ISMPO Guidelines for Diagnosis and Management of Early Breast Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(01): 001-023
DOI: 10.1055/s-0044-1786517
Abstract
The management of breast cancer has become increasingly complex and multidisciplinary in the recent past. Further, there are unique constraints and opportunities for cancer care delivery in India, including socioeconomic, geographic, and other disparities. Therefore, the Indian Society of Medical and Paediatric Oncology convened a panel of experts to create evidence and context-based guidelines for the management of early breast cancer.
Authors' Contributions
S.A., A.S., R.S. contributed in Concept, Design and Intellectual content whereas S.G., P.K., S.A. reviewed the manuscript. All authors were involved in Literature search, Clinical studies, Data analysis, Manuscript preparation and Editing.
Publication History
Article published online:
18 July 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Breast cancer: An overview of published Indian dataBharath Rangarajan, South Asian Journal of Cancer, 2016
- Indian Society of Medical and Paediatric Oncology (ISMPO)—Breast Cancer in Young GuidelinesJyoti Bajpai, Indian Journal of Medical and Paediatric Oncology
- Breast cancer risk factor evaluation in a Western Himalayan state: A case–control study and comparison with the Western WorldPurnima Thakur, South Asian Journal of Cancer, 2017
- Reproductive factors and breast cancer risk: A meta-analysis of case–control studies in Indian womenGayatri Vishwakarma, South Asian Journal of Cancer, 2019
- Breast cancer: Indian experience, data, and evidenceSudeep Gupta, South Asian Journal of Cancer, 2016
- Cost of breast cancer diagnosis and treatment in India: a scoping review protocolPriyanka Chandrakant Barathe, BMJ Open, 2022
- Comparative examination of breast cancer burden in sub-Saharan Africa, 1990–2019: estimates from Global Burden of Disease 2019 studyKenechukwu Kizito Igbokwe, BMJ Open, 2024
- EP027/#870 Breast cancer in UzbekistanSayde Djanklich, International Journal of Gynecological Cancer, 2022
- P99 Changing epidemiology and age-specific incidence of breast cancer in England, 1985–2018Alice Miller, J Epidemiol Community Health, 2021
- Increasing breast cancer awareness and breast examination practices among women through health education and capacity building of primary healthcare providers: ...Ranjan Kumar Prusty, BMJ Open, 2021
Addition of radiotherapy to BCS benefits survival, and reduces the absolute risk of any type of recurrence after 10 years by 15.7%-overall and by 15.4%-in patients with pN0 disease. Further, the risk of mortality from breast cancer at 15 years reduces by 3.8%-overall and by 3.3%-for patients with pN0 disease.[43] In pN+ patients, the 10-year recurrence risk and 15-year mortality risk from carcinoma breast are decreased by 21.2 and 8.5%, respectively.[44] ([Table 10])
Tumor Bed Boost
Ipsilateral breast tumor recurrence (IBTR), which mostly occurs in the proximity of tumor bed, can be as high as 16.4%- in the nonboost receiving patients versus 6.4%- in the boost group. However, the incidence of skin fibrosis is significantly higher in the boost RT patients. Younger patients benefit the most whereas patients >60 years of age benefit far less.[45]
Abstract
The management of breast cancer has become increasingly complex and multidisciplinary in the recent past. Further, there are unique constraints and opportunities for cancer care delivery in India, including socioeconomic, geographic, and other disparities. Therefore, the Indian Society of Medical and Paediatric Oncology convened a panel of experts to create evidence and context-based guidelines for the management of early breast cancer.
Keywords
early breast cancer - guidelines - ISMPO - medical oncologyIntroduction
The incidence of breast cancer has been gradually increasing in India in the past few decades and it overtook cervical cancer as the most common cancer among women in 2020.[1] Based on data from population-based cancer registries, the age-adjusted annual incidence of breast cancer in large urban locations in India is approximately 30 to 35/100,000, with an average annual percentage increase of about 1.1%, and in rural locations about 10 to 12/100,000.[2] [3]
The most important risk factors for breast cancer are increasing age, genetic predisposition,[4] obesity, lower parity, and exposure to estrogen, including use of hormone replacement therapy. Apart from the known high-penetrance germline mutations in a few genes like BRCA 1 and 2, research has evaluated that, in the Indian population, the association of body fat distribution (increased waist–hip ratio) is associated with breast cancer risk.[5] [6] There is some evidence that physical activity,[7] dietary modification,[7] and adequate breastfeeding[8] can be protective.
India is a large country with varied geography, different disease distribution pattern among rural/urban society, and socioeconomic constraints which make management of early breast cancer complex and different. Therefore, the Indian Society of Medical and Paediatric Oncology convened a panel of experts to create evidence and context-based guidelines for the management of early breast cancer. Various key opinion leaders from pathology, radiology, molecular oncology, medical, radiation, and surgical oncology have thoroughly discussed and put down the recommendations (Strength, Grade) applicable to our country.
Diagnosis of Early-stage Breast Cancer
Mammography is the gold standard worldwide for screening and diagnosis with the maximum benefit in some studies shown to be between 50 and 60 years. However, India has a high incidence (up to 33%- of all incident breast cancers in some studies) of cancer in younger females.[9] [10] There are currently no established screening standards that advocate for the routine screening of women under the age of 40 who are at average risk. In the population of women under the age of 40, the occurrence of breast cancer is relatively infrequent and young women have dense breast, hence the mammogram can be misleading and lead to false negatives. Additionally, there is a lack of randomized studies pertaining to breast cancer screening, and the efficacy of mammography in this context is suboptimal. For younger females, clinical and self-breast examination along with ultrasound (USG) breast can be adjuncts to mammography but cannot replace mammography. The implementation of biennial clinical breast examinations by primary health care providers resulted in a notable decrease in the stage of breast cancer at the time of diagnosis. Additionally, this intervention was associated with a reduction in breast cancer mortality, although the overall reduction was not statistically significant. However, a substantial reduction of almost 30%- in mortality was observed specifically among women aged 50 years and older. The inclusion of clinical breast examination as a component of breast cancer screening should be given due consideration in our country.[11]
In patients at high risk of developing breast cancer (estimated lifetime risk of >20%- such as—women with personal history of breast cancer, BRCA 1 and 2 gene mutation carriers or their first-degree relatives, women with a history of chest irradiation between 10 and 30 years of age, Li–Fraumeni syndrome [P53], or above syndromes in first-degree relatives); current evidence-based screening guidelines ([Table 1]) include earlier and more frequent screening, with the addition of annual breast MRI.[12] [13]
|
LoE |
GoR |
|
|---|---|---|
|
• It is advised that women between the ages of 50 and 69 get mammography screening. |
I |
A |
|
• Regular mammography screenings may also be conducted for women between the ages of 40 and 49, as well as those between the ages of 70 and 74. However, it should be noted that the data supporting the benefits of mammography in these age groups are not as well established. |
II |
B |
|
• In women with a strong familial history of breast cancer, with or without proven BRCA mutations, annual MRI and annual mammography (concomitant or alternating) are recommended. |
I |
A |
|
LoE |
GoR |
|
|---|---|---|
|
Familial breast cancer associated with BRCA mutations |
I |
A |
|
Lobular cancers |
I |
A |
|
Dense breasts |
II |
B |
|
Suspicion of multifocality/multicentricity (particularly in lobular breast cancer) |
I |
A |
|
Large discrepancies between conventional imaging and clinical examination |
III |
B |
|
When the findings of conventional imaging are inconclusive (such as a positive axillary lymph node status with an occult primary tumor in the breast) |
I |
A |
|
LoE |
GoR |
|
|---|---|---|
|
If preoperative systemic therapy is planned, a core needle biopsy is mandatory to ensure a diagnosis of invasive disease and assess biomarkers |
I |
A |
|
As a minimum, USG fine-needle aspiration or core biopsy of suspicious lymph nodes should be carried out, preferably followed by clip or carbon marking of biopsied lymph nodes |
III |
A |
|
Pathological evaluation includes histology from the primary tumor and cytology/histology of the axillary nodes (if involvement is suspected) |
I |
A |
|
Pathological report should include histological type, grade, IHC evaluation of ER, PgR (for invasive cancer), HER2 (for invasive cancer) |
I |
A |
|
[a]Ki-67 proliferative index below 5%- or above 30%- could be used as prognostic marker |
IV |
B |
|
Tumors should be grouped into surrogate intrinsic subtypes, defined by routine histology and IHC data |
I |
A |
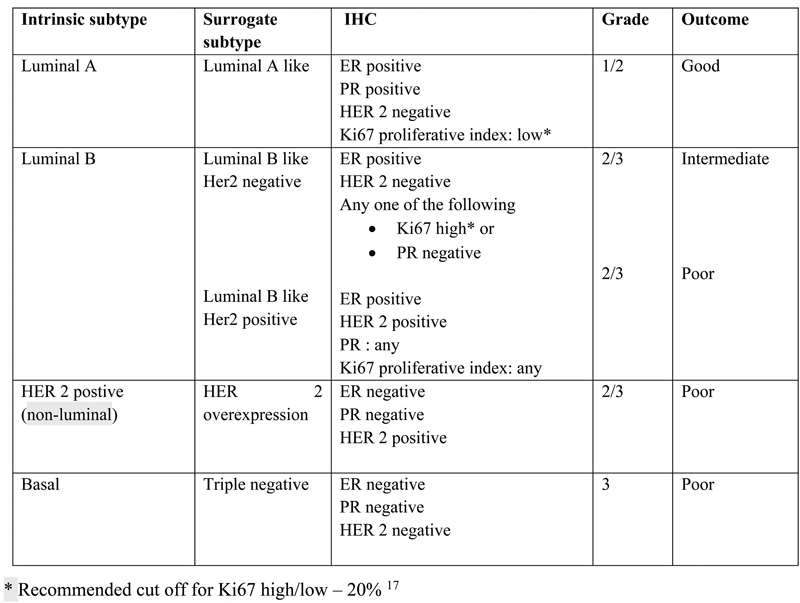
| Fig 1 Breast molecular and surrogate subtypes. Er, estrogen receptor; IHC, immunohistochemistry; PR, progesterone receptor.|
Predictive and Prognostic Multigene Assays in Hormone Receptor-positive/HER2-negative Early Breast Cancer
In patients with early-stage breast cancer, the decision of systemic adjuvant therapy is often based on the clinicopathological factors defining the risk of relapse, response prediction to specific treatment (endocrine vs. targeted), its benefits and toxicities. The risk of relapse particularly in HR-positive, HER2-negative tumors is assessed by age, menopause status, number of positive nodes, primary T stage, grade, Ki67, and multigene assays.
