Indian Council of Medical Research consensus document for the management of tongue cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2015; 36(03): 140-145
DOI: DOI: 10.4103/0971-5851.166712
E X E C U T I V E S U M M A R Y
The document is based on consensus among the experts and best available evidence pertaining to Indian population and is meant for practice in India. Early diagnosis is imperative in improving outcomes and preserving quality of life. High index of suspicion is to be maintained for leukoplakia (high risk site). Evaluation of a patient with newly diagnosed tongue cancer should include essential tests: Magnetic resonance imaging (MRI) is investigative modality of choice when indicated. Computed tomography (CT) scan is an option when MRI is unavailable. In early lesions when imaging is not warranted ultrasound may help guide management of the neck. Early stage cancers (stage I & II) require single modality treatment - either surgery or radiotherapy. Surgery is preferred. Adjuvant radiotherapy is indicated for T3/T4 cancers, presence of high risk features [lymphovascular emboli (LVE), perineural invasion (PNI), poorly differentiated, node +,close margins). Adjuvant chemoradiation (CTRT) is indicated for positive margins and extranodal disease. Locally advanced operable cancers (stage III & IVA) require combined multimodality treatment - surgery + adjuvant treatment. Adjuvant treatment is indicated in all and in the presence of high risk features as described above. Locally advanced inoperable cancers (stage IVB) are treated with palliative chemo-radiotherapy, chemotherapy, radiotherapy, or symptomatic treatment depending upon the performance status. Select cases may be considered for neoadjuvant chemotherapy followed by surgical salvage. Metastatic disease (stage IVC) should be treated with a goal for palliation. Chemotherapy may be offered to patients with good performance status. Local treatment in the form of radiotherapy may be added for palliation of symptoms. Intense follow-up every 3 months is required for initial 2 years as most recurrences occur in the first 24 months. After 2 nd year follow up is done at 4-6 months interval. At each follow up screening for local/ regional recurrence and second primary is done. Imaging is done only when indicated.
Publication History
Article published online:
12 July 2021
© 2015. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
EXECUTIVE SUMMARY
- The document is based on consensus among the experts and best available evidence pertaining to Indian population and is meant for practice in India.
- Early diagnosis is imperative in improving outcomes and preserving quality of life. High index of suspicion is to be maintained for leukoplakia (high risk site).
- Evaluation of a patient with newly diagnosed tongue cancer should include essential tests: Magnetic resonance imaging (MRI) is investigative modality of choice when indicated. Computed tomography (CT) scan is an option when MRI is unavailable. In early lesions when imaging is not warranted ultrasound may help guide management of the neck.
- Early stage cancers (stage I & II) require single modality treatment – either surgery or radiotherapy. Surgery is preferred. Adjuvant radiotherapy is indicated for T3/T4 cancers, presence of high risk features [lymphovascular emboli (LVE), perineural invasion (PNI), poorly differentiated, node +, close margins). Adjuvant chemoradiation (CTRT) is indicated for positive margins and extranodal disease.
- Locally advanced operable cancers (stage III & IVA) require combined multimodality treatment - surgery + adjuvant treatment. Adjuvant treatment is indicated in all and in the presence of high risk features as described above.
- Locally advanced inoperable cancers (stage IVB) are treated with palliative chemo-radiotherapy, chemotherapy, radiotherapy, or symptomatic treatment depending upon the performance status. Select cases may be considered for neoadjuvant chemotherapy followed by surgical salvage.
- Metastatic disease (stage IVC) should be treated with a goal for palliation. Chemotherapy may be offered to patients with good performance status. Local treatment in the form of radiotherapy may be added for palliation of symptoms.
- Intense follow-up every 3 months is required for initial 2 years as most recurrences occur in the first 24 months. After 2nd year follow up is done at 4-6 months interval. At each follow up screening for local/regional recurrence and second primary is done. Imaging is done only when indicated.
