Management of solitary and multiple brain metastases from breast cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(02): 87-93
DOI: DOI: 10.4103/0971-5851.158835
Abstract
As local and systemic control of breast cancer improves, metastasis to the brain remains a common event requiring a specialized management approach. Women diagnosed with breast cancer who develop brain metastases have superior overall survival compared to patients with other forms of metastatic carcinoma. This article summarizes some of the unique aspects of care for patients with breast cancer metastases to the brain.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
As local and systemic control of breast cancer improves, metastasis to the brain remains a common event requiring a specialized management approach. Women diagnosed with breast cancer who develop brain metastases have superior overall survival compared to patients with other forms of metastatic carcinoma. This article summarizes some of the unique aspects of care for patients with breast cancer metastases to the brain.
INTRODUCTION
Due to limited blood-brain permeability and a limited degree of the immune isolation, the brain remains an important sanctuary site for many malignancies, including breast cancer. The most common cancers that metastasize to the brain include lung, breast, melanoma, renal cell, and colorectal cancers.[1] As locoregional and systemic treatment options improve, distant metastasis to sanctuary sites such as the brain has become an increasingly more important issue.
It is estimated that 10-35% of patients diagnosed with breast cancer will develop metastasis to the brain.[2] Although patients with localized disease have a low probability of initial brain metastasis (BM), those with regional and metastatic disease have a 7.6% and 13.5% risk of BM at diagnosis, respectively,[3] while those with HER-2 positive disease or ER-negative disease may be at an even higher risk, especially in the context of other visceral metastases, such as the liver.[4] Current international guidelines for the evaluation of a new patient with breast cancer recommend screening for BM only if there are suspicious clinical symptoms, and not as a routine test.[5] For patients with neurological symptoms, such as progressive headache, motor dysfunction, or sensation changes, there should be a low threshold for ordering an MRI of the brain.[6] Treatment options for breast cancer brain metastases (BCBM) include surgery with/without adjuvant radiotherapy (whole brain or tumor-bed alone), whole-brain radiotherapy (with or without a focal boost, such as radiosurgery), radiosurgery alone, and potentially, systemic or targeted therapy, either used singly or in conjunction with other localized therapeutic approaches.
PROGNOSIS
Predicting individual patient survival after a diagnosis of BCBM has proven to be difficult, especially in identifying potential long-term survivors.[7] The most commonly used tool for estimating length of survival for a patient with BM is the recursive partitioning analysis (RPA) system published by Gaspar, et al. based on the experience of the Radiation Therapy Oncology Group (RTOG).[8] The RPA divides patients with brain metastases (BM) into three broad categories based on performance status, age, and degree of primary/systemic tumor control [Table 1]. One of the limitations of the RPA model for BM is that it is based on outcomes for patients largely treated in the 1980's whose length of survival is likely different than today. More recently, Sperduto, et al. have analyzed additional factors including the number of central nervous system (CNS) metastasis, primary histology, and molecular features, creating the graded prognostic assessment (GPA), and its successor, the Disease-Specific GPA.[9] One of the primary advantages of the GPA system is that it recognizes that some patients with limited, or oligometastatic disease, have a significantly longer observed overall survival (OS), which may warrant individualized therapy. Specifically, for women diagnosed with a breast malignancy with synchronous or subsequent metastasis to the brain, the disease-specific GPA analysis of more than 4,000 patients demonstrates that they will live longer than patients diagnosed with BM from other primary malignancies such as renal cell carcinoma or melanoma.[10]
Table 1
RPA stages for brain metastases[7]

Among patients with breast cancer in the initial GPA study, performance status was the only independent predictor of survival on multivariate analysis. Further analysis, using more variables revealed that breast cancer subtype,[9] as defined by estrogen (ER), progesterone, and HER-2/neu receptor status, influences both the time from initial diagnosis of metastasis to the brain as well as median survival following this diagnosis. For patients with favorable, ER positive tumors, time to BM is longer (55 months) with a better median survival (10-23 months) as compared to triple negative breast cancers (time to metastasis: 27.5 months; median OS: 7 months).[11] Other series have documented length of survival for patients with triple negative breast cancer who develop BM between to be as short as 3-4 months.[12] HER-2 positive patients, on average, have intermediate time until CNS involvement (34-47 months), depending on ER-negative or ER-positive histologic status, respectively, and a longer OS from time of diagnosis of BM (17.9 months).[10] Unlike triple negative breast cancer patients, women with HER-2 positive disease are more likely to develop isolated BCBM without systemic relapse and thus, are at higher risk of intracranial progression even with their systemic disease controlled with up to 50% of HER-2 positive BCBM patients dying from progressive CNS disease.[13] Other investigators have identified specific gene signatures as being predictive for defining a high-risk category[14] for the development of BM, earlier in the course of the disease.