Predictive and Prognostic Assays
Among various commercially available multigene-based assays, Oncotype DX is supported by clinical validation not only for estimating prognosis but also for predicting the recurrence risk reduction when chemotherapy is added to hormone therapy ([Fig. 2a]). Patients with more than 1.0 cm, node negative, hormone positive with recurrence score of 0 to 10, does not warrant adjuvant chemotherapy, only endocrine therapy (ET), as compared to high recurrence score (RS) (>25) which will demonstrate a clear benefit on adjuvant chemotherapy.[18] [19] Based on the TAILORx trial, RS 11 to 25 (intermediate risk) did not show any additional benefits of adjuvant chemotherapy over and above ET. However, for women aged less than 50 years, RS score from 16 to 25, did reveal lower risk of recurrence (ROR) when chemotherapy is added to postoperative hormone therapy.[19] As per Rx PONDER study, postmenopausal women with recurrence risk <26>
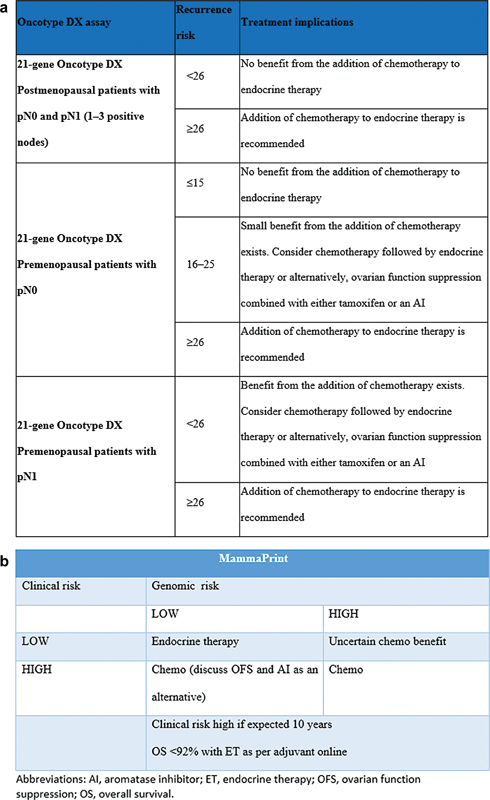
| Fig 2 (a) Oncotype DX-based risk stratification. (b) MammaPrint-based risk stratification.|
Prognostic Assays
70-gene assay (MammaPrint) can classify the patients into genomic low or high risk for distant recurrence. However, based on the randomized MINDACT published data, the utility of 70-gene signature in providing evidence for making recommendations regarding the use of adjuvant chemotherapy especially for patients at low clinical risk[20] is not present, thus having only prognostic significance ([Fig. 2b]).
50-gene assay (PAM50) has only prognostic clinical value and can identify the ROR and stratify patient into high-, medium-, and low-risk groups. Based on Danish Breast Cancer Cooperative Group database and TransATAC study, low ROR with either lymph node-negative or -positive tumors, had low risk for distant recurrence.[21] [22]
12-gene assay (EndoPredict) calculates the risk score and stratify the patients into low and high risk of distant recurrence. Patients with an RNA-based 12-gene low-risk score, predicts late recurrences risk among low-risk patients (less than 2 cm and more than 2 cm but node negative). TransATAC study, retrospectively validated endo predict (EP) and EP clin scores in patient with low risk of of distant recurrence,[22] suggest that adjuvant chemotherapy may not yield additional benefit in these patients.
Breast Cancer Index (BCI) is an RT PCR-based assay that combines gene expression of two biomarkers (the HOXB13:IL17BR ratio (H/I), and Molecular Grade Index (MGI)).[23] It helps us to predict risk of late distant recurrence (5 years postdiagnosis) and also cumulative risk of relapse at 10 years in female treated only with adjuvant ET for node-negative and chemo-ET in N1 patients.
Patients with a BCI low-risk score, the T1 and T2, and lymph node-negative tumors prognostic category is similar to T1a–T1b, N0, M0. BCI has only prognostic clinical value.
CanAssist Breast is an IHC-based test developed and validated on more than 2,000 patients primarily of Indian ethnic origin. It calculates the risk score and stratifies the patients into low and high risk of distant recurrence. This test assesses the expression of five biomarkers (CD44, ABCC4, ABCC11, N-Cadherin, pan-Cadherin) involved in tumor biology, namely metastasis, drug resistance, stemness, and arrives at a score predictive of distant recurrence, along with three clinical parameters—tumor size, grade, and node status.[24] The same has not been validated in any prospective randomized control trial. [Table 5] mentions recommendations for various genomic prognostic assay available to us for deciding adjuvant therapy for both node-negative and -positive (<4>
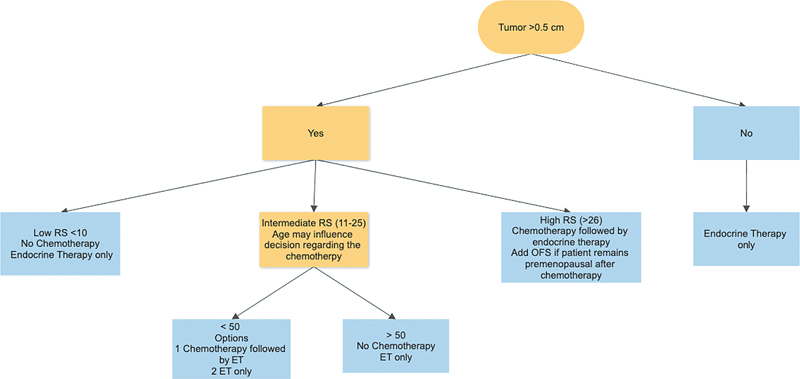
| Fig 3Algorithm for adjuvant therapy for HR + EBC > 0.5 cm. ET, endocrine therapy; OFS, ovarian function suppression.|
Management
Ductal Carcinoma In Situ
Ductal carcinoma in situ (DCIS) was a relatively rare entity till the advent of routine screening programs. DCIS now constitutes about 20 to 25%- of “Stage 0” breast cancer. National Cancer Institute study of 2020 reported a 36 to 100%- progression of DCIS to invasive cancer when not treated. The progression time was 0.2 to 2.5 years; however the overdiagnosis of DCIS was 3.1 to 65.8%.[25]
DCIS needs a multidisciplinary team approach. Mastectomy or breast conservation surgery (BCS) along with radiation are the cornerstones of management. Total mastectomy with clear margins along with reconstruction in DCIS is curative, and radiation therapy (RT) is usually not recommended. There is no general agreement on what is considered an optimal margin; however, recent consensus has determined that a 2-mm margin is adequate.[26] [Table 6] mentions the indication of adjuvant radiotherapy and/or hormone therapy, postsurgery for DCIS. RT is commonly used as the standard treatment for patients undergoing breast-conserving therapy (BCT). However, it may be justifiable to exclude certain individuals with advanced age, significant comorbidities, or small areas of low-grade disease that have been surgically removed with wide negative margins.
