Malignant Peripheral Nerve Sheath Tumor of the Pelvic Retroperitoneum Misdiagnosed as Carcinoma Ovary
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2018; 39(02): 234-236
DOI: DOI: 10.4103/ijmpo.ijmpo_47_17
Abstract
A 52-year-old postmenopausal female was found to have a large solid cystic adnexal
mass, compressing the right ureter, and was suspected to have carcinoma ovary. However, it turned
out to be a retroperitoneal neurogenic tumor. Malignant peripheral nerve sheath tumors (MPNST) may
have cystic areas and may be misdiagnosed as ovarian tumors. Treatment of MPNST involves complete
surgical excision of the tumor with a wide margin.
Keywords
Malignant peripheral nerve sheath tumor - neurogenic tumor - retroperitoneal tumorPublication History
23 June 2021
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
A 52-year-old postmenopausal female was found to have a large solid cystic adnexal mass, compressing the right ureter, and was suspected to have carcinoma ovary. However, it turned out to be a retroperitoneal neurogenic tumor. Malignant peripheral nerve sheath tumors (MPNST) may have cystic areas and may be misdiagnosed as ovarian tumors. Treatment of MPNST involves complete surgical excision of the tumor with a wide margin.
Introduction
Malignant peripheral nerve sheath tumor (MPNST) is a rare soft-tissue sarcoma that arises from peripheral nerve or its sheath.[1] Although majority of these tumors arise in the proximal portions of extremities, some may arise from the sacral plexus and thus be located in the pelvic retroperitoneum.[2] These pelvic tumors especially if associated with cystic changes may be mistaken for an ovarian tumor.
Case Report
A 52-year-old postmenopausal female came to our hospital with a complaint of pain abdomen for 2 years. There was no history of abdomen distension or postmenopausal bleeding. She had undergone laparotomy 2 years back in a private hospital, the details of which were not available. She said probably one of the ovaries was removed in that surgery. The pain had persisted, but she did not have any further hospital visits. On examination, we did not detect any ascites or mass in the abdomen. However, pelvic examination revealed 8 cm × 8 cm firm fixed mass in the right fornix. Uterus was anteverted and normal. Ultrasound showed 8 cm × 8 cm solid, cystic mass in the right adnexal region. Left ovary and uterus were normal. Right ovary was not separately visualized. Contrast-enhanced computed tomography (CECT) of pelvis revealed a 10 cm × 8 cm well-defined heterogeneously enhancing lesion with cystic components in the right side of pelvis, posterior to the uterus [Figure 1]. Right shrunken kidney and hydroureteronephrosis were also noted. Her CA 125 was within normal limits. In view of the patients' age and presence of solid, cystic adnexal mass, a provisional diagnosis of carcinoma ovary was made, and staging laparotomy was planned.
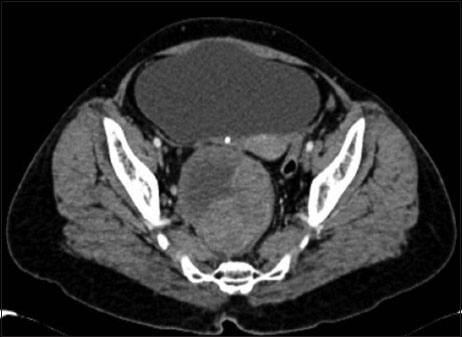
| Figure.1Contrast-enhanced computed tomography of pelvis showing a well-defined heterogeneously enhancing lesion with cystic components posterior to the uterus
Intraoperatively, right ovary was absent. Instead, there was a right-sided retroperitoneal mass adherent to sigmoid colon, uterus, and pelvic wall. Hysterectomy, left salpingo-oophorectomy, and excision of this retroperitoneal mass were done. The mass was stuck to sacrum, and when separating it, there was bleeding from a presacral vein; hemostasis was achieved with prolene sutures. Right external iliac lymph nodes were found to be enlarged, hence removed.
The retroperitoneal mass was a capsulated, yellowish mass with smooth surface. On the cut section, it was predominantly solid with focal cystic areas and calcification [Figure 2]. Histopathological examination revealed a well-circumscribed spindle cell tumor showing hypercellularity and nuclear atypia with foci of palisading, interlacing bundles [Figure 3]. No increase in mitosis or necrosis was seen. Immunohistochemistry showed positivity for S-100. Lymph nodes were free of tumor. The diagnosis of low-grade MPNST was made.