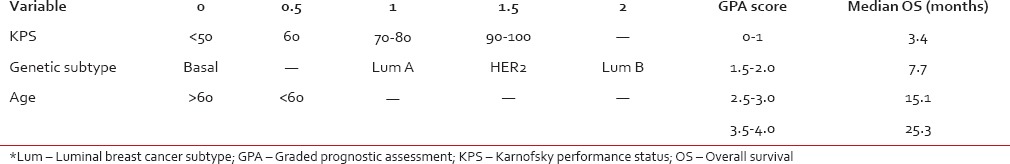
Table 2 summarizes survival as a function of diagnosis-specific-GPA score. Patients with the best prognostic variables (Breast-GPA 3.5-4.0) have a median survival of 25.3 months, much longer than previously predicted by histology-independent RPA and GPA indices for their best subclasses. Additional prognostic scales exist, which may be valuable in counseling individual patients and selecting treatment strategies, including a nomogram that predicts probability of both 6- and 12-month survival, as well as median survival in days; this is however also based on older RTOG clinical experience, as opposed to more modern patient cohorts.[15]
Table 2
Breast cancer specific graded prognostic assessment[11]
TREATMENT OPTIONS
Standard treatment of BM depends on the location, size, number of lesions, histology, severity of symptoms, and overall patient health. For patients with a solitary metastasis, especially if large (>3-4 cm) or those associated with significant cerebral edema and/or midline shift, or symptomatic due to significant mass effect, resection with or without adjuvant radiotherapy should be considered.[16] The role of resection was initially defined in a small randomized trial, wherein the addition of surgery to whole brain radiotherapy (WBRT) improved OS from 15 to 40 weeks for patients with a single BM.[17] For patients with multiple BM, the role of surgery has not been defined in randomized trials, but should be considered if there is a dominant lesion causing neurologic symptoms and/or significant vasogenic edema, especially if midline shift is present.[18] If the diagnosis of BM is in question (i.e., the patient has no history of malignancy or a primary tumor cannot be identified, or the interval from the diagnosis of the primary to the appearance of a new lesion in the brain is inordinately long), resection (and failing the ability to resect, at least a biopsy) should be considered for diagnostic purposes. In the initial randomized trial by Patchell et al., 11% of patients with a suspected single BM had either a primary brain tumor or nonmalignant inflammatory or infectious process, with the caveat that the majority of these were patients with lung cancer, and not breast cancer.[15] Unique to breast cancer patients, a dural-based lesion can present a diagnostic challenge as meningiomas are also common within this patient population. If diagnostic imaging is not clear, a surgical biopsy may be required to distinguish a dural-based metastasis from a benign meningioma.