|
LoE |
GoR |
|
|---|---|---|
|
BCS followed by whole breast radiotherapy or total mastectomy is acceptable treatment options for DCIS |
I |
A |
|
Whole breast radiotherapy is recommended for the majority of women with DCIS treated with BCS |
I |
A |
|
In patients with low-risk DCIS, omitting radiation is an option |
V |
B |
|
Both tamoxifen and Ais may be used after conservative, local treatment of DCIS to prevent local recurrence and to decrease the risk of development of a second primary breast cancer |
I |
B |
|
LoE |
GoR |
|
|---|---|---|
|
All patients must undergo definitive breast surgery BCT/mastectomy, after the completion of neoadjuvant therapy, taking into consideration the tumor characteristics and response posttherapy. |
II |
A |
|
SLNB is recommended in patients with node-negative axilla prior to systemic therapy |
II |
A |
|
SLNB may be performed in selected cases in patients with node-positive axilla converting to node-negative postsystemic therapy |
II |
B |
|
Completion ALND is recommended in patients with SLNB-positive patients postsystemic therapy |
II |
B |
|
LoE |
GoR |
|
|---|---|---|
|
Postoperative RT is strongly recommended after BCS (I, A) |
I |
A |
|
Boost RT is recommended to reduce the risk of in-breast relapse in patients: Exceptions are elderly (more than 60 years) with stage I tumors |
I |
A |
|
APBI is an acceptable treatment option in patients with a low risk for local recurrence |
III |
C |
|
LoE |
GoR |
|
|---|---|---|
|
PMRT is recommended for high-risk patients, including those with involved resection margins, involved axillary lymph nodes, and T3–T4 tumors |
I |
A |
|
It should also be considered in patients with one to three positive axillary lymph nodes |
I |
A |
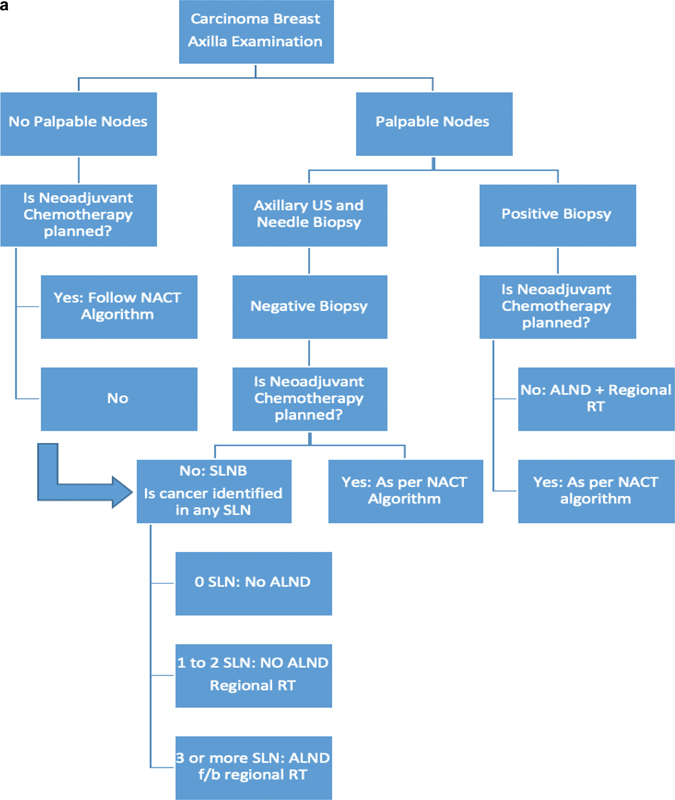
| Figure 4: (A) Algorithm for the role of radiation therapy in early breast cancer. (b) Algorithm for the role of radiation therapy after neoadjuvant therapy. ALND, axillary lymph node dissection; NACT, neoadjuvant chemotherapy; RT, radiation therapy; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; USG, ultrasound.|
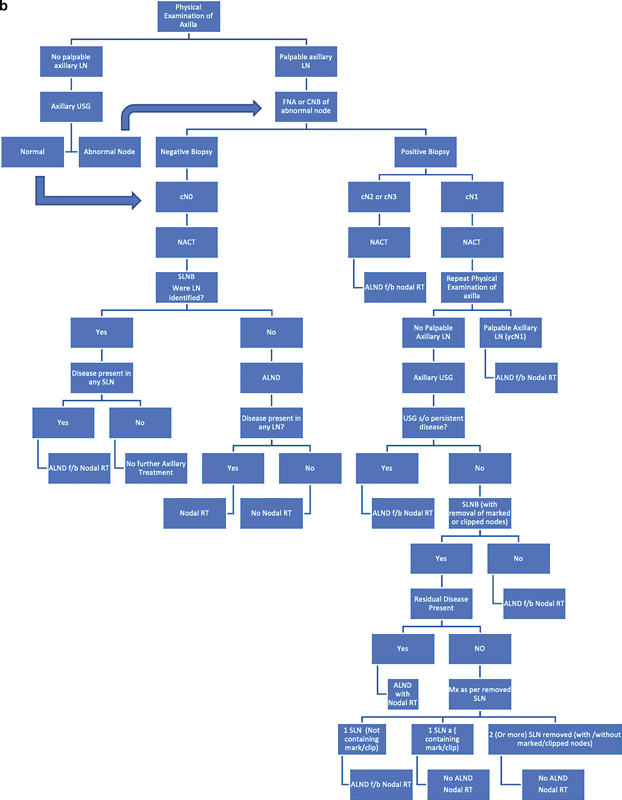
|
LoE |
GoR |
|
|---|---|---|
|
Comprehensive nodal RT is recommended for patients with involved lymph nodes |
I |
B |
|
After ALND, routine axillary irradiation should not be done to the |
I |
E |
|
LoE |
GoR |
|
|---|---|---|
|
For premenopausal women, tamoxifen for 5 to 10 years is a standard of care. |
I |
A |
|
In patients who turn postmenopausal during the first 5 years of tamoxifen, a switch to aromatase inhibitors (AIs) for 2 to 5 years shall be considered, depending on predicted risk of late recurrence. |
II |
A |
|
In high-risk patients requiring CT (node positive, tumor size more than 2 cm), addition of OFS to ET should be strongly considered. |
I |
A |
|
OFS during CT provides some protection of ovarian function and has no negative impact on oncological outcomes; thus, it should be proposed to patients. Patients must use barrier contraception along with medical ovarian suppression (recommend monthly LHRH analogs and not 3 monthly) |
I |
A |
|
Patients undergoing OFS and those taking AIs should be advised to have adequate calcium and vitamin D3 intake and undergo periodic assessment of bone mineral density (by DEXA scan) |
I |
A |
|
LoE |
GoR |
|
|---|---|---|
|
For postmenopausal women, AI (both nonsteroidal and steroidal) is the preferred adjuvant therapy. We recommend this treatment for those with hormone receptor-positive breast cancers regardless of size. |
I |
A |
|
For women receiving adjuvant ET, we recommend at least a 5-year course of treatment. |
I |
A |
|
For women with higher risk disease (stage II or node positive), we suggest extended endocrine treatment 7 to 10 years, although we recognize that some patients with poor tolerance may choose not to pursue extended treatment. |
I |
C |
|
For patients receiving adjuvant CT, we initiate ET after CT has completed (i.e., sequentially), in order to minimize toxicities. For women receiving adjuvant radiation therapy (RT) for breast cancer, ET may be initiated concurrently with RT or sequentially, following the completion of RT. |
I |
A |
|
We recommend addition of abemaciclib to adjuvant ET in high-risk patients. |
III |
C |
|
LoE |
GoR |
|
|---|---|---|
|
All HER2-positive tumors which are more than 2 cm in size or node positive shall be treated with neoadjuvant chemotherapy and HER2-targeted drugs |
I |
A |
|
We consider six cycles of taxane–carboplatin–trastuzumab (with or without pertuzumab) regimens as preferable alternatives to anthracycline-containing regimens (4AC or 4EC followed by weekly paclitaxel) as neoadjuvant therapy |
I |
A |
|
SC formulations are reasonable alternatives to IV formulations for both trastuzumab and pertuzumab |
III |
B |
|
For all pT1b/c, we recommend adjuvant chemotherapy/trastuzumab. For patients with pT1a tumors, we advise adjuvant chemotherapy/trastuzumab for 4- to 5-mm tumor if they are hormone negative ([Fig. 1]) |
II |
B |
|
For women with pT1b/c N0, we suggest to use weekly Paclitaxel for 12 weeks along with Trastuzumab for 1 year |
II |
A |
|
One year of (neo) adjuvant trastuzumab remains a standard for the vast majority of HER2-positive patients but in select subgroup,[a] shortening trastuzumab duration to 6 months shall be discussed |
I |
A |
|
For patients who had residual invasive disease after completion of neoadjuvant chemotherapy with anti-HER2 therapy (Trastuzumab with or without pertuzumab), substitute adjuvant trastuzumab with trastuzumab emtansine (T-DM1) for 14 cycles |
II |
B |
|
For those with pathologic complete response following HER2-directed therapy, we recommend adjuvant trastuzumab to complete a year of HER2-directed therapy |
III |
B |
|
For those with node-positive breast cancer, who have not received any neoadjuvant therapy, we advise trastuzumab with pertuzumab along with chemotherapy |
II |
B |
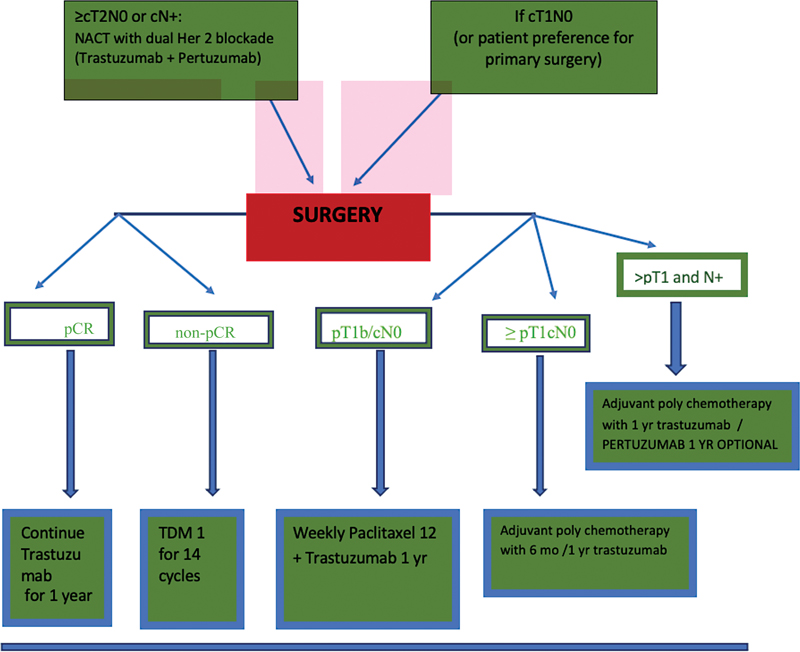
| Figure 5: Treatment algorithm for neoadjuvant therapy for HER2-positive EBC. pCR, yr, year.|
|
LoE |
GoR |
|
|---|---|---|
|
Patients with triple-negative breast cancer (TNBC; >0.5 cm)/node-positive require adjuvant chemotherapy |
I |
A |
|
AC/Taxane-based dose-dense chemotherapy is preferred for high-risk TNBC (more than 2 cm, node positive) |
I |
A |
|
Clinicians can individualize the decision to add Carboplatin to the chemotherapy regimen along with taxane. |
I |
C |
|
NACT does not offer a survival advantage in operable TNBC |
II |
A |
|
Carboplatin can be considered along with paclitaxel in neoadjuvant therapy to increase the pCR in BRCA1/2 patients |
I |
C |
|
Patients who do not achieve pCR after NACT should be treated with eight cycles of adjuvant capecitabine postoperatively |
I |
C |
|
Pembrolizumab for the treatment of patients with high-risk, early-stage TNBC[b] in combination with chemotherapy as neoadjuvant treatment, and then continued pembrolizumab as a single agent as adjuvant treatment after surgery for 1 year. |
I |
B |
|
Patients with a germline BRCA mutation: Olaparib is approved for the adjuvant treatment of adult patients with deleterious or suspected deleterious germline BRCA mutation, HER2-negative, high-risk early breast cancer.[b] |
I |
A |
|
LoE |
GoR |
|
|---|---|---|
|
Treatment of elderly early breast cancer patients should be adapted to biological (not chronological) age, with consideration of less aggressive regimens in frail patients. In patients suitable for standard ChT, a standard multidrug regimen should be used |
II |
B |
|
A geriatric assessment should be carried out before making treatment decisions in all patients more than 65 years |
II |
A |
|
LoE |
GoR |
|
|---|---|---|
|
Bisphosphonates for early breast cancer are recommended in women with low estrogen status (undergoing OFS or postmenopausal), especially if at high risk of relapse (I, A). |
I |
A |
|
Zoledronic acid six monthly for 2 to 5 years is recommended in patients with treatment-related bone loss |
I |
A |
|
Denosumab is not recommended in the adjuvant setting |
II |
B |
|
LoE |
GoR |
|
|---|---|---|
|
ChT and anti-HER2 therapy indications and regimens should follow the same recommendations as those for breast cancer in female patients |
IV |
A |
|
