Mammary Myofibroblastoma with Unusual Morphological and Immunohistochemical Features
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 223-225
DOI: DOI: 10.4103/ijmpo.ijmpo_115_16
Abstract
Mammary myofibroblastoma (MFB) is a rare mesenchymal tumor, derived from mammary stromal fibro/myofibroblasts, which has various morphological features and characteristic immunohistochemical staining. The epithelioid morphologic variant is defined, accordingly, as a proliferation of exclusively or predominantly (>50%) epithelioid cells, variably embedded in a myxoid to fibrous stroma. These histological and cytological features may pose a diagnostic challenge mainly with metaplastic carcinoma and invasive lobular carcinoma of the breast. Thus, immunohistochemical staining by myofibroblastic markers is helpful for confirming diagnosis. Herein, we present a case of MFB in a 43-year-old female. This case report emphasizes the role of immunohistochemistry as gold standard in the diagnosis of MFB. This case is also being presented because of its unusual radiologic findings, its epithelioid histologic variant mimicking malignancy, and its uncommon immunohistochemical phenotype.
Keywords
Breast neoplasms - diagnosis - immunohistochemistry - myofibroblastoma - staining and labelingPublication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Mammary myofibroblastoma (MFB) is a rare mesenchymal tumor, derived from mammary stromal fibro/myofibroblasts, which has various morphological features and characteristic immunohistochemical staining. The epithelioid morphologic variant is defined, accordingly, as a proliferation of exclusively or predominantly (>50%) epithelioid cells, variably embedded in a myxoid to fibrous stroma. These histological and cytological features may pose a diagnostic challenge mainly with metaplastic carcinoma and invasive lobular carcinoma of the breast. Thus, immunohistochemical staining by myofibroblastic markers is helpful for confirming diagnosis. Herein, we present a case of MFB in a 43-year-old female. This case report emphasizes the role of immunohistochemistry as gold standard in the diagnosis of MFB. This case is also being presented because of its unusual radiologic findings, its epithelioid histologic variant mimicking malignancy, and its uncommon immunohistochemical phenotype.
Introduction
Myofibroblastoma (MFB) of the breast is a rare benign mesenchymal tumor that belongs to the family of the “benign spindle cell tumors of the mammary stroma.” MFB, as its name reflects, is composed of neoplastic cells showing a variable fibro-myofibroblastic differentiation and, less frequently, myoid differentiation at morphological, immunohistochemical, and ultrastructural levels.[1] Since the original description, the morphologic spectrum of MFB has been expanded by the recognition of many unusual morphologic variants. Cellular and large-sized MFB, especially in its epithelioid histological pattern, can mimic various spindle cell tumors and metaplastic carcinomas of the breast.
The purpose of this case report is to emphasize characteristics and diagnostic pitfalls of this rare neoplasm.
Case Report
A 43-year-old female, known carrying a right breast nodule diagnosed radiologically and ultrasonographically 1 year ago, underwent evaluation for persistence of the lump. The former mammography and ultrasonography have revealed a well-circumscribed, hyperdense, hyperechogenous solid mass measuring 2 cm × 1 cm × 0.8 cm devoid of micro-calcifications and showing numerous cystic changes suggestive of hamartoma [Figure 1]. Current physical examination revealed a well-defined, nontender, and freely mobile tumor measuring 2 cm in diameter and occupying the upper outer quadrant of the right breast, without any nipple retraction or modification of the overlying skin. Axillary lymph nodes were not palpable. Control ultrasonography showed similar findings as the previous one in addition to the development of a 0.9 cm hyperdense nodule, suggesting an adenofibroma. A surgical excision biopsy of the 2 cm mass was performed.
