Indian Council of Medical Research consensus document for the management of gastrointestinal stromal tumors
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(04): 244-248
DOI: DOI: 10.4103/0971-5851.144983
E X E C U T I V E S U M M A R Y
This consensus statement was produced along with the gastric cancer discussions as stomach is the most common site for gastrointestinal stromal tumor (GIST). The recommendations apply to treatment of GIST.Evaluation of a patient with newly diagnosed GIST should include essential tests: A standard white light endoscopy with 6-8 biopsies (c-KIT testing on immunohistochemistry) from the tumor for confirmation of the diagnosis, a computed tomography (CT) scan (multi-detector or helical) of the abdomen and pelvis for staging with a CT chest or chest X-ray, and complete blood counts, renal function tests and liver function tests. Endoscopic ultrasonography (EUS)/magnetic resonance imaging (MRI)/positron emission tomography (PET)-CT are not recommended for all patients.For localized and resectable disease, surgery is recommended. The need for adjuvant treatment with imatinib would be guided by the risk stratification on the histopathological analysis of the resected specimen.For localized but borderline resectable tumors, upfront surgery may be considered only if complications due to the tumor are present such as major bleeding or gastric outlet obstruction. In all other patients, neoadjuvant imatinib should be considered to downstage the disease followed by surgery (with a curative intent, if feasible) in those with stable or partial response. This may be followed by adjuvant imatinib. In those patients with a poor response, further imatinib with dose escalation or sunitinib may be considered.Patients with metastatic disease must be assessed for treatment with imatinib as first-line therapy followed by sunitinib as second-line therapy versus best supportive care on an individual basis.
Publication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
EXECUTIVE SUMMARY
- This consensus statement was produced along with the gastric cancer discussions as stomach is the most common site for gastrointestinal stromal tumor (GIST). The recommendations apply to treatment of GIST.
- Evaluation of a patient with newly diagnosed GIST should include essential tests: A standard white light endoscopy with 6-8 biopsies (c-KIT testing on immunohistochemistry) from the tumor for confirmation of the diagnosis, a computed tomography (CT) scan (multi-detector or helical) of the abdomen and pelvis for staging with a CT chest or chest X-ray, and complete blood counts, renal function tests and liver function tests. Endoscopic ultrasonography (EUS)/magnetic resonance imaging (MRI)/positron emission tomography (PET)-CT are not recommended for all patients.
- For localized and resectable disease, surgery is recommended. The need for adjuvant treatment with imatinib would be guided by the risk stratification on the histopathological analysis of the resected specimen.
- For localized but borderline resectable tumors, upfront surgery may be considered only if complications due to the tumor are present such as major bleeding or gastric outlet obstruction. In all other patients, neoadjuvant imatinib should be considered to downstage the disease followed by surgery (with a curative intent, if feasible) in those with stable or partial response. This may be followed by adjuvant imatinib. In those patients with a poor response, further imatinib with dose escalation or sunitinib may be considered.
- Patients with metastatic disease must be assessed for treatment with imatinib as first-line therapy followed by sunitinib as second-line therapy versus best supportive care on an individual basis.
INCIDENCE
Gastrointestinal stromal tumors are uncommon mesenchymal smooth muscle tumors that may arise in any part of the GI tract. They account for <1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4264268/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_417381923" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1]. Among all the sites in the GI tract, gastric GISTs are the most common[1,2,3]. In one of the largest retrospective series of gastric GISTs[4], it was noted that these tumors occurred in patients above the age of 40 years, a finding confirmed by Indian studies[2,3]. These tumors present with a median diameter of 6 cm[4]. Gastric GISTs may present as part of the Carney triad syndrome (other lesions including paragangliomas and parachondromas). The incidence of GIST in India is unknown. Data regarding management strategies are largely derived from studies in Caucasian patients.
