patients developed metastasis later on during the course of follow-up. Of the 74 patients who had metastasis at presentation, 21 (28.37%) patients did not return after the first visit and a further 28 were lost to follow-up after one cycle.
Seventy-six (78.35%) patients received sunitinib at a dose of 50 mg/day or 37.5 mg/day based on PS (PS 0-1-50 mg and PS ≥2-37.5 mg).
In these 76 patients who are eligible for sunitinib, 28 (36.84%) received only one cycle and lost for follow-up may be due to toxicity or due to disease progression.
The remaining 48 (49.98%) patients had received at least two cycles of sunitinib (range: 2–36). Of the 48 patients, 40 (83.33%) were started on 4 weeks on and 2 weeks off schedule and 8 were started on 2 weeks on 1 week off schedule. The median number of cycles received was five. The median dose of sunitinib received was 50 mg/day.
The adverse effect profile of the patients is shown in Table 6. The most common adverse effect noticed in our population was mucositis in 31 patients (18.67%) followed by hand-foot syndrome in 25 patients (15.06%).
Table 6
Adverse effects

The treatment response is measured using RECIST criteria and response achieved is shown in Table 7. After disease progression, only seven patients received second-line treatment, three received sorafenib, and four received everolimus.
Table 7
Efficacy

DISCUSSION
RCC accounts for 85% of renal tumors. Cigarette smoking doubles the likelihood of renal-cell carcinoma and contributes to as many as one-third of all cases.[5,6] Obesity is also a risk factor, particularly in women, in whom there is a linear relation between increasing body weight and the increased risk of renal-cell carcinoma.[7,8] Kidney tumors usually are unilateral but may be bilateral in 2%–4% of cases.[9]
Up to 30% patients with RCC present with metastatic disease. Lung metastases are the most common sites of distant relapse, occurring in 50%–60% of patients.[10,11] The median time before a relapse after nephrectomy is 15–18 months, and 85% of relapses occur within 3 years.[10,11] Hormonal and chemotherapeutic agents have little or no effect against renal-cell carcinoma.[12]
Standard therapy for mRCC involves treatment with TKI. Approved agents for first-line use in clear cell RCC include pazopanib, sunitinib, and bevacizumab and interferon combination according to ESMO and NCCN guidelines. In nonclear cell variants, m- TOR inhibitor like temsirolimus is used as the first line.
This study was carried out in mRCC, and the objectives were to evaluate the demographic profile, sunitinib, adverse effects, response rates, and survival. There is only one study examining the use of sunitinib from India.
The data of these studies are summarized in Table 8.
Table 8
Comparison of our study with previous studies
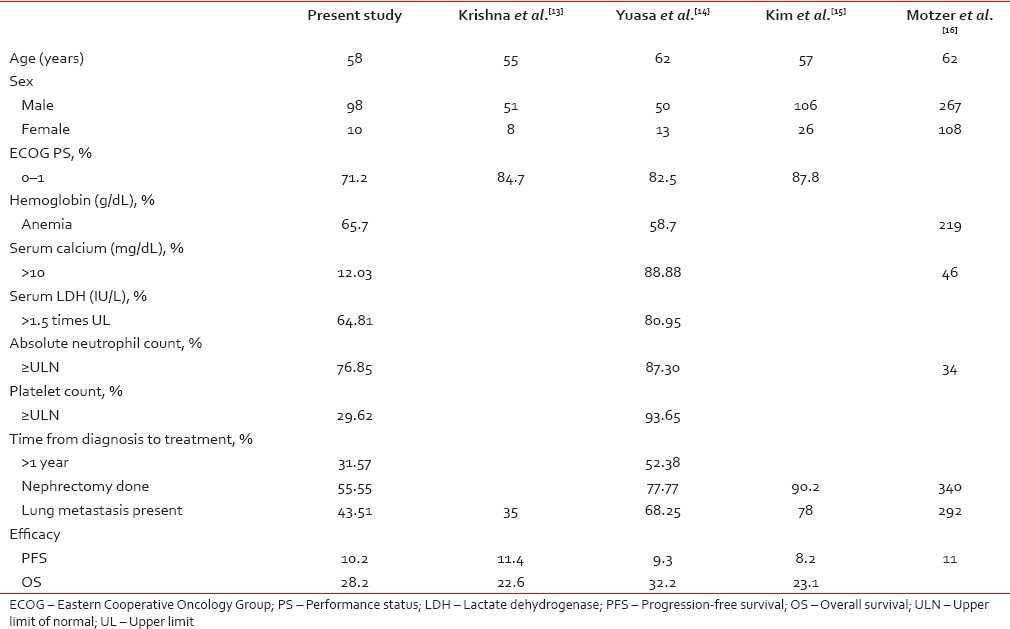
The median age of presentation in our study is 58 years. This is lower than other studies by Motzer et al. and Yuasa et al. suggesting that the age of onset of RCC might be earlier in our population. Similar to other studies the males were more than females in our study, however, the ratio of male to female was much higher (9.8:1) when compared to those studies.[13,14,15,16] The percentage of patients who were eligible for sunitinib in our study (i.e. with a performance score of 0–1) was lower when compared to other studies. Delay in diagnosis, poor follow-up after radical nephrectomy, compliance, lack of awareness, and lack of cancer care centers might be reasons for a low-performance score at presentation.