Tamoxifen is the standard adjuvant ET for male breast cancer patients |
IV |
A |
|
If a strong contraindication exists for the use of tamoxifen, a combination of an AI plus a luteinizing hormone-releasing hormone agonist may be considered, but its higher toxicity must be discussed with the patient to avoid compliance issues |
IV |
B |
|
An AI alone should not be used as adjuvant ET in male breast cancer patients |
IV |
E |
References
- Kulothungan V, Sathishkumar K, Leburu S. et al. Burden of cancers in India - estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer 2022; 22 (01) 527
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Dhillon PK, Yeole BB, Dikshit R, Kurkure AP, Bray F. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976-2005: an age-period-cohort analysis. Br J Cancer 2011; 105 (05) 723-730
- Mittal A, Deo SVS, Gogia A. et al. Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a North Indian tertiary care center. Ann Surg Oncol 2022; 29 (02) 1423-1432
- Yanes T, Young MA, Meiser B, James PA. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 2020; 22 (01) 21
- Nagrani R, Mhatre S, Rajaraman P. et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur J Cancer 2016; 66: 153-161
- Lammert J, Grill S, Kiechle M. Modifiable lifestyle factors: opportunities for (hereditary) breast cancer prevention - a narrative review. Breast Care (Basel) 2018; 13 (02) 109-114
- Fortner RT, Sisti J, Chai B. et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses' Health Studies. Breast Cancer Res 2019; 21 (01) 40
- Thangjam S, Laishram RS, Debnath K. Breast carcinoma in young females below the age of 40 years: a histopathological perspective. South Asian J Cancer 2014; 3 (02) 97-100
- Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol 2017; 13 (04) 289-295
- Mittra I, Mishra GA, Dikshit RP. et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ 2021; 372: n256
- Lad S, Bajaj P, Chandra M. et al. Standard reporting formats & basic imaging protocols for breast imaging ICRI sub-specialty group for breast imaging standard reporting formats & basic imaging protocols for mammogram, breast ultrasound & breast MRI. Accessed April 11, 2024 at: https://icri.iria.org.in/about-icri/radiology-imaging-guidelines/breast-imaging-reporting-formats
- Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018; 15 (3 Pt A): 408-414
- Hortobagyi GN, Connolly JL, D'Orsi CJ. et al. Breast. In: Amin MB, Edge S, Greene F. et al, eds. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed.. New York, NY: Springer; 2017: 589-636 . Last updated March 13, 2018. Accessed May 14, 2018 at: https://pubmed.ncbi.nlm.nih.gov/31928404/
- Allison KH, Hammond MEH, Dowsett M. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol 2020; 38 (12) 1346-1366
- Nielsen TO, Leung SCY, Rimm DL. et al. Assessment of Ki67 in breast cancer: updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2021; 113 (07) 808-819
- Coates AS, Winer EP, Goldhirsch A. et al; Panel Members. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26 (08) 1533-1546
- Paik S, Tang G, Shak S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24 (23) 3726-3734
- Sparano J, Gray RJ, Wood WC. et al. TAILORx: Phase III trial of chemo endocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2- negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score [Abstract]. J Clin Oncol 2018 ;36(18_suppl)
- Cardoso F, van't Veer LJ, Bogaerts J. et al; MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016; 375 (08) 717-729
- Sestak I, Buus R, Cuzick J. et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4 (04) 545-553
- Filipits M, Rudas M, Jakesz R. et al; EP Investigators. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011; 17 (18) 6012-6020
- Sgroi DC, Sestak I, Cuzick J. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013; 14 (11) 1067-1076
- Bakre MM, Ramkumar C, Attuluri AK. et al. Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients. Cancer Med 2019; 8 (04) 1755-1764
- Tomlinson-Hansen S, Khan M, Cassaro S. Breast ductal carcinoma in situ. In: StatPearls. StatPearls Publishing; Treasure Island (FL): ; 2023 Accesed January 5, 2024 at: https://europepmc.org/article/nbk/nbk567766
- Morrow M, Van Zee KJ, Solin LJ. et al. Society of Surgical Oncology- American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol 2016; 34 (33) 4040-4046
- Seijen M, Lips EH, Thompson AM. et al. PRECISION Team. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer 2019; 121 (04) 285-292
- Fisher B, Anderson S, Bryant J. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347 (16) 1233-1241
- Moran MS, Schnitt SJ, Giuliano AE. et al; Society of Surgical Oncology, American Society for Radiation Oncology. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014; 32 (14) 1507-1515
- Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014; 72 (02) 145-149
- Rosenkranz KM, Ballman K, McCall L. et al. Cosmetic outcomes following breast-conservation surgery and radiation for multiple ipsilateral breast cancer: data from the Alliance Z11102 Study. Ann Surg Oncol 2020; 27 (12) 4650-4661
- Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg 2014; 260 (06) 1000-1010
- Medina-Franco H, Vasconez LO, Fix RJ. et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg 2002; 235 (06) 814-819
- Duarte C, Bastidas F, de los Reyes A. et al. Randomized controlled clinical trial comparing radioguided occult lesion localization with wire-guided lesion localization to evaluate their efficacy and accuracy in the localization of nonpalpable breast lesions. Surgery 2016; 159 (04) 1140-1145
- Parmar V, Hawaldar R, Nair NS. et al. Sentinel node biopsy versus low axillary sampling in women with clinically node negative operable breast cancer. Breast 2013; 22 (06) 1081-1086
- Parmar V, Nair NS, Vanmali V. et al. Sentinel node biopsy Versus low axillary sampling in predicting nodal status of postchemotherapy axilla in women with breast cancer. JCO Glob Oncol 2020; 6: 1546-1553
- Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev 2017; 1 (01) CD004561
- Giuliano AE, Hunt KK, Ballman KV. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011; 305 (06) 569-575
- Kuehn T, Bauerfeind I, Fehm T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14 (07) 609-618
- Boughey JC, Suman VJ, Mittendorf EA. et al; Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310 (14) 1455-1461
- Boileau JF, Poirier B, Basik M. et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015; 33 (03) 258-264
- Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) 2021; 13 (07) 13
- Vinh-Hung V, Verschraegen C, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 2004; 96 (02) 115-121
- Darby S, McGale P, Correa C. et al; Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378 (9804) 1707-1716
- Bartelink H, Maingon P, Poortmans P. et al; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015; 16 (01) 47-56
- Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009; 90 (01) 1-13
- Vicini FA, Cecchini RS, White JR. et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019; 394 (10215): 2155-2164
- Coles CE, Griffin CL, Kirby AM. et al; IMPORT Trialists. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017; 390 (10099): 1048-1060
- Whelan TJ, Julian JA, Berrang TS. et al; RAPID Trial Investigators. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 2019; 394 (10215): 2165-2172
- Monticciolo DL, Malak SF, Friedewald SM. et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol 2021; 18 (09) 1280-1288
- Ott OJ, Strnad V, Hildebrandt G. et al; Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO). GEC-ESTRO multicenter phase 3-trial: Accelerated partial breast irradiation with interstitial multicatheter brachytherapy versus external beam whole breast irradiation: Early toxicity and patient compliance. Radiother Oncol 2016; 120 (01) 119-123
- Veronesi U, Orecchia R, Maisonneuve P. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013; 14 (13) 1269-1277
- Polgár C, Van Limbergen E, Pötter R. et al; GEC-ESTRO Breast Cancer Working Group. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010; 94 (03) 264-273
- McGale P, Taylor C, Correa C. et al; EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383 (9935) 2127-2135
