Isolated primary extranodal lymphoma of the oral cavity: A series of 15 cases and review of literature from a tertiary care cancer centre in India
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2011; 32(02): 76-81
DOI: DOI: 10.4103/0971-5851.89776
Abstract
Background: Non-Hodgkin′s lymphomas (NHL) have a great tendency to affect organs and tissues that do not ordinarily contain lymphoid cells. Involvement of the oral cavity by NHL is very rare. Materials and Methods: Retrospective analysis was carried out by chart review of patients who presented to our hospital between 1990 and 2008. All those patients whose histopathology at our hospital was confirmed as lymphoma were included. Results: Although we register nearly 2000 new oral cancers every year, most of which are squamous cell cancers, we could trace only 15 cases of oral lymphoma in the last 18 years. Of these, hard palate and alveolus were most common sites (5 each). The median age at presentation was 42.6 years. A vast majority (12/15) were NHL. Most patients (70%) reported with painless progressive swelling without systemic signs, such as fever, weight loss, and so on. Only 2 patients were HIV positive. Nearly two thirds received combinations of CT and RT. Cyclophosphamide, hydroxydaunorubicin, oncovin (vincristine), prednisolone regime was the most common regime offered (12/15). Most of them (67%) had good response to 6 cycles of CT that was followed by RT. 10/15 patients completed treatment. Follow-up data of more than 2 years of follow-up was present in 11/15 patients. With median follow-up of 27 months, 5 were disease free, 5 died, and 1 controlled following 2nd line of CT, 2 were lost to follow-up and 2 were alive with disease. Discussion: Head and neck lymphoma is the second most common region for extranodal lymphoma. The nasopharynx, tonsils, and base tongue are most often involved. Unlike the western world, oral cavity involvement is extremely rare. Interestingly, only 2 patients tested positive for HIV and most were young patients. Oral lymphoma may mimic benign oral conditions that often lead to misdiagnosis. Conclusion: Although oral cavity may be the preferred site of NHL in immunocompromised patients it does occur in immunocompetent patients as well. Isolated oral lymphoma is extremely rare and from our data we can say that oral NHL in Indian sub population is more aggressive compared with western literature.
Keywords
Chemotherapy - hard palate and alveolus - non-Hodgkin′s lymphoma - oral cavity - radiotherapyPublication History
Article published online:
06 August 2021
© 2011. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Non-Hodgkin's lymphomas (NHL) have a great tendency to affect organs and tissues that do not ordinarily contain lymphoid cells. Involvement of the oral cavity by NHL is very rare.
Materials and Methods:
Retrospective analysis was carried out by chart review of patients who presented to our hospital between 1990 and 2008. All those patients whose histopathology at our hospital was confirmed as lymphoma were included.
Results:
Although we register nearly 2000 new oral cancers every year, most of which are squamous cell cancers, we could trace only 15 cases of oral lymphoma in the last 18 years. Of these, hard palate and alveolus were most common sites (5 each). The median age at presentation was 42.6 years. A vast majority (12/15) were NHL. Most patients (70%) reported with painless progressive swelling without systemic signs, such as fever, weight loss, and so on. Only 2 patients were HIV positive. Nearly two thirds received combinations of CT and RT. Cyclophosphamide, hydroxydaunorubicin, oncovin (vincristine), prednisolone regime was the most common regime offered (12/15). Most of them (67%) had good response to 6 cycles of CT that was followed by RT. 10/15 patients completed treatment. Follow-up data of more than 2 years of follow-up was present in 11/15 patients. With median follow-up of 27 months, 5 were disease free, 5 died, and 1 controlled following 2nd line of CT, 2 were lost to follow-up and 2 were alive with disease.
Discussion:
Head and neck lymphoma is the second most common region for extranodal lymphoma. The nasopharynx, tonsils, and base tongue are most often involved. Unlike the western world, oral cavity involvement is extremely rare. Interestingly, only 2 patients tested positive for HIV and most were young patients. Oral lymphoma may mimic benign oral conditions that often lead to misdiagnosis.
Conclusion:
Although oral cavity may be the preferred site of NHL in immunocompromised patients it does occur in immunocompetent patients as well. Isolated oral lymphoma is extremely rare and from our data we can say that oral NHL in Indian sub population is more aggressive compared with western literature.