The percentage of patients who were anemic (low Hb-which is a risk stratification parameter) at presentation was more in our study when compared to other studies; this may be because of poor nutrition and low-socioeconomic status.
Our patients had fewer better prognostic factors when compared to previous studies. The number of patients who had calcium < 10 g/L was more in our study when compared with Yuasa et al.[14] The percentage of patients with LDH more than 1.5 times the upper limit of normal was 64.81%. It was less when compared with 80.95% in Yuasa et al.[13] In our study, 68.42% of patients presented earlier with metastatic disease, i.e., < 1 year when compared to 47.61% in Yuasa et al. study.[13]
The most common histology seen in our population was clear cell carcinoma seen in 84.25% which was similar to other studies. The most common site of metastasis was lung followed by bone and liver metastasis which was similar to other studies and the least common site of metastasis was the brain.
Only 55.55% of our patients underwent nephrectomy when compared to 77.77% in Yuasa et al.[13] and 90.2% in Kim et al.[15] The clinical efficacy rate (CR + PR + SD) was 86% in our study when it was 81% in Yuasa et al.[13] and 76% in Krishna et al.[13]
In our study, 12.5% of patients had PD after the use of sunitinib.
The median values of PFS [Figure 1] and OS [Figure 2] in our patients were similar to other previous studies. The median PFS was 10.2 months in our study, 11.4 months in Krishna et al.,[13] 9.3 months in Yuasa et al.,[13] 8.2 months in Kim et al.,[15] and 11.0 months in Motzer et al.[16]
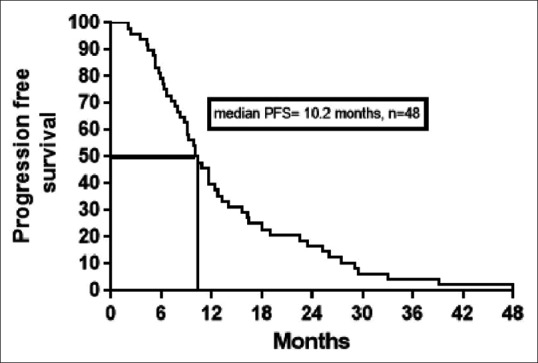
| Fig. 1 Kaplan–Meier estimates of progression-free survival (PFS)
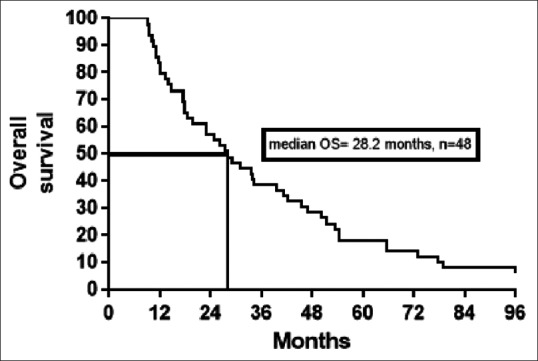
| Fig. 2 Kaplan–Meier estimates of overall survival (OS)
The median OS in our patients was similar to previous reports. The median OS in our patients was 28.2 months when compared to 22.6 months in Krishna et al.,[14] 32.2 months in Yuasa et al.[13] and 23.1 months in Kim et al.[15]
Despite our patients faring worse in nutritional parameters such as hemoglobin, body mass index, serum calcium, and PS, the median OS and PFS are similar to other studies probably hinting toward better disease biology among our patients which needs further validation in large-scale studies.
A few limitations of this study are its retrospective nature, small sample size, and high levels of attrition.
CONCLUSION
Median values of OS and PFS in this study are similar to other reported studies despite the presence of poor risk factors in the patient population. The pitfall in this study is significant attrition due to poor compliance to treatment and follow-up, which is a major factor in the clinic, limiting optimal, and standard treatment, thereby compromising outcomes. This poor follow-up may be due to toxicity, major concern which needs tailoring the dose of therapy, adequate counseling, and careful follow-up which are essential for better outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Kaplan–Meier estimates of progression-free survival (PFS)

| Fig. 2 Kaplan–Meier estimates of overall survival (OS)


 PDF
PDF  Views
Views  Share
Share