- Tabár L, Dean PB, Chen TH. et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019; 125 (04) 515-523
- Cosar R, Uzal C, Tokatli F. et al. Postmastectomy irradiation in breast in breast cancer patients with T1-2 and 1-3 positive axillary lymph nodes: is there a role for radiation therapy?. Radiat Oncol 2011; 6 (01) 28
- Zhang J, Wang C. Axillary radiotherapy: an alternative treatment option for adjuvant axillary management of breast cancer. Sci Rep 2016; 6: 26304
- Whelan TJ, Olivotto IA, Parulekar WR. et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015; 373 (04) 307-316
- Poortmans PM, Collette S, Kirkove C. et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015; 373 (04) 317-327
- Haviland JS, Owen JR, Dewar JA. et al; START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013; 14 (11) 1086-1094
- Smith BD, Bellon JR, Blitzblau R. et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018; 8 (03) 145-152
- Murray Brunt A, Haviland JS, Wheatley DA. et al; FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020; 395 (10237): 1613-1626
- Francis PA, Pagani O, Fleming GF. et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018; 379 (02) 122-137
- Regan MM, Fleming GF, Walley B, Francis PA, Pagani O. Adjuvant systemic treatment of premenopausal women with hormone receptor-positive early breast cancer: lights and shadows. J Clin Oncol 2019; 37 (11) 862-866
- Dowsett M, Cuzick J, Ingle J. et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010; 28 (03) 509-518
- Coombes RC, Kilburn LS, Snowdon CF. et al; Intergroup Exemestane Study. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 2007; 369 (9561) 559-570
- Morden JP, Alvarez I, Bertelli G. et al. Long-term follow-up of the Intergroup Exemestane Study. J Clin Oncol 2017; 35 (22) 2507-2514
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386 (10001): 1341-1352
- Johnston SRD, Harbeck N, Hegg R. et al; monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38 (34) 3987-3998
- Gianni L, Pienkowski T, Im YH. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13 (01) 25-32
- van Ramshorst MS, van der Voort A, van Werkhoven ED. et al; Dutch Breast Cancer Research Group (BOOG). Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018; 19 (12) 1630-1640
- Joerger M, Thürlimann B, Huober J. Small HER2-positive, node-negative breast cancer: who should receive systemic adjuvant treatment?. Ann Oncol 2011; 22 (01) 17-23
- Curigliano G, Viale G, Bagnardi V. et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 2009; 27 (34) 5693-5699
- Bahl A, Singh R, Wadhwa J. et al. Practical consensus recommendations regarding the management of HER2 neu positive early breast cancer. South Asian J Cancer 2018; 7 (02) 102-105
- Tolaney SM, Barry WT, Dang CT. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015; 372 (02) 134-141
- Tolaney SM, Tayob N, Dang C. et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J Clin Oncol 2021; 39 (21) 2375-2385
- Manuprasad A, Shenoy PK, Jones J, Vinin NV, Dharmarajan A, Muttath G. Short-course adjuvant trastuzumab in breast cancer: Experience from a tertiary cancer center in rural India. Cancer Res Stat Treat 2020; 3: 69-73
- Gulia S, Kannan S, Badwe R, Gupta S. Evaluation of 1-year vs shorter durations of adjuvant trastuzumab among patients with early breast cancer: an individual participant data and trial-level meta-analysis. JAMA Netw Open 2020; 3 (08) e2011777
- von Minckwitz G, Huang CS, Mano MS. et al; KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380 (07) 617-628
- Loibl S, Jassem J. Sonnenblick, et al. Adjuvant pertuzumab and trastuzumab in patients with HER2 positive breast cancer in APHINITY: 8.4 years follow up. Ann Oncol 2022; 33 (09) 986-987 . Accessed August 1, 2022 at: https://www.annalsofoncology.org/article/S0923-7534(22)01738-0/fulltext
- Citron ML, Berry DA, Cirrincione C. et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21 (08) 1431-1439
- Blum JL, Flynn PJ, Yothers G. et al. Anthracyclines in early breast cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017; 35 (23) 2647-2655
- Pathak N, Sharma A, Elavarasi A. et al. Moment of truth-adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level Meta-analysis. Breast 2022; 64: 7-18
- Gupta S, Nair NS, Hawaldar R. et al. Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: A phase III randomized controlled trial [abstract]. In: Proceedings of the 2022 San Antonio Breast Cancer Symposium; 2022 Dec 6–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2023;83(5 suppl):Abstract nr GS5–01
- Hahnen E, Lederer B, Hauke J. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary Analysis of the GeparSixto randomized clinical trial. JAMA Oncol 2017; 3 (10) 1378-1385
- Masuda N, Lee SJ, Ohtani S. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376 (22) 2147-2159
- Schmid P, Cortes J, Pusztai L. et al; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382 (09) 810-821
- Tutt ANJ, Garber JE, Kaufman B. et al; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 2021; 384 (25) 2394-2405
- Parikh PM, Bhattacharyya GS, Biswas G. et al. Practical consensus recommendations for optimizing risk versus benefit of chemotherapy in patients with HR positive Her2 negative early breast cancer in India. South Asian J Cancer 2021; 10 (04) 213-219
- Banerjee J, Behal P, Satapathy S. et al. Implementing and validating a care protocol for older adults with cancer in resource limited settings with a newly developed screening tool. J Geriatr Oncol 2021; 12 (01) 139-145
- Brufsky A, Bundred N, Coleman R. et al; Z-FAST and ZO-FAST Study Groups. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist 2008; 13 (05) 503-514
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G. et al; Austrian Breast and Colorectal Cancer Study Group. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2007; 25 (07) 820-828
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015; 386 (10001): 1353-1361
- Gnant M, Pfeiler G, Steger GG. et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20 (03) 339-351
- Coleman R, Finkelstein DM, Barrios C. et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2020; 21 (01) 60-72
- Bajpai J, Shylasree T. Pregnancy-associated breast cancer: controversies and consensus!. Oncobiology and Targets 2016; 3: 6
- Langer A, Mohallem M, Berment H. et al. Breast lumps in pregnant women. Diagn Interv Imaging 2015; 96 (10) 1077-1087
- Bajpai J, Simha V, Shylasree TS. et al. Pregnancy associated breast cancer (PABC): Report from a gestational cancer registry from a tertiary cancer care centre, India. Breast 2021; 56: 88-95
- Amant F, Lefrère H, Borges VF. et al. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol 2021; 22 (06) 753-754
- Loibl S, Schmidt A, Gentilini O. et al. Breast cancer diagnosed during pregnancy adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol 2015; 1 (08) 1145-1153
- Paris I, Di Giorgio D, Carbognin L. et al. Pregnancy-associated breast cancer: a multidisciplinary approach. Clin Breast Cancer 2021; 21 (01) e120-e127
- Vashi R, Hooley R, Butler R, Geisel J, Philpotts L. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol 2013; 200 (02) 321-328
- Partridge AH, Niman SM, Ruggeri M. et al; International Breast Cancer Study Group, POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med 2023; 388 (18) 1645-1656
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68 (01) 7-30
- Noone AM, Howlader N, Krapcho M. et al, Eds. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; 2018. . Accessed December 4, 2018 at: https://seer.cancer.gov/csr/1975_2015
- Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat 2009; 115 (02) 429-430
- Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer?. Breast Cancer Res Treat 2004; 83 (01) 77-86
- Sathwara J, Bobdey SBG. Breast cancer survival studies in India: a review. Int J Res Med Sci 2017; 4 (08) 3102-3108 . Accessed 2023 October 29, 2023 at: https://www.msjonline.org/index.php/ijrms/article/view/1084
- Mustian KM, Alfano CM, Heckler C. et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer- related fatigue: a meta-analysis. JAMA Oncol 2017; 3 (07) 961-968
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol 2002; 20 (04) 1128-1143
-
Holmberg L,
Iversen OE,
Rudenstam CM.
et al;
HABITS Study Group.
Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 2008; 100 (07) 475-482
Address for correspondence
Randeep Singh, MD/DMOncomed Clinic, X-10, Basement, Hauz Khas, New Delhi 110016IndiaEmail: randeeptmh@gmail.comPublication History
Article published online:
18 July 2024© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, IndiaWe recommend- Breast cancer: An overview of published Indian dataBharath Rangarajan, South Asian Journal of Cancer, 2016
- Indian Society of Medical and Paediatric Oncology (ISMPO)—Breast Cancer in Young GuidelinesJyoti Bajpai, Indian Journal of Medical and Paediatric Oncology
- Breast cancer risk factor evaluation in a Western Himalayan state: A case–control study and comparison with the Western WorldPurnima Thakur, South Asian Journal of Cancer, 2017
- Reproductive factors and breast cancer risk: A meta-analysis of case–control studies in Indian womenGayatri Vishwakarma, South Asian Journal of Cancer, 2019
- Breast cancer: Indian experience, data, and evidenceSudeep Gupta, South Asian Journal of Cancer, 2016
- Breast cancer : the importance of early diagnosis and treatmentMedical Chronicle, 2018
- Early detection saves lives : oncology - breast cancerJustus Apffelstaedt, The Specialist Forum, 2015
- Assessment of HER2 status in breast cancer : CPD : oncologyThe Specialist Forum, 2016
- Early detection : options and recommendations : CPD : oncology - breast cancerJustus Apffelstaedt, The Specialist Forum, 2016
- When breast is not bestThe Specialist Forum, 2020
- Breast cancer: An overview of published Indian data

| Fig 1 Breast molecular and surrogate subtypes. Er, estrogen receptor; IHC, immunohistochemistry; PR, progesterone receptor.|

| Fig 2 (a) Oncotype DX-based risk stratification. (b) MammaPrint-based risk stratification.|

| Fig 3 Algorithm for adjuvant therapy for HR + EBC > 0.5 cm. ET, endocrine therapy; OFS, ovarian function suppression.|

| Figure 4: (A) Algorithm for the role of radiation therapy in early breast cancer. (b) Algorithm for the role of radiation therapy after neoadjuvant therapy. ALND, axillary lymph node dissection; NACT, neoadjuvant chemotherapy; RT, radiation therapy; SLN, sentinel lymph node; SLNB, sentinel lymph node biopsy; USG, ultrasound.|


| Figure 5: Treatment algorithm for neoadjuvant therapy for HER2-positive EBC. pCR, yr, year.|
References
- Kulothungan V, Sathishkumar K, Leburu S. et al. Burden of cancers in India - estimates of cancer crude incidence, YLLs, YLDs and DALYs for 2021 and 2025 based on National Cancer Registry Program. BMC Cancer 2022; 22 (01) 527
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Dhillon PK, Yeole BB, Dikshit R, Kurkure AP, Bray F. Trends in breast, ovarian and cervical cancer incidence in Mumbai, India over a 30-year period, 1976-2005: an age-period-cohort analysis. Br J Cancer 2011; 105 (05) 723-730
- Mittal A, Deo SVS, Gogia A. et al. Profile of pathogenic mutations and evaluation of germline genetic testing criteria in consecutive breast cancer patients treated at a North Indian tertiary care center. Ann Surg Oncol 2022; 29 (02) 1423-1432
- Yanes T, Young MA, Meiser B, James PA. Clinical applications of polygenic breast cancer risk: a critical review and perspectives of an emerging field. Breast Cancer Res 2020; 22 (01) 21
- Nagrani R, Mhatre S, Rajaraman P. et al. Central obesity increases risk of breast cancer irrespective of menopausal and hormonal receptor status in women of South Asian Ethnicity. Eur J Cancer 2016; 66: 153-161
- Lammert J, Grill S, Kiechle M. Modifiable lifestyle factors: opportunities for (hereditary) breast cancer prevention - a narrative review. Breast Care (Basel) 2018; 13 (02) 109-114
- Fortner RT, Sisti J, Chai B. et al. Parity, breastfeeding, and breast cancer risk by hormone receptor status and molecular phenotype: results from the Nurses' Health Studies. Breast Cancer Res 2019; 21 (01) 40
- Thangjam S, Laishram RS, Debnath K. Breast carcinoma in young females below the age of 40 years: a histopathological perspective. South Asian J Cancer 2014; 3 (02) 97-100
- Malvia S, Bagadi SA, Dubey US, Saxena S. Epidemiology of breast cancer in Indian women. Asia Pac J Clin Oncol 2017; 13 (04) 289-295
- Mittra I, Mishra GA, Dikshit RP. et al. Effect of screening by clinical breast examination on breast cancer incidence and mortality after 20 years: prospective, cluster randomised controlled trial in Mumbai. BMJ 2021; 372: n256
- Lad S, Bajaj P, Chandra M. et al. Standard reporting formats & basic imaging protocols for breast imaging ICRI sub-specialty group for breast imaging standard reporting formats & basic imaging protocols for mammogram, breast ultrasound & breast MRI. Accessed April 11, 2024 at: https://icri.iria.org.in/about-icri/radiology-imaging-guidelines/breast-imaging-reporting-formats
- Monticciolo DL, Newell MS, Moy L, Niell B, Monsees B, Sickles EA. Breast cancer screening in women at higher-than-average risk: recommendations from the ACR. J Am Coll Radiol 2018; 15 (3 Pt A): 408-414
- Hortobagyi GN, Connolly JL, D'Orsi CJ. et al. Breast. In: Amin MB, Edge S, Greene F. et al, eds. American Joint Committee on Cancer. AJCC cancer staging manual. 8th ed.. New York, NY: Springer; 2017: 589-636 . Last updated March 13, 2018. Accessed May 14, 2018 at: https://pubmed.ncbi.nlm.nih.gov/31928404/
- Allison KH, Hammond MEH, Dowsett M. et al. Estrogen and progesterone receptor testing in breast cancer: ASCO/CAP Guideline Update. J Clin Oncol 2020; 38 (12) 1346-1366
- Nielsen TO, Leung SCY, Rimm DL. et al. Assessment of Ki67 in breast cancer: updated recommendations from the International Ki67 in Breast Cancer Working Group. J Natl Cancer Inst 2021; 113 (07) 808-819
- Coates AS, Winer EP, Goldhirsch A. et al; Panel Members. Tailoring therapies–improving the management of early breast cancer: St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2015. Ann Oncol 2015; 26 (08) 1533-1546
- Paik S, Tang G, Shak S. et al. Gene expression and benefit of chemotherapy in women with node-negative, estrogen receptor-positive breast cancer. J Clin Oncol 2006; 24 (23) 3726-3734
- Sparano J, Gray RJ, Wood WC. et al. TAILORx: Phase III trial of chemo endocrine therapy versus endocrine therapy alone in hormone receptor-positive, HER2- negative, node-negative breast cancer and an intermediate prognosis 21-gene recurrence score [Abstract]. J Clin Oncol 2018 ;36(18_suppl)
- Cardoso F, van't Veer LJ, Bogaerts J. et al; MINDACT Investigators. 70-gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016; 375 (08) 717-729
- Sestak I, Buus R, Cuzick J. et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor–positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4 (04) 545-553
- Filipits M, Rudas M, Jakesz R. et al; EP Investigators. A new molecular predictor of distant recurrence in ER-positive, HER2-negative breast cancer adds independent information to conventional clinical risk factors. Clin Cancer Res 2011; 17 (18) 6012-6020
- Sgroi DC, Sestak I, Cuzick J. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013; 14 (11) 1067-1076
- Bakre MM, Ramkumar C, Attuluri AK. et al. Clinical validation of an immunohistochemistry-based CanAssist-Breast test for distant recurrence prediction in hormone receptor-positive breast cancer patients. Cancer Med 2019; 8 (04) 1755-1764
- Tomlinson-Hansen S, Khan M, Cassaro S. Breast ductal carcinoma in situ. In: StatPearls. StatPearls Publishing; Treasure Island (FL): ; 2023 Accesed January 5, 2024 at: https://europepmc.org/article/nbk/nbk567766
- Morrow M, Van Zee KJ, Solin LJ. et al. Society of Surgical Oncology- American Society for Radiation Oncology-American Society of Clinical Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in ductal carcinoma in situ. J Clin Oncol 2016; 34 (33) 4040-4046
- Seijen M, Lips EH, Thompson AM. et al. PRECISION Team. Ductal carcinoma in situ: to treat or not to treat, that is the question. Br J Cancer 2019; 121 (04) 285-292
- Fisher B, Anderson S, Bryant J. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med 2002; 347 (16) 1233-1241
- Moran MS, Schnitt SJ, Giuliano AE. et al; Society of Surgical Oncology, American Society for Radiation Oncology. Society of Surgical Oncology-American Society for Radiation Oncology consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I and II invasive breast cancer. J Clin Oncol 2014; 32 (14) 1507-1515
- Losken A, Dugal CS, Styblo TM, Carlson GW. A meta-analysis comparing breast conservation therapy alone to the oncoplastic technique. Ann Plast Surg 2014; 72 (02) 145-149