INTRODUCTION
Lymphomas are heterogenous group of clonal malignant diseases, which are known for their spectrum of behavior ranging from relatively indolent to highly aggressive and potentially fatal, which share the same single character of arising as a result of somatic mutation in lymphocyte progenitor cells–either B cell or T cell or both.[1]
Lymphomas can be classified as Hodgkin's lymphoma (HL) or non-Hodgkin's lymphoma (NHL).[2] HL rarely shows extranodal disease (1% cases) in contrast to NHL (23–30% cases).[3] Typical location of extranodal NHL include, gastrointestinal tract, Waldayer's ring, skin, and others. Waldayer's ring is the 2nd most common area to gastrointestinal tract. Oral cavity as a primary site constitutes only 2% of all extranodal NHL.[4]
Majority of adult non-Hodgkin's lymphoma are of B cell origin.[5] According to Yin, 79% are B-cell lymphomas while rest belong to T cell or NK cell types but according to other studies incidence of B cell lymphoma is even higher.[6] There is a controversy existing for the nature of lymphoma being either multicentric or unicentric.[7]
Till recently, only a few cases have been reported in the international literature of extranodal oral NHL and paucity of cases makes diagnosis, understanding of biological behavior, and therapeutic options difficult. The present paper reviews 15 cases of extranodal oral NHL reported at our Hospital, which is a tertiary cancer care center.
MATERIALS AND METHODS
Retrospective analysis of patients who presented to our hospital form 1990 to 2008 have been reviewed total 15 cases could be traced, in which oral mass was initial presentation and diagnosed- as primary NHL. The patient data, including gender, age, oral subsite of presentation, and clinical staging were analyzed. All the basic routine investigations were carried out. Positivity for LCA, CD20, CD3, CD30, CD10, and CD13 was checked. Mib 1 labeling index was checked. Immunotyping was done based on that. CT/MRI scan of local area was done to assess the extension of the lesion and bone involvement. CT thorax and abdomen was carried out for staging. Bone marrow biopsy was done to rule out lymphoma infiltration. Specific treatment CT, RT, or combination was given according to staging.
RESULTS
We were able to identify a total of 15 cases of primary oral NHL in the last 18 years. The median age at presentation was 42.6 years with male to female ratio of 3:2. The gingivobuccal complex was the most common site of involvement (12/15), of which lower gingivobuccal complex was involved in 7 patients. Buccal mucosa was involved site in 1, anterior tongue in 1, and labial mucosa in 1 patient. Most patients reported with painless progressive swelling and B symptoms (systemic) were not present in any of the patients. All patients fell into stage IE A according to Ann Arbor staging. Serological test revealed that only 2 patients were HIV positive.
Two thirds received a combination of chemotherapy and radiotherapy. Cyclophosphamide, hydroxydaunorubicin, oncovin (vincristine), prednisolone (CHOP) was the most common regime that was offered to these patients (12/15). Majority of the patients (67%) had good response to 6 cycles of CT that was followed by RT. Of the 15 patients, 11 patients completed the full course of treatment. Detailed follow-up for more than 2 years was available in only 11 patients. With median follow-up of 27 months, 5 were disease free, 5 died, and 1 controlled following 2nd line of CT, 2 were lost to follow-up, and 2 were alive with disease.
DISCUSSION
The annual incidence of head and neck NHL has increased since the last few years. Extranodal NHL as a distinct entity was first described by Isaacson and Wright in 1983.[8] The incidence of oral manifestation of NHL according to international literature is approximately 2% of all extranodal lymphomas.[4] However; in our study we found it to be an extremely rare disease. Alshemari,[9] suggested racial disproportion with extranodal NHL [Table 1].
Table 1
A review of literature about more than 2 cases presented
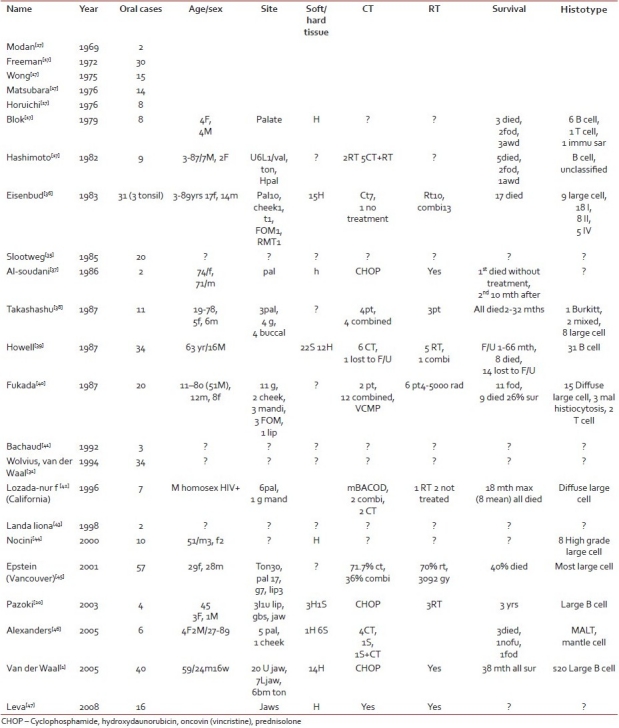
Lymphoma arises due to mutation of any progenitor cell that can be determined by immunophenotyping and gene arrangement studies and various etiologies have been suggested. Gulley et al.[10] suggested the role of EBV in oral lymphomas. Because EBV leads to chronic inflammation, which drives lymphoid cells to proliferate, it is expected that therapies leading to their suppression or withdrawal will weaken or stop genesis and progression of NHL.[11]