WHOLE BRAIN RADIOTHERAPY
The standard of care for most patients with BM, especially those with many metastases, a large burden of either intracranial or extracranial disease, and those with limited systemic therapy options, is WBRT. This treatment consists of opposed lateral radiotherapy fields that block out the anterior orbits, nasopharynx, oropharynx, and throat. Typical dose and fractionation schedule range from 20 Gray (Gy) in five fractions to 30 Gy in ten fractions or up to 37.5 Gy in fifteen fractions. The regimen that has the highest published rates of intracranial control is from the control arm of RTOG 9508, published by Andrews et al. which showed a rate of 70% in patients who received WBRT alone to a total dose of 37.5 Gy using 2.5 Gy fractions.[19] Aside from good local control, a significant clinical response (tumor shrinkage >45%) after WBRT has also been correlated with reduced deterioration of neurocognitive function and actually improvement in certain domains.[20]
The addition of WBRT to surgery has been shown to improve local control, decrease elsewhere failures within the brain, and reduce death from an intracranial progression.[21] Adjuvant radiotherapy should be considered after craniotomy due to an estimated local failure rate between 45% and 60% following resection alone[19,22] and a decrease in the rate of CNS-related death when WBRT is added.[19] Increasingly, both pre and postoperative tumor bed radiosurgery, in lieu of WBRT is being considered, and a single randomized trial testing this concept is nearing accrual completion, but no level 1 data or evidence is available to date.
Ongoing research in the application of WBRT includes whether side effects associated with this technique, such as neurocognitive dysfunction, can be ameliorated. Due to the longer relative lifespan of patients with BCBM, quality of life following WBRT is especially important. Possible short-term toxicities of WBRT include otitis media, otitis externa, dermatitis, and alopecia while long term toxicities include neurocognitive decline, decrease in cerebellar function, cataracts and blindness.[23] Gondi et al. have demonstrated the ability to reduce the dose to the bilateral hippocampi with intensity-modulated radiotherapy (IMRT) resulting in a clinically significant reduction in early neurocognitive decline following therapy compared to historical controls.[24] Following the success of the initial phase II multi-institutional study, a randomized phase III trial to compare WBRT with hippocampal avoidance to standard WBRT is being developed.[25] Other investigators have also attempted to apply IMRT to WBRT to evaluate endpoints other than neurocognitive function including a recent publication by De Puysseleyr et al. that showed a significant reduction of dose to the scalp, the so-called hair-sparing WBRT technique.[26]
RADIOSURGERY (STEREOTACTIC RADIOSURGERY)
Stereotactic radiosurgery (SRS) is a precise form of external beam radiotherapy typically utilizing a head frame system coupled with either a hemi-spherical array of cobalt - 60 sources or a linear accelerator-based system delivering highly conformal treatment using multiple arcs or 360° linac rotation.[27,28] This treatment modality is often a cooperative effort between neurosurgery, radiation oncology, and medical physics, which may be used alone or in conjunction with another therapy in the form of a definitive or postsurgical boost. Due to the high dose per fraction, it is thought that high-dose/hypofractionated radiosurgery may have an additional indirect mechanism of cell death through vascular damage of the tumor environment,[29] possibly leading to increased efficacy. Due to the size limitations of SRS, patients with a large solitary metastasis may receive initial surgical resection followed by radiosurgery to the cavity.[30] In patients with more than one BM, radiosurgery alone or WBRT followed by a radiosurgical boost may be employed.[17] Patients with greater than four BM are traditionally treated with WBRT, although patients with primary tumors classically considered to be radioresistant could be considered candidates for radiosurgical boost or primary therapy,[31] especially after failing WBRT. More recent publications, such as a multi-institutional observational study from Japan have reported treating patients with up to 10 BM with SRS alone. Yamamoto, et al. showed survival was noninferior between patients treated with SRS alone for two to four BM compared to the same treatment for patients with five to ten BM (OS: 10.8 months, both cohorts, P = 0.94).[32] Each treatment group had excellent local control (89% vs. 90%, P = 0.78) and required low rates of salvage WBRT (10 vs. 8%, P = 0.48, for 2-4 vs. 5-10 metastases, respectively).[30]
Most series have documented an excellent rate of local control with the use of SRS alone[23,33] or in combination with WBRT.[34,20] In the recently published EORTC 22952 trial, SRS alone produced a local control rate greater than surgery alone with a local control rate at 1-year of 69% versus 41% for surgery, which improved to 81% when SRS was combined with WBRT.[30] RTOG 95-08 also demonstrated an improved rate of local control when an SRS boost was added to whole brain radiotherapy.[17]
Radiosurgery alone may have the benefit of neurocognitive preservation over standard whole brain radiotherapy. In a randomized clinical trial, Chang et al. demonstrated that patients who received radiosurgery alone had less neurocognitive decline at 4 months postradiotherapy versus those who received both SRS and WBRT.[35] These results are thought provoking because reduction of long-term toxicities (such as neurocognitive decline) may be a clinical endpoint worth pursing in the treatment of BM patients. A potential concern of utilizing SRS alone is the decreased rate of local control and higher rate of elsewhere failure in the brain, which would require additional interventions incusing repeat craniotomy, additional SRS, and/or whole brain radiotherapy at a later date. In addition, the cognitive impact of allowing disease to progress in the brain is believed to be significant and of some concern, and a recently completed randomized trial has evaluated this question, results of which are pending.