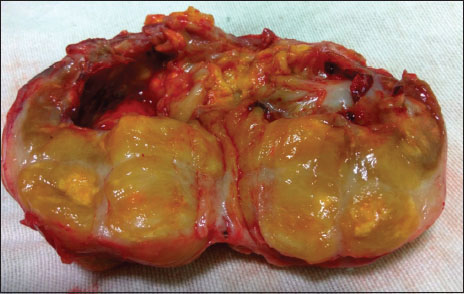
| Figure.2Cut section of the tumor showing focal cystic areas and calcification
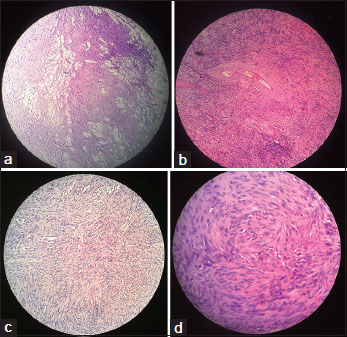
| Figure.3(a) Low-power view showing aggregates of foamy histiocytes between the tumor cells. (b) Presence of hyalinized vessels within the tumor. (c) Low-power view showing fascicles of spindle-shaped cells. (d) High-power view of tumor cells showing atypia
Postoperative course of the patient was uneventful. CECT chest and bone scan were normal. As the patient did not undergo wide excision of the mass, adjuvant radiotherapy was given to the patient. The patient is on regular follow-up with no evidence of recurrence.
Discussion
The term MPNST has now replaced earlier terminologies such as neurogenic sarcoma, malignant schwannoma, neurofibrosarcoma, and malignant neurilemmoma.[2] MPNST accounts for 5%–10% of all soft-tissue sarcomas.[3] Almost 50% of cases are observed in patients with neurofibromatosis type 1 (NF 1), the rest are sporadic.[4] Our patient did not have any features of NF 1. The sporadic MPNST occurs in equal frequency in men and women and the peak incidence is in the seventh decade of life.[2]
The common presenting complaints in these patients are pain, neurological deficits, and paresthesia.[5] Sometimes, they can present with back or abdominal pain or symptoms due to compression of surrounding structures such as ureter. Surprisingly, our patient despite having a large tumor did not have any neurological complaints but was found to have contracted right kidney due to ureteric obstruction.
Imaging techniques such as CT, magnetic resonance imaging (MRI), and positron emission tomography have been used to differentiate MPNST from benign tumors of the nerve sheath.[1] In a study by Benz et al., CT scan was used to differentiate between these tumors and they reported that MPNST were bigger than their benign counterparts (mean size 7.4 ± 4.1 cm vs. 4.8 ± 2.7 cm).[6] MRI is considered the gold standard in evaluating these tumors; areas of necrosis, hemorrhage, heterogeneous enhancement, intratumoral lobulation, peritumoral edema, and cystic areas support a diagnosis of MPNST.[7] In our patient, only CT scan was done, and it showed a 10 cm × 8 cm heterogeneously enhancing lesion with cystic areas.
The histologic appearance of MPNST usually consists of spindle cells showing a fascicular growth pattern with alternating hyper- and hypocellular areas. The spectrum of findings may vary from a picture resembling neurofibroma to fibrosarcoma; MPNST still lacks a widely accepted diagnostic criteria. The presence of generalized nuclear atypia, diffuse cellularity, and low levels of mitotic activity supports a diagnosis of low-grade MPNST.[8] Scheithauer et al. have proposed alternative minimal criteria for the diagnosis of low-grade MPNST: hypercellularity, nuclear enlargement, and hyperchromasia, independent of the presence of mitotic figures.[9] Immunohistochemistry may also help in the diagnosis; focal S-100 positivity may be present in 50%–90% of MPNST.[1]
Majority of MPNSTs (almost 85%) are high-grade tumors associated with poor prognosis and managed by complete surgical resection with a wide negative margin.[2] On the other hand, low-grade MPNSTs have a good prognosis; surgical margins may not have a significant effect on the clinical outcome in these patients.[10] In a study involving 23 patients who underwent surgical resection for low-grade MPNST, 78% were found to have microscopically positive margin.[10] Although 16.7% of them had local recurrence, there was no metastatic disease or mortality at the end of 4 years in this group.
Regional lymphadenectomy is not recommended in this condition as nodal involvement is uncommon. In our patient, we did pelvic lymphadenectomy as we found few enlarged external iliac lymph nodes. However, on microscopy, these lymph nodes were found to be normal.
Radiotherapy may delay the recurrence but has little long-term effect on survival. Adjuvant radiotherapy is administered to all patients with high-grade MPNST and some patients with low-grade MPNST especially those with a positive margin.[2] The use of chemotherapy is restricted to those with metastatic disease and unresectable lesions.