INCIDENCE
The incidence of tongue cancer is rising in the country. As per Indian Council of Medical Research population based cancer registries (PBCRs), males in Ahmedabad Urban had the highest age adjusted rates (AAR) (12.2) among all the PBCRs as compared to mouth cancers, which is 17.1. Kamrup Urban district showed highest AAR (9.4) among the North East registries. Among females, the north eastern registry areas of Kamrup Urban district and East Khasi Hills of Meghalaya shared the top place with Ahmedabad urban for the highest AAR of 3.2 per 100,000 among all the PBCRs. Males in Indian PBCRs had the highest AARs (given in parentheses) in cancers of the tongue in males among all the Indian and international PBCRs (Ahmedabad Urban [12.2], Kamrup Urban district [9.4], Ahmedabad Rural [9.3], Bhopal [9.0] and Delhi [8.0]). The top five positions were occupied by five Indian PBCRs. Amongst females, South Karachi in Pakistan had highest AAR (6.6). Three Indian PBCRs, East Khasi Hills of Meghalaya State, Ahmedabad Urban and Kamrup Urban district followed next with an AAR of 3.2.[1]
PURPOSE
Though International Consensus Guidelines are available for the management of tongue cancer, it is not entirely feasible to apply these guidelines to the Indian population owing to differences in incidence of the disease in different parts of India, socioeconomic factors, and availability of Resources.[2,3,4,5]
While the broad principles of management are the same, there are finer nuances in the management of individual cancers that comprise the oral cavity. It is an endeavor in these guidelines to highlight these differences to help the treating clinician to manage cancer of oral tongue.
There is a paucity of randomized controlled trials addressing tongue cancers specifically. This endeavor has attempted to combine the best available evidence put together by the experts on the task force. It is our belief that this information is the collation of the best available evidence. However, these guidelines may be modified when required in the best interest of patient care given the paucity of level I evidence in certain situations. Taking into consideration peripheral oncology centers, regional cancer centers and tertiary cancer centers in major cities, the set of recommendations includes two categories, viz.
Desirable/ideal
Tests and treatments that may not be available at all centers but the centers should aspire to have them in the near future.
Essential
Bare minimum that should be offered to all the patients by all the centers treating cancer patients.
DIAGNOSIS
Essential features in the history to be noted are duration, pain (may be referred), difficulty in swallowing movement of the tongue, alteration in speech, dental history along with details of addictions. Assessment of comorbidities, prior treatment and family history should be recorded.
Examination details should include size and location. Other important features are the posterior extent to base of tongue/vallecula, tonsil involvement, lateral/deep extent and relationship to midline, extrinsic musculature and root of tongue and mandible. Ankyloglossia and hypoglossal nerve palsy indicate advanced disease and are signs of relative inoperability. Evaluation under anesthesia is a useful adjunct when clinical examination is difficult in view of pain/trismus/prior treatment. Examination of upper aero-digestive tract is done to rule out synchronous primary. Neck to be properly evaluated as tongue cancers have a high propensity to neck metastasis, skip metastasis and contralateral metastasis.
Punch biopsy easily establishes diagnosis and should avoid obviously necrotic areas (Essential). Incisional biopsy is recommended for submucosal/infiltrative and verrucous lesions.
For T1/early T2 lesions, imaging is usually not advocated for primary particularly for lesions that are well palpated in its entirety. However the neck may be investigated for nodal involvement due to high incidence of occult metastases (27-40%).[6] USG is cost-effective and widely available, but it should be noted that both USG and USG guided fine-needle aspiration cytology have lower accuracy in the N0 neck as compared to N+ neck. For large T2/T3/T4 tumors, MRI is preferred due to its superior soft tissue delineation and information on deep extent (posterior and inferior) of the primary lesion and could give information on the status of neck nodes (Desirable).
Positron emission tomography-computed tomography is not indicated in the initial workup for tongue cancers.[7,8] It may be indicated for evaluating distant metastases in stage III and IV tongue cancers particularly with large nodes in the lower neck.[2]
Staging for tongue cancer is similar to that of oral cavity cancer (American Joint Committee on Cancer updated 7th edition 2010)[9] [Figure 1].