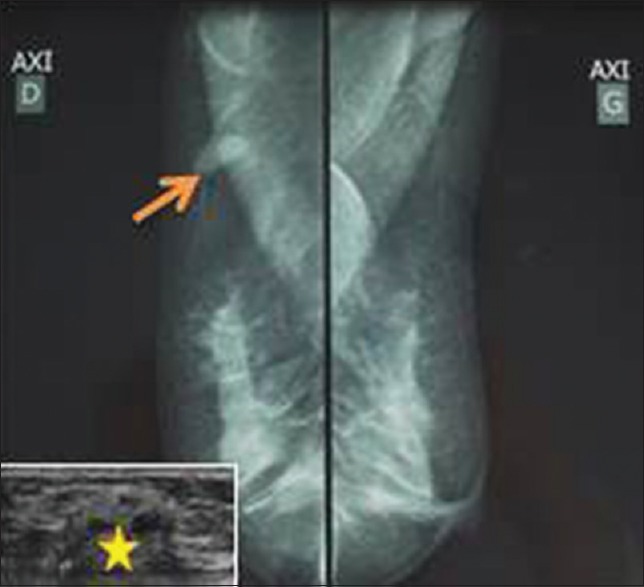
| Figure 1:Mammographic findings: A well-circumscribed hyperdense solid mass (arrow) measuring 2 cm × 1 cm × 0.8 cm devoid of calcifications (ultrasonography in the lower left corner, the mass was hyperechogenous showing numerous cystic changes [star])
Grossly, the lump was well circumscribed, un-encapsulated measuring 2 cm × 1 cm × 1 cm with pushing borders and a whitish homogeneous cut surface, but no necrosis was seen.
Microscopically, the tumor was composed of predominantly (>70%) oval-to-polygonal mononucleated and binucleated eosinophilic epithelioid cells with eccentric nuclei having small nucleoli, arranged either as single cells or in small clusters [Figure [Figure2a2a and andb].b]. A minority of neoplastic cells were spindle shaped and closely packed in short intersecting fascicles and forming whorls occasionally [Figure 2c]. Fascicles and clusters of tumor cells were separated by bands of thick hyalinized collagen [Figure 2d]. Infrequent mitotic figures (<2 xss=removed>high-power field [HPF]) and few entrapped adipocytes were noticed, but neither necrosis nor entrapment of breast glands was observed. Histological diagnosis of atypical spindle cell lesion, especially MFB or metaplastic carcinoma, was suggested.
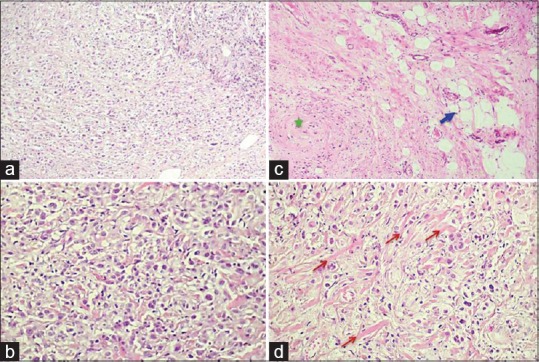
| Figure 2:High and low microscopic view: (a) Epithelioid cells are arranged either as single cells or in small clusters (H and E, ×100). (b) Mononucleated and binucleated epithelial-like cells having abundant eosinophilic cytoplasm and eccentric nuclei with small nucleoli (H and E, ×400). (c) Some neoplastic cells were spindle shaped occasionally forming whorls (star), few entrapped adipocytes were noticed (arrow) (H and E, ×100). (d) Tumor cells were separated by bands of thick hyalinized collagen (arrows) (H and E, ×400)
Immunohistochemically, tumor cells were diffusely positive for vimentin, calponin, and desmin, focally positive for smooth muscle actin (SMA) and S-100 protein, but no immunostaining by cytokeratin, CD34, or H-caldesmon was objectified [Figure 3]. A diagnosis of epithelioid-MFB was finally made after correlating morphological and immunohistochemical findings. Recurrence or metastases were not detected within a 7-month follow-up.