- Rosenkranz KM, Ballman K, McCall L. et al. Cosmetic outcomes following breast-conservation surgery and radiation for multiple ipsilateral breast cancer: data from the Alliance Z11102 Study. Ann Surg Oncol 2020; 27 (12) 4650-4661
- Fayanju OM, Stoll CR, Fowler S, Colditz GA, Margenthaler JA. Contralateral prophylactic mastectomy after unilateral breast cancer: a systematic review and meta-analysis. Ann Surg 2014; 260 (06) 1000-1010
- Medina-Franco H, Vasconez LO, Fix RJ. et al. Factors associated with local recurrence after skin-sparing mastectomy and immediate breast reconstruction for invasive breast cancer. Ann Surg 2002; 235 (06) 814-819
- Duarte C, Bastidas F, de los Reyes A. et al. Randomized controlled clinical trial comparing radioguided occult lesion localization with wire-guided lesion localization to evaluate their efficacy and accuracy in the localization of nonpalpable breast lesions. Surgery 2016; 159 (04) 1140-1145
- Parmar V, Hawaldar R, Nair NS. et al. Sentinel node biopsy versus low axillary sampling in women with clinically node negative operable breast cancer. Breast 2013; 22 (06) 1081-1086
- Parmar V, Nair NS, Vanmali V. et al. Sentinel node biopsy Versus low axillary sampling in predicting nodal status of postchemotherapy axilla in women with breast cancer. JCO Glob Oncol 2020; 6: 1546-1553
- Bromham N, Schmidt-Hansen M, Astin M, Hasler E, Reed MW. Axillary treatment for operable primary breast cancer. Cochrane Database Syst Rev 2017; 1 (01) CD004561
- Giuliano AE, Hunt KK, Ballman KV. et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA 2011; 305 (06) 569-575
- Kuehn T, Bauerfeind I, Fehm T. et al. Sentinel-lymph-node biopsy in patients with breast cancer before and after neoadjuvant chemotherapy (SENTINA): a prospective, multicentre cohort study. Lancet Oncol 2013; 14 (07) 609-618
- Boughey JC, Suman VJ, Mittendorf EA. et al; Alliance for Clinical Trials in Oncology. Sentinel lymph node surgery after neoadjuvant chemotherapy in patients with node-positive breast cancer: the ACOSOG Z1071 (Alliance) clinical trial. JAMA 2013; 310 (14) 1455-1461
- Boileau JF, Poirier B, Basik M. et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015; 33 (03) 258-264
- Swarnkar PK, Tayeh S, Michell MJ, Mokbel K. The evolving role of marked lymph node biopsy (MLNB) and targeted axillary dissection (TAD) after neoadjuvant chemotherapy (NACT) for node-positive breast cancer: systematic review and pooled analysis. Cancers (Basel) 2021; 13 (07) 13
- Vinh-Hung V, Verschraegen C, Verschraegen C. Breast-conserving surgery with or without radiotherapy: pooled-analysis for risks of ipsilateral breast tumor recurrence and mortality. J Natl Cancer Inst 2004; 96 (02) 115-121
- Darby S, McGale P, Correa C. et al; Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials. Lancet 2011; 378 (9804) 1707-1716
- Bartelink H, Maingon P, Poortmans P. et al; European Organisation for Research and Treatment of Cancer Radiation Oncology and Breast Cancer Groups. Whole-breast irradiation with or without a boost for patients treated with breast-conserving surgery for early breast cancer: 20-year follow-up of a randomised phase 3 trial. Lancet Oncol 2015; 16 (01) 47-56
- Offersen BV, Overgaard M, Kroman N, Overgaard J. Accelerated partial breast irradiation as part of breast conserving therapy of early breast carcinoma: a systematic review. Radiother Oncol 2009; 90 (01) 1-13
- Vicini FA, Cecchini RS, White JR. et al. Long-term primary results of accelerated partial breast irradiation after breast-conserving surgery for early-stage breast cancer: a randomised, phase 3, equivalence trial. Lancet 2019; 394 (10215): 2155-2164
- Coles CE, Griffin CL, Kirby AM. et al; IMPORT Trialists. Partial-breast radiotherapy after breast conservation surgery for patients with early breast cancer (UK IMPORT LOW trial): 5-year results from a multicentre, randomised, controlled, phase 3, non-inferiority trial. Lancet 2017; 390 (10099): 1048-1060
- Whelan TJ, Julian JA, Berrang TS. et al; RAPID Trial Investigators. External beam accelerated partial breast irradiation versus whole breast irradiation after breast conserving surgery in women with ductal carcinoma in situ and node-negative breast cancer (RAPID): a randomised controlled trial. Lancet 2019; 394 (10215): 2165-2172
- Monticciolo DL, Malak SF, Friedewald SM. et al. Breast cancer screening recommendations inclusive of all women at average risk: update from the ACR and Society of Breast Imaging. J Am Coll Radiol 2021; 18 (09) 1280-1288
- Ott OJ, Strnad V, Hildebrandt G. et al; Groupe Européen de Curiethérapie of European Society for Radiotherapy and Oncology (GEC-ESTRO). GEC-ESTRO multicenter phase 3-trial: Accelerated partial breast irradiation with interstitial multicatheter brachytherapy versus external beam whole breast irradiation: Early toxicity and patient compliance. Radiother Oncol 2016; 120 (01) 119-123
- Veronesi U, Orecchia R, Maisonneuve P. et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): a randomised controlled equivalence trial. Lancet Oncol 2013; 14 (13) 1269-1277
- Polgár C, Van Limbergen E, Pötter R. et al; GEC-ESTRO Breast Cancer Working Group. Patient selection for accelerated partial-breast irradiation (APBI) after breast-conserving surgery: recommendations of the Groupe Européen de Curiethérapie-European Society for Therapeutic Radiology and Oncology (GEC-ESTRO) breast cancer working group based on clinical evidence (2009). Radiother Oncol 2010; 94 (03) 264-273
- McGale P, Taylor C, Correa C. et al; EBCTCG (Early Breast Cancer Trialists' Collaborative Group). Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials. Lancet 2014; 383 (9935) 2127-2135
- Tabár L, Dean PB, Chen TH. et al. The incidence of fatal breast cancer measures the increased effectiveness of therapy in women participating in mammography screening. Cancer 2019; 125 (04) 515-523
- Cosar R, Uzal C, Tokatli F. et al. Postmastectomy irradiation in breast in breast cancer patients with T1-2 and 1-3 positive axillary lymph nodes: is there a role for radiation therapy?. Radiat Oncol 2011; 6 (01) 28
- Zhang J, Wang C. Axillary radiotherapy: an alternative treatment option for adjuvant axillary management of breast cancer. Sci Rep 2016; 6: 26304
- Whelan TJ, Olivotto IA, Parulekar WR. et al; MA.20 Study Investigators. Regional nodal irradiation in early-stage breast cancer. N Engl J Med 2015; 373 (04) 307-316
- Poortmans PM, Collette S, Kirkove C. et al; EORTC Radiation Oncology and Breast Cancer Groups. Internal mammary and medial supraclavicular irradiation in breast cancer. N Engl J Med 2015; 373 (04) 317-327
- Haviland JS, Owen JR, Dewar JA. et al; START Trialists' Group. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol 2013; 14 (11) 1086-1094
- Smith BD, Bellon JR, Blitzblau R. et al. Radiation therapy for the whole breast: executive summary of an American Society for Radiation Oncology (ASTRO) evidence-based guideline. Pract Radiat Oncol 2018; 8 (03) 145-152
- Murray Brunt A, Haviland JS, Wheatley DA. et al; FAST-Forward Trial Management Group. Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial. Lancet 2020; 395 (10237): 1613-1626
- Francis PA, Pagani O, Fleming GF. et al; SOFT and TEXT Investigators and the International Breast Cancer Study Group. Tailoring adjuvant endocrine therapy for premenopausal breast cancer. N Engl J Med 2018; 379 (02) 122-137
- Regan MM, Fleming GF, Walley B, Francis PA, Pagani O. Adjuvant systemic treatment of premenopausal women with hormone receptor-positive early breast cancer: lights and shadows. J Clin Oncol 2019; 37 (11) 862-866
- Dowsett M, Cuzick J, Ingle J. et al. Meta-analysis of breast cancer outcomes in adjuvant trials of aromatase inhibitors versus tamoxifen. J Clin Oncol 2010; 28 (03) 509-518
- Coombes RC, Kilburn LS, Snowdon CF. et al; Intergroup Exemestane Study. Survival and safety of exemestane versus tamoxifen after 2-3 years' tamoxifen treatment (Intergroup Exemestane Study): a randomised controlled trial. Lancet 2007; 369 (9561) 559-570
- Morden JP, Alvarez I, Bertelli G. et al. Long-term follow-up of the Intergroup Exemestane Study. J Clin Oncol 2017; 35 (22) 2507-2514
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Aromatase inhibitors versus tamoxifen in early breast cancer: patient-level meta-analysis of the randomised trials. Lancet 2015; 386 (10001): 1341-1352
- Johnston SRD, Harbeck N, Hegg R. et al; monarchE Committee Members and Investigators. Abemaciclib combined with endocrine therapy for the adjuvant treatment of HR+, HER2-, node-positive, high-risk, early breast cancer (monarchE). J Clin Oncol 2020; 38 (34) 3987-3998