PURPOSE
Several International Consensus Guidelines are available for the management of GISTs[5,6]. It is essential to analyze the evidence pertaining to GISTs from India and the rest of the world with an aim to formulate reliable, evidence-based guidelines that could be applicable to Indian patients bearing in mind the socio-cultural diversity, the distribution of resources and the availability and accessibility to health-care. Taking into consideration peripheral oncology centers, regional cancer centers and tertiary cancer centers in major cities, the set of recommendations includes two categories, viz:
- Desirable/Ideal: Tests and treatments that may not be available at all centers but the centers should aspire to have them in the near future; and
- Essential: Bare minimum that should be offered to all the patients by all the centers treating cancer patients.
DIAGNOSIS AND STAGING
Evaluation of a patient presenting with a gastric GIST should be aimed at pathological confirmation of the diagnosis and an accurate staging of the disease.
Essential tests which need to be done in all patients include:
- Standard white light endoscopy with 6-8 biopsies from the tumor for confirmation of the diagnosis (c-kit/DOG-1 testing on immunohistochemistry)[7].
- CT scan (multi-detector or helical) of the abdomen and pelvis which consists of a nonenhanced phase, an arterial phase, and a portal venous phase. Patients may receive a negative/water-equivalent oral contrast agent for the detection of GI tract wall lesions. If the patient is allergic to contrast media, then an MRI of the abdomen is recommended along with a noncontrast CT thorax.
- CT Thorax or X-ray for staging of the chest, and
- Routine blood investigations-complete blood counts, renal function tests, and liver function tests.
Desirable investigations (when indicated) include:
- 2-[18 F] fluoro 2-deoxyD-glucose-PET or PET-CT-if metastatic disease is suspected.
- EUS may be used for gastric GISTs to stage and accurately identify subjects with an early GIST in whom endoscopic therapy could be planned.
RISK ASSESSMENT
The TNM classification is not able to risk stratify patients with GIST, hence, its routine use is not recommended. Over the years, the key prognostic criteria for GIST are the size of primary tumor, mitotic rate, the location of primary (gastric GIST have a better prognosis than small bowel or rectal GISTs), and rupture at the time of surgery. After curative resections, patients with a mitotic rate of ≥10/50 high-power fields (HPFs) have a poorer survival rate as compared to smaller lesions[8]. Fletcher et al.[9] stratified the risk of aggressive or malignant behavior in GISTs, based on size and mitotic rate, and this is widely followed:
- Very low risk <2>
- Low risk 2-5 cm and <5>
- Intermediate risk either[1] <5 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4264268/#ref2" rid="ref2" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_417381925" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>2] 5-10 cm and <5>
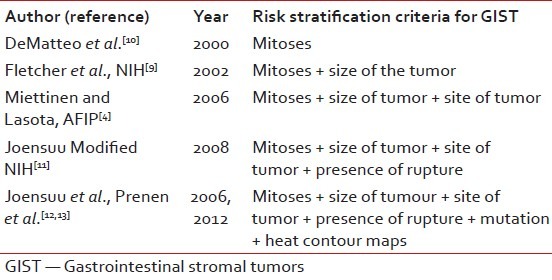
Table 1 shows the evolution of risk criteria over the years. Patients with gastric GISTs do significantly better compared to others. Recently, heat and contour maps give added information and may be used routinely in the future.
Table 1
Risk stratification criteria for GIST over the years
|
TREATMENT PLAN
All patients should be discussed at the multidisciplinary team (MDT) or tumor board meetings, and a care plan advised. The MDT should comprise of surgical, medical, and radiation oncologists; gastroenterologists; pathologists; radiologists (including interventional and nuclear medicine radiologists); nurse specialists; and palliative care physicians. Treatment decisions are based on the extent of disease. The intent of treatment is “curative” for patients with localized resectable disease and “palliative” for patients with metastatic disease. In patients with locally advanced disease, surgical resection may be undertaken following neoadjuvant imatinib[14].
