Isolated humeral recurrence in endometrial carcinoma
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 199-201
DOI: DOI: 10.4103/0971-5851.190363
Abstract
Isolated skeletal metastasis in endometrial carcinoma at recurrence is very rare. We report a 52-year-old woman diagnosed to have FIGO Stage 1b, Grade 1 endometrioid adenocarcinoma, presenting with isolated distal humerus metastasis, 2 years after surgery and adjuvant radiotherapy for primary disease. Imaging, bone scintigraphy, and cytology confirmed the diagnosis of poorly differentiated metastatic adenocarcinoma. She was treated with local radiotherapy followed by six cycles of paclitaxel and carboplatin chemotherapy along with zoledronic acid, monthly. She is symptom-free after the treatment and at a first follow-up visit after 3 months.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Isolated skeletal metastasis in endometrial carcinoma at recurrence is very rare. We report a 52-year-old woman diagnosed to have FIGO Stage 1b, Grade 1 endometrioid adenocarcinoma, presenting with isolated distal humerus metastasis, 2 years after surgery and adjuvant radiotherapy for primary disease. Imaging, bone scintigraphy, and cytology confirmed the diagnosis of poorly differentiated metastatic adenocarcinoma. She was treated with local radiotherapy followed by six cycles of paclitaxel and carboplatin chemotherapy along with zoledronic acid, monthly. She is symptom-free after the treatment and at a first follow-up visit after 3 months.
INTRODUCTION
Endometrial cancer (EC) is the 7th most common cancer worldwide and 4th most common cancer in women in developed countries.[1] It is diagnosed in an early stage in 70% of the patients. The 5-year overall survival (OS) is 90%.[2] It recurs mostly occurs in the pelvis. Sometimes, distant metastases are seen in lymph nodes, lung, or liver.[3] Bone metastasis is rare, and the prevalence is variously reported as 0–15%.[4,5] Again, it is generally restricted to the pelvic bones and vertebrae. Isolated peripheral skeletal metastasis is very unusual.[6] This is a report of isolated humeral metastasis in a treated patient of Stage 1b, Grade 1 endometrioid adenocarcinoma, and its management.
CASE REPORT
A woman aged 52 years, presented in May 2015 with pain and swelling of the left elbow of 5 months duration. Movements at the elbow were restricted.
At the age of 50 years, 4 years after menopause, she had an episode of heavy vaginal bleeding. Endometrial curettings showed well-differentiated endometrioid adenocarcinoma. She underwent a total abdominal hysterectomy and bilateral salpingo-oophorectomy in July 2013. Histopathological examination confirmed a well differentiated endometrioid adenocarcinoma, with myometrial invasion (Stage Ib, Grade 1). She received adjuvant external beam radiotherapy to pelvis (5040 cGy) and brachytherapy to vault thrice. She completed her treatment in December 2013 and since then has been on regular follow-up.
At presentation, her performance status was 1. She weighed 59 kg, and her height was 1.4 m. Her body surface area was 1.48 m2 and body mass index is 29.3 kg/m2. Breast and abdomen examinations were unremarkable. Pelvic examination did not reveal any local recurrence. A soft, tender swelling was noted over the lateral aspect of the left elbow toward the lower end of the humerus. Rest of the skeletal examination was normal.
Plain X-ray showed an osteolytic lesion in the distal humerus with soft tissue swelling. The joint space was well preserved. There was no fracture [Figure 1].
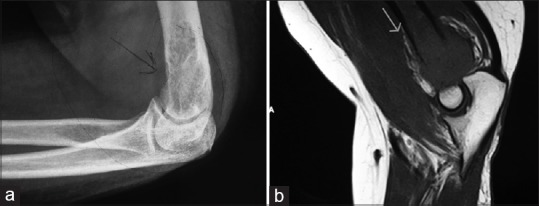
| Fig. 1 (a) Plain radiograph and (b) magnetic resonance imaging showing the humeral lesion
Magnetic resonance imaging (MRI) of left elbow showed a lytic expansile lesion in distal metaphyseal region of humerus with cortical erosion. The zone of transition of the lesion with normal bone marrow appears to be wide with irregular margins. There was edema in the muscles around the elbow both anterior and posterior compartments [Figure 1].
A technetium bone scan showed focal increased tracer uptake in the distal end of the left humerus. Rest of the skeletal survey was normal.
A sonography-guided fine needle aspiration cytology showed metastatic deposits of poorly differentiated adenocarcinoma.
Sonography and contrast enhanced computed tomography scan of abdomen and pelvis did not reveal any local or distant metastasis.