An alternate approach to preserving neurocognitive function, although not universally adopted, is the use of Memantine, an NMDA receptor agonist agent which dampens inflammatory injury in the brain, especially hippocampal injury, and in the context of using it with WBRT, it has demonstrated a decreased rate of neurocognitive decline.[36]
SYSTEMIC/TARGETED THERAPY
The use of concurrent chemotherapy with brain radiotherapy has largely been avoided due to the fear of additive or synergistic neurotoxicity.[37] Although most systemic agents are thought to not cross the blood-brain barrier (BBB) in significant concentrations to treat BM, there is evidence that disruption of the BBB occurs with brain tumors[38] and moderate success with some targeted agents has been reported. Challenges in using systemic therapy in the treatment of BM include the intrinsic resistance of BM to chemotherapy and potential for progression of concurrent systemic disease if agents specific only to brain treatment are utilized.[1] Intrinsic resistance (or metastatic disease resistant to systemic therapy) may be more prominent if disease progression within the CNS develops while a patient is being treated with systemic therapy. In this setting, additional systemic therapy or targeted therapy may be less effective due to the development of cross-resistance. An additional challenge is a lack of uniformity of how response rates to systemic agents are reported, which makes interpreting efficacy of chemotherapy and targeted agents more difficult.
Despite these challenges, objective responses to traditional chemotherapy for BCBM have been reported. Initial reports of partial efficacy with cyclophosphamide-based regimens were presented by Rosner et al. who showed a 50% overall response (10% complete response) in 100 patients.[39] Boogerd et al. also evaluated cyclophosphamide-based chemotherapy in a smaller cohort of patients, documenting a 76% response rate with a median duration of CNS remission of 30 weeks.[40] More recently, a phase II trial investigating a regimen involving the irinotecan showed a modest 30% response rate but with only a limited 2 month time interval to CNS progression.[41] Reports of some responses to hormonal agents, such as tamoxifen exist, however ER positive patients who develop BCBM often have hormone refractory disease at the time of BM and anti-ER therapy is not considered standard of care for ER + BCBM.
Because the incidence of BM is higher in patients with HER-2 positive tumors, a specific interest in this subset exists. Standard systemic treatment of HER-2 positive disease typically includes trastuzumab, which has been shown to cross a disrupted BBB in some patients (albeit in limited amounts)[42] and is associated with both longer time to the development of BM and survival after a diagnosis of BCBM.[43] Current clinical trials to assess efficacy of anti-HER-2 therapy for BCBM include high-dose trastuzumab as well as combination trastuzumab-emtansine, with the latter having demonstrated convincing anecdotal responses (Chumsri S, personal communication, 2014).