Nonmetastatic, resectable gastric cancer (including loco-regionally advanced disease)
Role of surgery
The ideal treatment for a nonmetastatic GIST ≥2 cm[4] or even tumors <2 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4264268/#ref10" rid="ref10" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_417381921" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>10]. In patients with tumors that are ≥10 cm or that have ruptured (where there is a high-risk of cells being shed), every attempt must be made to resect all visible tumor. Such tumors that have been removed with a microscopically positive margin (R1) recurrence-free survival at a median of 4 years was found to be not different from those patients who underwent an R0 resection[15]. Gastric GISTs generally arise from the wall and grow outward. As a result, rather than standard resections, often a wedge resection with negative margins may be adequate. In the case of advanced GISTs that have invaded surrounding structures, en bloc resections of the involved organs, is recommended.
Lymphadenectomy is not indicated as part of surgery for GISTs as they seldom metastasize to lymph nodes[16]. However, enlarged lymph nodes that appear suspicious of malignant invasion may be sampled at the time of surgery.
For localized but borderline resectable GIST, upfront surgery may be considered only if complications due to the tumor are present such as major bleeding or gastric outlet obstruction. In all other patients, neoadjuvant imatinib should be considered to downstage the disease followed by surgery in those with stable or partial response[14,17].
SYSTEMIC THERAPY IN NONMETASTATIC GASTROINTESTINAL STROMAL TUMOURS
Neoadjuvant imatinib
Neoadjuvant imatinib for treating a localized GIST is a matter of surgical and medical discretion and should be made on an individual basis. Preoperative imatinib may be used in the following settings: If an R0 resection is unlikely, very large localized, but potentially resectable GIST that may bleed or rupture, poorly located small GISTs that are difficult to resect, nonmetastatic, but localized, GISTs deemed unresectable.
In unresectable or locally advanced GISTs, preoperative imatinib could be useful to improve resectability and reduce surgical morbidity[17]. The optimal duration of preoperative therapy is unknown; hence, imatinib may be continued until maximal response. Early response assessment after 8 weeks of initiation of therapy should be done. Each new cross-sectional imaging should prompt multidisciplinary reappraisal of the surgery timing or continuation of preoperative imatinib. If the progression is confirmed with CT scan, surgery is recommended after discontinuing imatinib.
Adjuvant imatinib
Standard care for primary resectable localized gastric GISTs is surgery followed by postoperative radiologic surveillance for recurrence. However, because many patients develop recurrence after resection, imatinib is indicated in the postoperative setting to reduce recurrence. Adjuvant imatinib 400 mg daily for resectable GIST should be considered in high-risk disease (tumor size >10 cm and any mitotic index; any tumor size and mitotic index >10, tumor size 5 cm and mitotic index >5, tumor size ≤5 cm and mitotic index >5 (nongastric site), tumor size 5.1-10 cm and mitotic index ≤5 (nongastric site), any tumor size and any mitotic index in the presence of tumor rupture). Prospective randomized evidence shows that this treatment must be continued for 3 years in patients with c-kit/CD-117 positive GIST[18]. Ideally, PDGFRA D-842V mutation testing should also be considered as these patients do not respond to imatinib and patients with exon 9 kit mutation need higher doses of imatinib (800 mg)[19].
Metastatic gastrointestinal stromal tumors
Role of surgery
Palliative resections may need to be undertaken in patients who have uncontrolled bleeding or gastric outlet obstruction who are otherwise well with a projected longer life expectancy. A gastrojejunostomy may be helpful in patients with distally obstructing tumors with distant metastases. In those patients with short-life expectancy, endoscopic stenting or an endoscopically-placed nasojejunal tube for feeding may be useful for palliation. In patients with recurrent or metastatic GIST, cytoreductive surgery may need to be considered in the following settings-stable disease or disease responsive to imatinib therapy when complete gross resection is possible, progression of isolated clones on therapy after initial response (indicative of secondary drug resistance), while other disease sites remain stable (limited disease progression) and in case of emergencies, including hemorrhage, perforation, obstruction, or abscess formation. Surgery should also be considered for patients with impending emergencies, including those with significant cystic degeneration who are at potential risk for perforation. Complete excision of residual metastatic disease has been associated with a good prognosis, but there are no randomized data to support this. Hence, each decision should be individualized within an MDT setting[20].