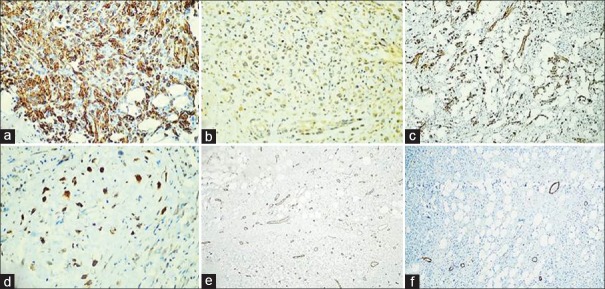
| Figure 3:Immunohistochemical stain showing diffuse positivity for desmin (a) and calponin (b); focal positivity for smooth muscle actin (c) and S-100 protein (d) but no immunostaining by CD34 (e) or H-caldesmon (f)
Discussion
MFB is a distinctive benign stromal tumor of the breast, first described by Wargotz et al. in 1987.[2] This tumor is composed of bland-looking spindle cells probably derived from stromal fibroblasts and mammary myoepithelial cells.[1] Currently, it occurs more commonly in adults of both sexes with ages ranging from 25 to 87 years.[3] Classically, MFB appears as a solitary, slow-growing painless lump with well-circumscribed margins and firm-to-hard consistency. Average size of the tumor is 1–4 cm but can reach larger dimensions.[4,5] Ultrasonography and mammographic outcomes usually consist of a well-circumscribed homogeneous hypoechoic mass devoid of calcifications.[3] Grossly, MFB presents as a solid, well-circumscribed, un-encapsulated tumor with foci of mucoid and lipomatous changes. Cystic change, hemorrhage, and necrosis are scarcely seen.[4] The diagnosis of MFB can be rendered preoperatively on fine-needle aspiration cytology. The presence of coffee bean-shaped nuclear grooves, which was first described by Wargotz et al.,[2] is a cytological clue for diagnosis in fine-needle aspiration smears.[6]
Histologically, classic-type MFB is usually composed of spindle-to-oval cells, exhibiting varying degrees of myogenic and fibroblastic differentiations, closely packed in short haphazardly intersecting fascicles, and interrupted by thick hyalinized collagen bundles.[1,3]
Typically, there is neither necrosis nor entrapment of mammary ducts or lobules within the tumor; mitoses are numbered up to 2 per 10 HPF.[3,7]
Several histomorphological variants of MFB have been documented in the past two decades such as epithelioid, fibrous, deciduoid, cellular, collagenized, lipomatous, myxoid, and infiltrative MFB.[3]
The term epithelioid MFB should be reserved to those tumors composed exclusively or predominantly (>50%) of epithelioid cells.[3,8] In these cases, medium-sized mononucleated, binucleated, or multinucleated neoplastic cells with well-defined cell borders are oval to polygonal, with abundant eosinophilic cytoplasm, and round to oval, with eccentrically placed nuclei containing small evident nucleoli. Epithelioid cells are usually arranged in clusters or in alveolar or trabecular growth patterns and even in isolated single cells.[3] A single-cell file arrangement, as seen in invasive lobular carcinoma, can be observed.[3]
Thus, immunohistochemistry plays a fundamental role in asserting the definite diagnosis. In fact, most cases of MFBs show strong immunostaining by vimentin, desmin, and CD34. Immunoreactivity for SMA, bcl-2, CD10, and CD99 is variably and focally obtained. Moreover, tumor cells are weakly reactive for S-100 protein, estrogen receptor, progesterone receptor, and negative for cytokeratin.[1,3,7] Smooth muscle differentiation attested by H-caldesmon expression was reported in 2%–10% of MFB cells and exclusively in half of the cases.[7,9] In our case, neoplastic cells were unexpectedly negative for CD34, which is a rare circumstance that may suggest the diagnosis of fibromatosis instead of MFB.
Recognition of atypical variants of MFB is crucial to avoid diagnostic dilemma with other benign or malignant breast tumors. The main differential diagnosis is fibromatosis which is distinguished from MFB by its locally aggressive behavior, its recurrence, and its negativity for CD34.[4,5] Other pitfalls in the diagnosis needed to be considered are other breast spindle cell lesions such as nodular fasciitis, myoepithelial lesions, schwannoma, leiomyoma, spindle cell lipoma, phyllodes, hemangiopericytoma, and metaplastic carcinoma.[1,4] Furthermore, MFB in its epithelioid variant may mimic an invasive lobular carcinoma or a metaplastic carcinoma. Finally, several similarities between mammary-type MFB and stromal tumors arising from the lower female genital tract (i.e., superficial MFB, angiomyofibroblastoma, and cellular angiofibroma) have recently been reported.[8] The optimal treatment is surgical excision of the lump. Typically, MFB shows no tendency to recur or metastasize, thanks to its benign nature.[7]
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Shivali B, Kataria S, Chandramouleeswari K, Anita S. Myofibroblastoma breast with unusual morphological features. Cytohistopathogical diagnostic pitfalls and role of immunohistochemistry-review of literature. J Clin Diagn Res 2013;7:2323-5.
- Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol 1987;11:493-502.
- Magro G. Mammary myofibroblastoma: A tumor with a wide morphologic spectrum. Arch Pathol Lab Med 2008;132:1813-20.