Sankaranarayanan,[12] discussed about maxillary lymphoma and suggested that there is a reversal of the incidence of NHL in HIV-positive patients and they carry a risk 60 times more than the general population. Plasmablastic lymphomas (PBL) have been reported as the most common histotype seen in gingivobuccal sulcus; majority are associated with HIV disease; and are difficult to diagnose if unaware and carry poor prognosis.[13] Hicks et al,[14] talked about intraoral presentation of anaplastic large cell Ki-l lymphoma in association with HIV infection and concluded that approximately 3% of HIV-positive cases develop lymphoma. Wood et al,[15] talked about human immunodeficiency virus (HIV)-associated extranodal T-cell nonHodgkin's lymphoma of the oral cavity. However, in our study only 2 patients were HIV positive and both showed cutaneour large B cel lymphoma (CLBL) or PBL. One of the HIV-positive patient died after 3rd cycle of chemotherapy and the other was lost to follow-up.
Unlike HL, in head and neck NHL there is a logarithmic increase in the incidence with increase in age[16] and generally older adults are involved.[6] Vander Waal et al.,[1] published largest number (40) of primary extranodal NHL of the oral cavity and according to his study mean age was 59 years (3–88 years) and male gender was commonly involved. He and Hashimoto,[17] (9 cases) in their study of oral lymphoma reported oral lymphoma in a 3-year-old child. In our study average age was 42.6 years and male gender was commonly affected.
The most common site involved in oral cavity is palate and gingiva,[1] however, lymphomas at other sites have been reported. Maheshwari et al.[18] reported a case of primary NHL in cheek and reviewed 27 cases of cheek lymphoma in the literature; in 2001,[19] he reported a case of tongue NHL and reviewed 12 cases of tongue lymphoma in the literature. Alexander et al.,[20] reported 4 cases of primary extranodal NHL of jaws and reviewed literature about hard tissue extranodal NHL. Lesions involving bone show diffuse bone destruction and disappearance of lamina dura or may appear as a solitary radiolucency.[1] In our study hard palate and alveolus (gingivobuccal complex) were the most common sites. Multicentric as well as unicentric locations have been reported in head and neck.[7] In our study 1 case had involvement of bilateral cheek, the opposite was noted a few days after appearance of the first.
Primary occurrence of NHL in oral mucosa is rare and when oral soft tissue lesions first appear they generally appear as nontender soft to firm swelling of the area often with overlying ulcerations,[5] which may often lead to misdiagnosis. According to Enrique, 2004,[21] the incidence of involvement of cervical lymph nodes in HL is 100% and in NHL is 86.6%. Abdominal adenopathies may be found in 50% of head and neck NHL patients.[21] Other B symptoms are rare finding with head and neck NHL cases according to most publications.[1,21] However, Enrique, 2004[21] noted B symptoms in 27% patients of head and neck NHL in his series of 31 cases.
Various classifications and staging systems have been suggested include: working formulation classification, REAL classification, WHO classification, IPI and Ann Arbor staging system, and NCI proposed grading.[22–26]
WHO/REAL classification[23] divided lymphomas into 4 major types: Indolent lymphomas, Aggressive lymphomas, Highly aggressive lymphomas, and Special group of localized indolent lymphomas. Ann Arbor staging system[22] has four stages. Most extranodal head and neck NHL fall into stage I E if localized and additional suffix A–absence of systemic signs or B–unexplained weight loss >10% or fever or night sweats.
The diagnosis of oral extranodal lymphoma is challenging due to low index of clinical suspicion. Incisional biopsy is a definitive diagnostic modality. Fine needle aspiration cytology (FNAC) shows infiltration of polymorphonuclear cells (PMNs), plasma cells, and lymphocytes and gives inconclusive appearance. Gross appearance of lesion may be whitish translucent, pale colored soft to firm. The most common histotype in head and neck is large B cell lymphoma high or intermediate grade in 40% of immunocompetent adults; while in immunocompromised host PBL is the most common type.[1,29] In our study also B cell lymphoma was the most common variety.
Rappaport[27] and Leukes and Collins[28] contributed to immunotyping classifications. Histologically they can also be classified into low (indolent), intermediate, and high grades (aggressive).