Role of biological therapy
For recurrent or metastatic GIST, the current standard of care is imatinib 400 mg for the patient who is c-kit positive[21,22]. Patients with kit exon nine mutations do better on the higher dose of imatinib 800 mg (19). The treatment in metastatic patients has to be continued indefinitely. It is important to discuss compliance and drug interactions with the patient at the time of commencing therapy. Tumor response should be assessed at 3 monthly intervals to begin with and then 6 monthly if response is ongoing. If patients demonstrate progressive disease on imatinib, the standard approach is to increase the dose from 400 mg to 800 mg daily[22].
In the case of progression or intolerance on imatinib, second-line treatment with sunitinib can be considered. Sunitinib at the dose of 50 mg has been shown to be effective in terms of progression-free survival using a ‘4 weeks on-2 weeks off’ regimen though nonrandomized data has shown that continuous dosing at 37.5 mg is better tolerated and equally effective[23]. After failing on sunitinib, patients with metastatic GIST can be considered for third-line treatment with regorafenib or be considered for participation in a clinical trial[24].
FOLLOW-UP
There are no data on optimal follow-up for patients with GIST. The aim of follow-up is to detect recurrences early as well as to assess any complication due to surgery/radiotherapy. Follow-up can be risk stratified, and patients with high-risk disease should be followed-up every 3-4 months for the first 2 years and then 6 monthly. Patients with low-risk disease may be followed-up 6 monthly. A repeat endoscopy after 6 months to a year postsurgery is advised. CT scans can be done at annual intervals or sooner if the patient develops symptoms suspicious of recurrent disease. Further research should focus on epidemiologic and genetic markers in Indian patients as there is some suggestion that the biology may be different in Indian patients.[25]
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Roggin KK, Posner MC. Modern treatment of gastric gastrointestinal stromal tumors. World J Gastroenterol 2012;18:6720-8.
- Rajappa S, Muppavarapu KM, Uppin S, Digumarti R. Gastrointestinal stromal tumors: A single institution experience of 50 cases. Indian J Gastroenterol 2007;26:225-9.
- Lakshmi VA, Chacko RT, Kurian S. Gastrointestinal stromal tumors: A 7-year experience from a tertiary care hospital. Indian J Pathol Microbiol 2010;53:628-33.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83.
- von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Casper ES, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw 2014;12:853-62.
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii49-55.
- Rubin BP, Blanke CD, Demetri GD, Dematteo RP, Fletcher CD, Goldblum JR, et al. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med 2010;134:165-70.
- Dougherty MJ, Compton C, Talbert M, Wood WC. Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic groups by mitotic count. Ann Surg 1991;214:569-74.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65.
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8.
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9.
- Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74.
- Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schöffski P, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res 2006;12:2622-7.
- Shrikhande SV, Marda SS, Suradkar K, Arya S, Shetty GS, Bal M, et al. Gastrointestinal stromal tumors: Case series of 29 patients defining the role of imatinib prior to surgery. World J Surg 2012;36:864-71.
- McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: Analysis of risk factors and tumor recurrence. J Am Coll Surg 2012;215:53-9.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993;217:72-7.
- Fiore M, Palassini E, Fumagalli E, Pilotti S, Tamborini E, Stacchiotti S, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45.
- Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012;307:1265-72.
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53.
- Gronchi A, Fiore M, Miselli F, Lagonigro MS, Coco P, Messina A, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6.
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80.
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32.
- George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959-68.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329-38.
- Singh A, Chatterjee P, Pai MC, Chacko RT. Gastrointestinal stromal tumours: A clinico-radiologic review from a single centre in South India. J Med Imaging Radiat Oncol 2009;53:522-9
References
- Roggin KK, Posner MC. Modern treatment of gastric gastrointestinal stromal tumors. World J Gastroenterol 2012;18:6720-8.
- Rajappa S, Muppavarapu KM, Uppin S, Digumarti R. Gastrointestinal stromal tumors: A single institution experience of 50 cases. Indian J Gastroenterol 2007;26:225-9.