Based on the clinical, radiological, and cytological examination, a diagnosis of isolated humerus metastasis following primary endometrial carcinoma was confirmed.
She received a single fraction of radiation (800 cGy) locally to distal humerus to reduce fracture risk. Later, she was given six cycles of paclitaxel (175 mg/m2) and carboplatin (area under the curve 5) at 3-weekly intervals. She was also given intravenous zoledronic acid 4 mg once in 4 weeks. She tolerated chemotherapy well with good response to her symptoms.
A repeat bone scan showed the persistence of isotope uptake in the left humerus. She is symptom-free and on regular, 3 monthly follow-ups.
DISCUSSION
Bone metastases with EC are infrequent. Their real incidence is unknown, and medical literature is limited to case reports. It is reported to vary between 0 and 15%.[4,5] Although metastases to the bone are thought to result from hematologic dissemination, the mechanism of bone metastasis is not clearly understood. It can be associated with primary tumor behavior, vascular supply, immune system, and bone environment.[7]
A few case reports are available wherein bone metastasis was the primary presentation in EC.[8,9] The overall incidence of bone metastases at a presentation in EC is reported to be between 0.12 and 0.3% in two case series.[5,10] A few other case reports showed bone metastasis at recurrence.[11] Abdul-Karim et al. reported the highest incidence of 25%, in a study of bone metastasis from 67 autopsy cases with EC.[12] Uccella et al., in their series, reported an incidence of 0.8% while Yoon et al. reported it to be 1.8%.[5,10] Bone metastasis either at primary diagnosis of EC or recurrence is observed in all stages and all grades of adenocarcinoma.[13]
Most patients present with pain (81%) or fracture (9.5%) at the time of diagnosis of bone metastasis.[10] Our patient also presented with bone pain and swelling. Plain radiograph, bone scan, MRI, positron emission tomography (PET), aspiration cytology, and bone biopsy help in the diagnosis.[10,11] Except PET and bone biopsy, other investigations were done in our patient. She underwent computed tomography scan of abdomen and pelvis to look for other sites of metastasis.
The optimal elective treatment of bone metastasis in EC is not clear. This uncertainty is not only due to small number of cases reported in medical literature but also of various osseous sites being involved. It is equally important to identify extraosseous sites of metastasis for planning optimal treatment.[5] Treatment options include directed radiation, surgical resection, systemic chemotherapy, and hormonal therapy if hormone receptor positive. In our patient, hormone receptors could not be ascertained as only needle aspiration cytology was obtained.
Selection of therapy is based on the sites and number of bones involved, concomitant extraosseous metastasis, type of previous treatment and patients’ performance status.[10] All these treatment modalities were used in patients’ series analyzed by Uccella et al. and Yoon et al.[5,10] Based on literature from other solid tumors such as prostate and breast, bisphosphonates could be considered as the treatment modality.[8,14] Our patient received directed radiation followed by chemotherapy and bisphosphonate zoledronic acid. The indication for systemic chemotherapy is an extrapolation from the management of high-risk locoregional disease in EC, where a combination of paclitaxel and carboplatin is the standard of practice.
In the series reported by Yoon et al. the median time of recurrence to the bone was 9 months (2–43 months). The median OS and survival after bone metastasis was 33 months (range, 9–57 months) and 15 months (range, 12–17 months), respectively. Patients with bone metastasis at recurrence had significantly longer OS than those patients with bone metastasis at diagnosis of EC (36 vs. 13 months; P = 0.042). Metastasis to extra-pelvic bone was associated with significantly longer OS (46 vs. 19 months; P = 0.001) and longer survival after bone metastasis [10] (25 vs. 12 months; P = 0.002).
In the series from Uccella et al., the median time to recurrence was 19.5 months (range 3–114 months) and the median survival after metastasis was 12 months (range 2–267 months). Isolated bone metastasis with no extraosseous spread is associated with longer survival (26 vs. 6 months; P = 0.008).[5] In our patient, bone metastasis occurred 22 months after the primary diagnosis. As there is no extraosseous spread, survival chances may be good. Only time will answer this question.
CONCLUSION
Isolated humerus metastasis in early stage EC is a rare entity. Till date, only one such case is reported with Stage 1a, Grade 3 disease. Even though bone metastasis is rare in EC, due attention to bone pain or joint swelling will help in prompt diagnosis. Multimodality treatment can often provide good palliation and prolong survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 (a) Plain radiograph and (b) magnetic resonance imaging showing the humeral lesion


 PDF
PDF  Views
Views  Share
Share