According to Hicks and Flaitz, 2001[29] in nonimmuno-compromised individuals most common type seen was diffuse large B-cell lymphoma (DLBL) in head and neck region, but mantle cell lymphoma, marginal zone B-cell lymphoma, Burkitt's lymphoma–lymphomablastic lymphoma, peripheral T-cell lymphoma, and anaplastic large cell lymphoma can also occur. While according to Teruya-Feldstein et al.,[30] the most common histotype seen in immunocompromised individuals is plasmablastic variety. Hashimoto, 1982[17] reviewed pathological characteristics of oral NHL and according to his 9 cases and review of literature he concluded that B-cell lymphoma is the most common histotype in oral NHL. However, Alexander et al,[20] reported diffuse histiocytic lymphoma (old term for DLBL) as the most common histotype in jaw bones.
Oral lymphoma can spread by three routes: Lymphatic, Contiguous spread to adjacent structures, Blood borne distant metastases in the later stages of the disease.
Pratibha et al,[8] suggested genetic profile for extranodal follicular (mixed type) lymphoma of head and neck: characteristics CD20+, CD3–, immunoprofile CD10+, CD5, most frequent t (14:18), cytogenetic site q32 q21, and associated oncogene bcl-2. Van der Waal et al,[1] used following antibodies for differential diagnosis of 40 cases of oral NHL: L26 (CD20, a pan B-cell marker), CD 79a (the immunoglobulin anchoring molecule so a B-cell marker), CD3 and UCHL 1 (CD45RO) (both pan T-cell markers), BerH2 (CD30), and Mib1 (staining predominantly B cells).
Treatment options for head and neck NHL are chemotherapy, radiation, or both. Different chemotherapeutic regimens are proposed. Van der Waal et al,[1] treated 40 cases of oral NHL. He used only radiotherapy for stage I cases (28–40 Gy fractionated over 2–4 weeks), and combination for higher stages. Chemotherapy for indolent was Chlorambucil with or without Prednisolone and for aggressive CHOP. The mortality rate was zero and average survival time for 34 patients was 38 months. This study and study of same center 1994 (34 oral NHL)[31] did not observe any difference in survival time between bone versus soft tissue involvement.
Gustavsson, 2003[32] form Sweden reviewed role of radiotherapy from 64 previous studies, including 13,305 patients with NHL. He suggested radiotherapy alone for indolent stage I NHL as combination of chemotherapy did not show any improvement in the outcome (follow-up more than 15 years). For aggressive head and neck NHL he supported combination therapy. He also supported radioimmunotherapy for advanced NHL.
We used 6 cycles of CHOP chemotherapy in our patients. However, different regimens of chemotherapy have been proposed. Maheshwari et al,[18] used 6 cycles of cyclophosphamide, vincristine, prednisolone (CVP) regimen, which was followed by radiotherapy of 50 Gy/25 fractions/5 weeks with 19-month disease-free follow-up. Yokobayashi, 1981[33] used vincristine, epirubicin, methylprednisolone (VEMP) regimen for his patient, however, patients died after chemotherapy. We used 45 Gy/25 fractions radiotherapy for our patients.
Role of anti-retroviral therapy (ART) in cases of HIV-positive NHL is studied by Spina and Tirelli .[34] Along with continuous infusion CT (R-CDE regimen), they studied the role of concomitant HAART (highly active ART), that is, rituximab and improvement in disease-free survival was noted.
Survival depends on the extent of the disease, presence of HIV disease, histopathology, and Ann Arbor staging. According to Alexander et al,[20] for extranodal head and neck lymphoma 5-year survival rate is approximately 50%, whereas median survival rate for stage IE is 10 years. Hermans, 1995[25] reviewed 755 cases of NHL and suggested the following survival rates: stage I–IV: 59, 34, 14, and 10%. Slootweg, 1985[35] reviewed 20 cases of oral NHL and showed survival rate of 70% for stage I and 20% for stage II–IV. However, in our study 4 patients died out of 15 that had aggressive disease and 2 of them were HIV positive.
CONCLUSION
Although, we register 2000 new oral cancers every year we could collect only 15 cases in 18 years. The authors would like to conclude that although oral cavity may be the preferred site of NHL in immunocompromised patients, it also occurs in immunocompetent patients, however index of suspicion is every low in case of oral cavity hence, diagnosis can be missed or delayed (particularly in PBL, which mimics carcinoma), which ultimately effects prognosis. The role or ART is important for response to CT and survival in HIV positive cases. Although the incidence of isolated oral disease is miniscule and our data of 15 patients is admittedly as small number, we can still say that the oral NHL in Indian subpopulations more aggressive as compared with western literature.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared.


 PDF
PDF  Views
Views  Share
Share