- Bharathi K, Chandrasekar VA, Hemanathan G, Anuradha S. Myofibroblastoma of female breast masquerading as scirrhous malignancy – A rare case report with review of literature. J Clin Diagn Res 2014;8:ND10-1.
- Mele M, Jensen V, Wronecki A, Lelkaitis G. Myofibroblastoma of the breast: Case report and literature review. Int J Surg Case Rep 2011;2:93-6.
- Landeyro J, DÁaz ML, Raventós A, Vadillo J, MartÁnez MS. Cytological diagnostic clues in fine needle aspiration of breast myofibroblastoma: A case report. Diagn Cytopathol 2012;40:1107-11.
- Magro G, Fletcher CD, Eusebi V. Myofibroblastoma. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours of the Breast. 4th ed. Lyon, France: IARC Press; 2012. p. 130-1.
- Magro G. Stromal tumors of the lower female genital tract: Histogenetic, morphological and immunohistochemical similarities with the “benign spindle cell tumors of the mammary stroma.” Pathol Res Pract 2007;203:827-9.
- Magro G, Gurrera A, Bisceglia M. H-caldesmon expression in myofibroblastoma of the breast: Evidence supporting the distinction from leiomyoma. Histopathology 2003;42:233-8.

| Figure 1:Mammographic findings: A well-circumscribed hyperdense solid mass (arrow) measuring 2 cm × 1 cm × 0.8 cm devoid of calcifications (ultrasonography in the lower left corner, the mass was hyperechogenous showing numerous cystic changes [star])

| Figure 2:High and low microscopic view: (a) Epithelioid cells are arranged either as single cells or in small clusters (H and E, ×100). (b) Mononucleated and binucleated epithelial-like cells having abundant eosinophilic cytoplasm and eccentric nuclei with small nucleoli (H and E, ×400). (c) Some neoplastic cells were spindle shaped occasionally forming whorls (star), few entrapped adipocytes were noticed (arrow) (H and E, ×100). (d) Tumor cells were separated by bands of thick hyalinized collagen (arrows) (H and E, ×400)

| Figure 3:Immunohistochemical stain showing diffuse positivity for desmin (a) and calponin (b); focal positivity for smooth muscle actin (c) and S-100 protein (d) but no immunostaining by CD34 (e) or H-caldesmon (f)
References
- Shivali B, Kataria S, Chandramouleeswari K, Anita S. Myofibroblastoma breast with unusual morphological features. Cytohistopathogical diagnostic pitfalls and role of immunohistochemistry-review of literature. J Clin Diagn Res 2013;7:2323-5.
- Wargotz ES, Weiss SW, Norris HJ. Myofibroblastoma of the breast. Sixteen cases of a distinctive benign mesenchymal tumor. Am J Surg Pathol 1987;11:493-502.
- Magro G. Mammary myofibroblastoma: A tumor with a wide morphologic spectrum. Arch Pathol Lab Med 2008;132:1813-20.
- Bharathi K, Chandrasekar VA, Hemanathan G, Anuradha S. Myofibroblastoma of female breast masquerading as scirrhous malignancy – A rare case report with review of literature. J Clin Diagn Res 2014;8:ND10-1.
- Mele M, Jensen V, Wronecki A, Lelkaitis G. Myofibroblastoma of the breast: Case report and literature review. Int J Surg Case Rep 2011;2:93-6.
- Landeyro J, DÁaz ML, Raventós A, Vadillo J, MartÁnez MS. Cytological diagnostic clues in fine needle aspiration of breast myofibroblastoma: A case report. Diagn Cytopathol 2012;40:1107-11.
- Magro G, Fletcher CD, Eusebi V. Myofibroblastoma. In: Lakhani SR, Ellis IO, Schnitt SJ, Tan PH, van de Vijver MJ, editors. WHO Classification of Tumours of the Breast. 4th ed. Lyon, France: IARC Press; 2012. p. 130-1.
- Magro G. Stromal tumors of the lower female genital tract: Histogenetic, morphological and immunohistochemical similarities with the “benign spindle cell tumors of the mammary stroma.” Pathol Res Pract 2007;203:827-9.
- Magro G, Gurrera A, Bisceglia M. H-caldesmon expression in myofibroblastoma of the breast: Evidence supporting the distinction from leiomyoma. Histopathology 2003;42:233-8.


 PDF
PDF  Views
Views  Share
Share