Besides monoclonal antibodies such as trastuzumab, small molecule inhibitors have been used to target HER-2 positive tumors. These small molecule inhibitors such as lapatinib target the kinase domains of epidermal growth factor receptor and HER-2. In preclinical studies, lapatinib has been shown to prevent progression of microscopic deposits of breast-cancer cell within the brain.[44] Geyer et al. found combination therapy of lapatinib with capecitabine superior to capecitabine alone for treatment of BM in HER2-positive patients who had received trastuzumab.[45] The recent results of the LANDSCAPE trial provide convincing evidence for the activity of the lapatinib-capecitabine combination regimen. Patients were limited to those who had not previously had WBRT or SRS and were not currently receiving radiation or systemic therapy for breast cancer.[46]65.9% of patients showed an objective CNS response defined as a reduction in volume of at least 50% without experiencing new brain lesions, progressive extra-CNS disease, worsening neurologic symptoms or the need for increased corticosteroids.[46] About 84% experienced tumor volume reduction when compared to baseline.[46] This study also showed another benefit of the lapatinib-capecitabine regimen as delaying WBRT and its associated toxicities with a median time to WBRT of 8.3 months.[46] A randomized Phase II clinical trial through the RTOG combining WBRT with Lapatinib is also currently being conducted.[47] For patients with HER-2-positive breast cancer, a trial of systemic therapy alone could be considered for those with limited brain only disease or for patients with BM mixed with advanced systemic progression.[6]
Outside of breast cancer, the efficacy of systemic therapy up front or targeted agents for primary intracranial therapy or radiosensitization has been mixed. In the recently reported RTOG 0320 trial, the addition of temozolomide or erlotinib to WBRT plus SRS was not beneficial (and possibly harmful).[48] Other results, including a small randomized trial reported at the 2014 ASCO meeting, show a modest rate of regression of limited BM from NSCLC with the use of platinum-based chemotherapy up front; however, the arms of the study were not balanced for the number of BM.[49]
Immune checkpoint inhibitors, especially in combination with radiotherapy represent a rationale exploratory strategy, and some preliminary data supporting this approach as an investigational method exist for other disease types, but no major effort for BCBM has been mounted to date.
The potential detriment of relying on systemic therapy to treat intracranial disease could be disease progression within the brain leading to worsened neurologic symptoms or, potentially, worse prognosis if additional small deposits of disease progress into more clinically significant lesions. If initial therapy for limited intracranial disease is being considered, consultation with a radiation oncologist up-front with close multi-disciplinary observation should be employed for timely access to radiotherapy.
SUMMARY OF GUIDELINES
For patients with a solitary BM from breast cancer, consideration should be given to treat with surgical resection followed by adjuvant whole brain radiotherapy (level 1 evidence) or SRS to the resection cavity. For solitary lesions in eloquent areas or those in close proximity to or within the brainstem, radiosurgery alone (in addition to WBRT) could be considered. Radiosurgery alone can also be considered in patients with one to three BCBM or, potentially, patients with additional metastases with excellent performance status and/or those who refuse whole brain radiotherapy. Whole brain radiotherapy should, however, be considered the standard of care for all other patients or those who have obvious failure after radiosurgery alone. In patients with HER2-positive disease, systemic therapy shows promise and a trial of systemic therapy alone, especially as part of a clinical trial, could be considered on a case-by-case basis with close MRI-based surveillance.
FUTURE DIRECTIONS
As systemic therapy improves control of extracranial disease and lengthens OS, refinement of current treatment strategies for BCBM to be both more efficacious and less toxic will be important. Quality of life for breast cancer patients, who are expected to live longer than traditionally quoted averages for a general diagnosis of BM, will also continue to increase in importance. Patient outcomes other than local and distant control will continue to influence treatment strategy and techniques such as radiosurgery alone and hippocampal-avoidance whole brain radiotherapy will likely be used to help maintain cognitive function, and to improve quality of life while maintaining treatment efficacy. Although the surgery followed by adjuvant radiotherapy or radiotherapy/radiosurgery alone remain the standard of care, promising work in systemic therapy may also minimize the frequency of use of traditional whole brain radiotherapy in the future.
Footnotes
Source of Support: Nil.


 PDF
PDF  Views
Views  Share
Share