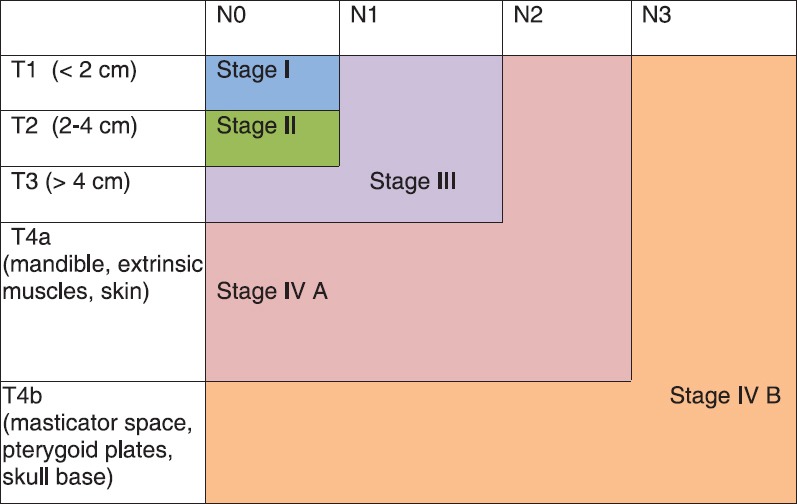
| Fig. 1 Stage grouping: Stage IV A – Moderately advanced cancers, Stage IV B – Very advanced cancers
TREATMENT PLAN
Treatment of each patient should ideally be undertakenby a multidisciplinary team. The intent of treatment is “curative” for patients with stage I-IVA and “palliative” for patients with stage IVB/C disease. Treatment decisions are based on the clinico-radiological staging of the tumor. Occasionally a stage IVB tumor may respond to initial treatment and subsequently be amenable for treatment with curative intent. However, this occurs in a small percentage of properly selected patients.
EARLY STAGE (STAGE I AND II)
Early stage disease is treated with single modality therapy and has similar control rates with either surgery or radiotherapy. Surgery is preferred because of its simplicity, low cost, minimal alteration in function or cosmesis and the fact that it can be repeated (Essential).
Primary
Aim at surgery is to achieve wide local resection of the tumor with adequate margins in all dimensions (Essential). Assessment of depth of tumor by digital palpation and imaging is an essential prerequisite for obtaining appropriate deep margin. It is preferable to have a 1 cm clear margin around the tumor in all dimensions at surgery as margins shrink 20-30% after resection. Any margin < 5 mm is compromised and warrants adjuvant radiotherapy and adds an extra treatment modality, which could have been avoided with adequate surgery.
Early lesions are approached per orally. Mandibulotomy is required occasionally in the presence of trismus or posteriorly located lesions.
Neck
In clinically node negative early lesions, neck can either be observed or treated electively. Elective treatment is recommended for tumors with a higher propensity to metastasis (T2, tumor thickness >4 mm, adverse histological features - high grade, LVE, PNI) or when follow-up is unreliable. The same criteria are applied for elective neck irradiation should the primary be treated by radiotherapy.
The extent of neck dissection for node negative patients is selective neck dissection with removal of levels I-III (up to omohyoid muscle). As tongue cancers are known to harbor skip metastasis as high as 15.8%,[10] some surgeons recommend clearing level IV as well (extended supraomohyoid neck dissection). The risk for level V metastasis is usually < 5 mm is compromised and warrants adjuvant radiotherapy and adds an extra treatment modality, which could have been avoided with adequate surgery.
Another debatable issue is the dissection of level IIB in node negative tongue cancers. Dissection of this area is associated with increased risk of nerve dysfunction. Incidence of occult metastasis in IIB area in N0 neck is very low (2-6%). Our group, as well as others, have shown the importance of level IIA in deciding the extent of surgery in the neck.[11] Level IIA is an area bounded superiorly by skull base, inferiorly by hyoid bone, anteriorly by stylohyoid muscle and posteriorly by a vertical plane defined by spinal accessory nerve (SAN). Presence of IIA involvement increases the risk of IIB, IV and V metastasis, which then need to be addressed.
Reconstruction
Defect following excision of early tongue cancers is usually closed primarily. Leaving a raw wound is usually not advisable due to the risk of infection, pain, and secondary hemorrhage. Use of split thickness skin graft has been described, but not used due to poor take. They are occasionally used for dorsal tongue defects. The working dictum is a defect greater than 30% of mobile tongue is reconstructed by tissue replacement, the free flap being the procedure of choice (free radial artery forearm flap [FRAFF], lateral arm etc.). In situations where free flap facility is not available, submental island flap, nasolabial flap, pectoralis major myofacial flap (PMMF) can be used.