Conclusion
MPNST should be considered in the differential diagnosis of pelvic retroperitoneal tumors. MPNST may have cystic areas and may be mistaken for an ovarian tumor. The treatment of this condition involves complete surgical excision with a wide margin.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published, and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- James AW, Shurell E, Singh A, Dry SM, Eilber FC. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am 2016; 25: 789-802
- Gupta G, Mammis A, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am 2008; 19: 533-43
- Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant Peripheral Nerve Sheath Tumor: Molecular pathogenesis and current management considerations. J Surg Oncol 2008; 97: 340-9
- Bradtmöller M, Hartmann C, Zietsch J, Jäschke S, Mautner VF, Kurtz A. et al. Impaired Pten expression in human malignant peripheral nerve sheath tumours. PLoS One 2012; 7: e475954
- Goertz O, Langer S, Uthoff D, Ring A, Stricker I, Tannapfel A. et al. Diagnosis, treatment and survival of 65 patients with malignant peripheral nerve sheath tumors. Anticancer Res 2014; 34: 777-83
- Benz MR, Czernin J, Dry SM, Tap WD, Allen-Auerbach MS, Elashoff D. et al. Quantitative F18-fluorodeoxyglucose positron emission tomography accurately characterizes peripheral nerve sheath tumors as malignant or benign. Cancer 2010; 116: 451-8
- Demehri S, Belzberg A, Blakeley J, Fayad LM. Conventional and functional MR imaging of peripheral nerve sheath tumors: Initial experience. AJNR Am J Neuroradiol 2014; 35: 1615-20
- Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss's Soft Tissue Tumors. Philadelphia: Saunders/Elsevier 2014; 6: 1155
- Scheithauer BW, Woodruff JM, Spinner RJ. Peripheral nerve sheath tumors. In: Practical Surgical Neuropathology. Philadelphia: Elsevier 2010; p: 235-85
- Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol 2014; 110: 813-6
Address for correspondence
Publication History
Article published online:
23 June 2021
© 2018. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2,
Noida-201301 UP, India

| Figure.1Contrast-enhanced computed tomography of pelvis showing a well-defined heterogeneously enhancing lesion with cystic components posterior to the uterus

| Figure.2Cut section of the tumor showing focal cystic areas and calcification

| Figure.3(a) Low-power view showing aggregates of foamy histiocytes between the tumor cells. (b) Presence of hyalinized vessels within the tumor. (c) Low-power view showing fascicles of spindle-shaped cells. (d) High-power view of tumor cells showing atypia
References
- James AW, Shurell E, Singh A, Dry SM, Eilber FC. Malignant peripheral nerve sheath tumor. Surg Oncol Clin N Am 2016; 25: 789-802
- Gupta G, Mammis A, Maniker A. Malignant peripheral nerve sheath tumors. Neurosurg Clin N Am 2008; 19: 533-43
- Grobmyer SR, Reith JD, Shahlaee A, Bush CH, Hochwald SN. Malignant Peripheral Nerve Sheath Tumor: Molecular pathogenesis and current management considerations. J Surg Oncol 2008; 97: 340-9
- Bradtmöller M, Hartmann C, Zietsch J, Jäschke S, Mautner VF, Kurtz A. et al. Impaired Pten expression in human malignant peripheral nerve sheath tumours. PLoS One 2012; 7: e475954
- Goertz O, Langer S, Uthoff D, Ring A, Stricker I, Tannapfel A. et al. Diagnosis, treatment and survival of 65 patients with malignant peripheral nerve sheath tumors. Anticancer Res 2014; 34: 777-83
- Benz MR, Czernin J, Dry SM, Tap WD, Allen-Auerbach MS, Elashoff D. et al. Quantitative F18-fluorodeoxyglucose positron emission tomography accurately characterizes peripheral nerve sheath tumors as malignant or benign. Cancer 2010; 116: 451-8
- Demehri S, Belzberg A, Blakeley J, Fayad LM. Conventional and functional MR imaging of peripheral nerve sheath tumors: Initial experience. AJNR Am J Neuroradiol 2014; 35: 1615-20
- Goldblum JR, Folpe AL, Weiss SW. Enzinger and Weiss's Soft Tissue Tumors. Philadelphia: Saunders/Elsevier 2014; 6: 1155
- Scheithauer BW, Woodruff JM, Spinner RJ. Peripheral nerve sheath tumors. In: Practical Surgical Neuropathology. Philadelphia: Elsevier 2010; p: 235-85
- Bernthal NM, Putnam A, Jones KB, Viskochil D, Randall RL. The effect of surgical margins on outcomes for low grade MPNSTs and atypical neurofibroma. J Surg Oncol 2014; 110: 813-6


 PDF
PDF  Views
Views  Share
Share