- Gianni L, Pienkowski T, Im YH. et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol 2012; 13 (01) 25-32
- van Ramshorst MS, van der Voort A, van Werkhoven ED. et al; Dutch Breast Cancer Research Group (BOOG). Neoadjuvant chemotherapy with or without anthracyclines in the presence of dual HER2 blockade for HER2-positive breast cancer (TRAIN-2): a multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2018; 19 (12) 1630-1640
- Joerger M, Thürlimann B, Huober J. Small HER2-positive, node-negative breast cancer: who should receive systemic adjuvant treatment?. Ann Oncol 2011; 22 (01) 17-23
- Curigliano G, Viale G, Bagnardi V. et al. Clinical relevance of HER2 overexpression/amplification in patients with small tumor size and node-negative breast cancer. J Clin Oncol 2009; 27 (34) 5693-5699
- Bahl A, Singh R, Wadhwa J. et al. Practical consensus recommendations regarding the management of HER2 neu positive early breast cancer. South Asian J Cancer 2018; 7 (02) 102-105
- Tolaney SM, Barry WT, Dang CT. et al. Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 2015; 372 (02) 134-141
- Tolaney SM, Tayob N, Dang C. et al. Adjuvant trastuzumab emtansine versus paclitaxel in combination with trastuzumab for stage I HER2-positive breast cancer (ATEMPT): a randomized clinical trial. J Clin Oncol 2021; 39 (21) 2375-2385
- Manuprasad A, Shenoy PK, Jones J, Vinin NV, Dharmarajan A, Muttath G. Short-course adjuvant trastuzumab in breast cancer: Experience from a tertiary cancer center in rural India. Cancer Res Stat Treat 2020; 3: 69-73
- Gulia S, Kannan S, Badwe R, Gupta S. Evaluation of 1-year vs shorter durations of adjuvant trastuzumab among patients with early breast cancer: an individual participant data and trial-level meta-analysis. JAMA Netw Open 2020; 3 (08) e2011777
- von Minckwitz G, Huang CS, Mano MS. et al; KATHERINE Investigators. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med 2019; 380 (07) 617-628
- Loibl S, Jassem J. Sonnenblick, et al. Adjuvant pertuzumab and trastuzumab in patients with HER2 positive breast cancer in APHINITY: 8.4 years follow up. Ann Oncol 2022; 33 (09) 986-987 . Accessed August 1, 2022 at: https://www.annalsofoncology.org/article/S0923-7534(22)01738-0/fulltext
- Citron ML, Berry DA, Cirrincione C. et al. Randomized trial of dose-dense versus conventionally scheduled and sequential versus concurrent combination chemotherapy as postoperative adjuvant treatment of node-positive primary breast cancer: first report of Intergroup Trial C9741/Cancer and Leukemia Group B Trial 9741. J Clin Oncol 2003; 21 (08) 1431-1439
- Blum JL, Flynn PJ, Yothers G. et al. Anthracyclines in early breast cancer: The ABC Trials-USOR 06-090, NSABP B-46-I/USOR 07132, and NSABP B-49 (NRG Oncology). J Clin Oncol 2017; 35 (23) 2647-2655
- Pathak N, Sharma A, Elavarasi A. et al. Moment of truth-adding carboplatin to neoadjuvant/adjuvant chemotherapy in triple negative breast cancer improves overall survival: An individual participant data and trial-level Meta-analysis. Breast 2022; 64: 7-18
- Gupta S, Nair NS, Hawaldar R. et al. Addition of platinum to sequential taxane-anthracycline neoadjuvant chemotherapy in patients with triple-negative breast cancer: A phase III randomized controlled trial [abstract]. In: Proceedings of the 2022 San Antonio Breast Cancer Symposium; 2022 Dec 6–10; San Antonio, TX. Philadelphia (PA): AACR; Cancer Res 2023;83(5 suppl):Abstract nr GS5–01
- Hahnen E, Lederer B, Hauke J. et al. Germline mutation status, pathological complete response, and disease-free survival in triple-negative breast cancer: Secondary Analysis of the GeparSixto randomized clinical trial. JAMA Oncol 2017; 3 (10) 1378-1385
- Masuda N, Lee SJ, Ohtani S. et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N Engl J Med 2017; 376 (22) 2147-2159
- Schmid P, Cortes J, Pusztai L. et al; KEYNOTE-522 Investigators. Pembrolizumab for early triple-negative breast cancer. N Engl J Med 2020; 382 (09) 810-821
- Tutt ANJ, Garber JE, Kaufman B. et al; OlympiA Clinical Trial Steering Committee and Investigators. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. N Engl J Med 2021; 384 (25) 2394-2405
- Parikh PM, Bhattacharyya GS, Biswas G. et al. Practical consensus recommendations for optimizing risk versus benefit of chemotherapy in patients with HR positive Her2 negative early breast cancer in India. South Asian J Cancer 2021; 10 (04) 213-219
- Banerjee J, Behal P, Satapathy S. et al. Implementing and validating a care protocol for older adults with cancer in resource limited settings with a newly developed screening tool. J Geriatr Oncol 2021; 12 (01) 139-145
- Brufsky A, Bundred N, Coleman R. et al; Z-FAST and ZO-FAST Study Groups. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist 2008; 13 (05) 503-514
- Gnant MF, Mlineritsch B, Luschin-Ebengreuth G. et al; Austrian Breast and Colorectal Cancer Study Group. Zoledronic acid prevents cancer treatment-induced bone loss in premenopausal women receiving adjuvant endocrine therapy for hormone-responsive breast cancer: a report from the Austrian Breast and Colorectal Cancer Study Group. J Clin Oncol 2007; 25 (07) 820-828
- Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Adjuvant bisphosphonate treatment in early breast cancer: meta-analyses of individual patient data from randomised trials. Lancet 2015; 386 (10001): 1353-1361
- Gnant M, Pfeiler G, Steger GG. et al; Austrian Breast and Colorectal Cancer Study Group. Adjuvant denosumab in postmenopausal patients with hormone receptor-positive breast cancer (ABCSG-18): disease-free survival results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20 (03) 339-351
- Coleman R, Finkelstein DM, Barrios C. et al. Adjuvant denosumab in early breast cancer (D-CARE): an international, multicentre, randomised, controlled, phase 3 trial. Lancet Oncol 2020; 21 (01) 60-72
- Bajpai J, Shylasree T. Pregnancy-associated breast cancer: controversies and consensus!. Oncobiology and Targets 2016; 3: 6
- Langer A, Mohallem M, Berment H. et al. Breast lumps in pregnant women. Diagn Interv Imaging 2015; 96 (10) 1077-1087
- Bajpai J, Simha V, Shylasree TS. et al. Pregnancy associated breast cancer (PABC): Report from a gestational cancer registry from a tertiary cancer care centre, India. Breast 2021; 56: 88-95
- Amant F, Lefrère H, Borges VF. et al. The definition of pregnancy-associated breast cancer is outdated and should no longer be used. Lancet Oncol 2021; 22 (06) 753-754
- Loibl S, Schmidt A, Gentilini O. et al. Breast cancer diagnosed during pregnancy adapting recent advances in breast cancer care for pregnant patients. JAMA Oncol 2015; 1 (08) 1145-1153
- Paris I, Di Giorgio D, Carbognin L. et al. Pregnancy-associated breast cancer: a multidisciplinary approach. Clin Breast Cancer 2021; 21 (01) e120-e127
- Vashi R, Hooley R, Butler R, Geisel J, Philpotts L. Breast imaging of the pregnant and lactating patient: imaging modalities and pregnancy-associated breast cancer. AJR Am J Roentgenol 2013; 200 (02) 321-328
- Partridge AH, Niman SM, Ruggeri M. et al; International Breast Cancer Study Group, POSITIVE Trial Collaborators. Interrupting endocrine therapy to attempt pregnancy after breast cancer. N Engl J Med 2023; 388 (18) 1645-1656
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68 (01) 7-30
- Noone AM, Howlader N, Krapcho M. et al, Eds. SEER Cancer Statistics Review, 1975–2015. National Cancer Institute; 2018. . Accessed December 4, 2018 at: https://seer.cancer.gov/csr/1975_2015
- Speirs V, Shaaban AM. The rising incidence of male breast cancer. Breast Cancer Res Treat 2009; 115 (02) 429-430
- Anderson WF, Althuis MD, Brinton LA, Devesa SS. Is male breast cancer similar or different than female breast cancer?. Breast Cancer Res Treat 2004; 83 (01) 77-86
- Sathwara J, Bobdey SBG. Breast cancer survival studies in India: a review. Int J Res Med Sci 2017; 4 (08) 3102-3108 . Accessed 2023 October 29, 2023 at: https://www.msjonline.org/index.php/ijrms/article/view/1084
- Mustian KM, Alfano CM, Heckler C. et al. Comparison of pharmaceutical, psychological, and exercise treatments for cancer- related fatigue: a meta-analysis. JAMA Oncol 2017; 3 (07) 961-968
- Chlebowski RT, Aiello E, McTiernan A. Weight loss in breast cancer patient management. J Clin Oncol 2002; 20 (04) 1128-1143
- Holmberg L, Iversen OE, Rudenstam CM. et al; HABITS Study Group. Increased risk of recurrence after hormone replacement therapy in breast cancer survivors. J Natl Cancer Inst 2008; 100 (07) 475-482


 PDF
PDF  Views
Views  Share
Share