Radiotherpy
Brachytherapy can be given in selected early, accessible superficial lesions preferably less than 2-3 cm, situated well away from the bone with node negative status. It may be delivered using low dose rate (LDR) or high dose rate (HDR) systems. LDR brachytherapy is given in the doses of 65-70 Gy/6-7 days and HDR brachytherapy is given in doses of 48 Gy/12 Fr 4 Gy 1 BD × 6 days. Generally dose prescription encompasses the primary with 1-1.5 cm margins.
Patients not suitable for brachytherapy alone (i.e., large or bulky primary disease) may be treated with external beam radiotherapy (EBRT), followed by brachytherapy boost. EBRT is delivered using conventional planning/three-dimensional conformal radiotherapy/intensity modulated radiotherapy to primary and neck. Dose of EBRT is restricted to 45-50 Gy and brachytherapy boost (dose of 20-25 Gy [LDR] or equivalent HDR) is also given.
LOCALLY ADVANCED STAGE (STAGE III-IVA)
Locally advanced operable cancers are treated with combined modality therapy, surgery followed by postoperative radiotherapy or chemo-radiation.
Primary
Principles of surgical resection are same as that for early stage tumors. Adequate margins to be achieved in mucosal as well as in soft tissue and bony margins. Adequate surgical access is key to obtaining an en-bloc resection with clear margins. Access may be per oral, mandibulotomy (paramedian with preservation of the mental nerve) or pull through technique-combined neck and intraoral approach [Table 1].
Table 1
Various approaches for excision of primary

Marginal mandibulectomy is indicated when there is no direct invasion of mandible by tumor, but tumor is in close proximity of mandible and would result in inadequate margin. However, it is relatively contraindicated in postradiation settings as it may be oncologically unsafe and associated with risk of osteo-radionecrosis in remnant mandible. If the mandible is invaded by the tumor or there is paramandibular soft tissue then segmental mandibulectomy is indicated.
Neck
Modified neck dissection is indicated in node positive cases. The philosophy of treatment for node negative cases is as described earlier. Radical neck dissection is to be discouraged. SAN, internal jugular vein and sternocleidomastoid muscle are removed only if directly involved by the disease. Contralateral neck is to be addressed if tumor is approximating or crossing the midline especially with ipsilateral node positive.[12]
Reconstruction
Reconstruction of the defect is done by the free radial artery forearm flap (FRAFF) or free anterolateral thigh (FALT) flap. The latter is preferred when larger volume replacement is needed. Free fibula osteocutaneous flap (FFOCF) with skin island is sometimes needed in the presence of an adjacent mandibular defect that needs reconstruction. If free flap facility is not available then pectoralis major myo-cutaneous flap (PMMC) or pectoralis major myo-fascial flap (PMMF) is another option available.
Adjuvant treatment
Locally advanced disease requires either postoperative radiotherapy or chemoradiotherapy. Adjuvant radiotherapy is indicated in T3/T4 primary, high grade tumor, LVE/PNI positive, close margins and node positive disease.[13,14] Positive margins and extracapsular spread are indications of adjuvant chemoradiotherapy.[15] Minimum dose of radiotherapy in adjuvant settings should be 56 Gy with 2 Gy/fraction × 5 days a week. The dose should be escalated to 60-66 Gy in high risk areas. Chemotherapy is given in concurrent setting. Single agent cisplatin 100 mg/m2 every 3 weeks or 30-40 mg/m2/week for the entire course of radiotherapy is recommended. It is suggested that the total dose of 200 mg/m2 needs to be maintained. Additionally anti-emetics and hydration are to be carefully used.
ADVANCED STAGE (IVB)
Moderately advanced stage IVB disease is usually inoperable. Patients with ankyloglossia with deep infiltration into the root of the tongue, skin involvement due to direct extension and extension to the infratemporal fossa/masticator space and base of the skull are contraindications to surgery. If the patient has good performance status and can tolerate CTRT, response is most durable compared to other modalities of treatment. Evidence exists for 100 mg/m2 cisplatin administered three weekly on days 1 and 22 and 43 of radiation as concurrent chemotherapy. Smaller weekly dose of cisplatin between 30 and 40 mg/m2 is a widely accepted practice in India as well as elsewhere, and this may also be recommended. However, it is imperative to achieve a total dose of 200 mg/m2 of cisplatin. If the patient is not a suitable candidate for chemotherapy, biological agents (cetuximab, nimotuzimab) are often used in place of cisplatin although the data in support of this is not robust.[16,17] Carboplatin is another alternative. EBRT can be used in those not suitable for CTRT. Conservative portals of EBRT with smaller margins should be used. Various fractionation regimes are used. The commonly practiced regimes are 40 Gy/16 Fr/4 weeks or 30 Gy/10 Fr/2 weeks or 20 Gy/5 Fr/1-week, however in responders the dose may be escalated to consolidate the response.[18]
Concurrent chemo-radiation is associated with significant short and long-term toxicities. It is strongly recommended that this treatment be offered only at the high volume centers with adequate multimodality team capable of handling the toxicities. Maintenance of nutrition during therapy results in better compliance and use of nasogastric tube feeding should be considered when needed.
The role of neoadjuvant chemotherapy is a debatable issue in tongue cancers. Induction chemotherapy has been used in inoperable T4b cancers and responders considered for surgical salvage.[19] Patients most likely to benefit with such an approach are those in whom upfront surgery would have resulted in positive margins. The best regimen for induction therapy is the three drug regimen that includes a taxane, cisplatin, and 5 flourouracil. Usually, three cycles are given. Following induction chemotherapy, if the tumor is found to be resectable surgery is offered followed by appropriate adjuvant treatment. This treatment needs a motivated patient with good performance status without co-morbidities.
Large fungating nodes, orocutaneous fistula, and low performance status are indications for symptomatic treatment to avoid toxicity of definitive therapies.
RECURRENT/METASTATIC DISEASE (STAGE IV C)
The mainstay of the treatment of patients with recurrent/metastatic tongue cancer is palliation. Pain relief and maintaining the nutrition takes priority in the overall management of these patients. Chemotherapy may be offered to patients with good performance status. Before planning the treatment for recurrent tongue cancer, efforts should be made to identify the occasional patient who may be a candidate for surgical salvage or re-radiation.
Palliative chemotherapy is usually a two drug regimen (Essential). The most widely used and recommended doublet is cisplatin and 5-flurouracil. The combination of carboplatin and paclitaxel or docetaxel has been described. Addition of cetuximab has demonstrated improvement in progression free as well as overall survival.[20] The regimen is cisplatin, 5-FU and cetuximab for three cycles followed by cetuximab weekly till the progression of the disease. Although there is evidence to suggest benefit of this regimen, it is costly, and the cost benefits ratio is not established. Patients with compromised performance status or those with comorbidities may be offered single agent chemotherapy or only palliative care.
FOLLOW-UP AND REHABILITATION
Patient should be counseled regarding abstinence from tobacco and alcohol. Dental evaluation for appropriate extraction of carious teeth and restoration of dental health with dental filling along with fluoride prophylaxis before and after radiotherapy should be done. Nutritional evaluation and support and psychosocial support is also important. Shoulder physiotherapy should be started in all cases of neck dissections. Patients should be taught jaw stretching exercises to prevent postoperative trismus. Swallowing and speech rehabilitation is very important to improve the quality of life. All patients to be followed-up every 2-3 months for first 2 years, 3-4 months for the 3rd year, six monthly for next 2 years and annually thereafter. On every follow-up thorough examination for loco-regional control, second primary tumor and late sequelae of treatment should be done. Investigation should be done only if indicated by symptoms and positive clinical findings. Chest X-ray can be done annually. Serum T3, T4, thyroid-stimulating hormone should be done annually if neck has been irradiated.
Footnotes
Source of Support: Nil.
Conflicts of Interest: None declared.
REFERENCES

| Fig. 1 Stage grouping: Stage IV A – Moderately advanced cancers, Stage IV B – Very advanced cancers


 PDF
PDF  Views
Views  Share
Share