- Lakshmi VA, Chacko RT, Kurian S. Gastrointestinal stromal tumors: A 7-year experience from a tertiary care hospital. Indian J Pathol Microbiol 2010;53:628-33.
- Miettinen M, Lasota J. Gastrointestinal stromal tumors: Pathology and prognosis at different sites. Semin Diagn Pathol 2006;23:70-83.
- von Mehren M, Randall RL, Benjamin RS, Boles S, Bui MM, Casper ES, et al. Gastrointestinal stromal tumors, version 2.2014. J Natl Compr Canc Netw 2014;12:853-62.
- ESMO/European Sarcoma Network Working Group. Gastrointestinal stromal tumors: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2012;23 Suppl 7:vii49-55.
- Rubin BP, Blanke CD, Demetri GD, Dematteo RP, Fletcher CD, Goldblum JR, et al. Protocol for the examination of specimens from patients with gastrointestinal stromal tumor. Arch Pathol Lab Med 2010;134:165-70.
- Dougherty MJ, Compton C, Talbert M, Wood WC. Sarcomas of the gastrointestinal tract. Separation into favorable and unfavorable prognostic groups by mitotic count. Ann Surg 1991;214:569-74.
- Fletcher CD, Berman JJ, Corless C, Gorstein F, Lasota J, Longley BJ, et al. Diagnosis of gastrointestinal stromal tumors: A consensus approach. Hum Pathol 2002;33:459-65.
- DeMatteo RP, Lewis JJ, Leung D, Mudan SS, Woodruff JM, Brennan MF. Two hundred gastrointestinal stromal tumors: Recurrence patterns and prognostic factors for survival. Ann Surg 2000;231:51-8.
- Joensuu H. Risk stratification of patients diagnosed with gastrointestinal stromal tumor. Hum Pathol 2008;39:1411-9.
- Joensuu H, Vehtari A, Riihimäki J, Nishida T, Steigen SE, Brabec P, et al. Risk of recurrence of gastrointestinal stromal tumour after surgery: An analysis of pooled population-based cohorts. Lancet Oncol 2012;13:265-74.
- Prenen H, Cools J, Mentens N, Folens C, Sciot R, Schöffski P, et al. Efficacy of the kinase inhibitor SU11248 against gastrointestinal stromal tumor mutants refractory to imatinib mesylate. Clin Cancer Res 2006;12:2622-7.
- Shrikhande SV, Marda SS, Suradkar K, Arya S, Shetty GS, Bal M, et al. Gastrointestinal stromal tumors: Case series of 29 patients defining the role of imatinib prior to surgery. World J Surg 2012;36:864-71.
- McCarter MD, Antonescu CR, Ballman KV, Maki RG, Pisters PW, Demetri GD, et al. Microscopically positive margins for primary gastrointestinal stromal tumors: Analysis of risk factors and tumor recurrence. J Am Coll Surg 2012;215:53-9.
- Fong Y, Coit DG, Woodruff JM, Brennan MF. Lymph node metastasis from soft tissue sarcoma in adults. Analysis of data from a prospective database of 1772 sarcoma patients. Ann Surg 1993;217:72-7.
- Fiore M, Palassini E, Fumagalli E, Pilotti S, Tamborini E, Stacchiotti S, et al. Preoperative imatinib mesylate for unresectable or locally advanced primary gastrointestinal stromal tumors (GIST). Eur J Surg Oncol 2009;35:739-45.
- Joensuu H, Eriksson M, Sundby Hall K, Hartmann JT, Pink D, Schütte J, et al. One vs three years of adjuvant imatinib for operable gastrointestinal stromal tumor: A randomized trial. JAMA 2012;307:1265-72.
- Gastrointestinal Stromal Tumor Meta-Analysis Group (MetaGIST). Comparison of two doses of imatinib for the treatment of unresectable or metastatic gastrointestinal stromal tumors: A meta-analysis of 1,640 patients. J Clin Oncol 2010;28:1247-53.
- Gronchi A, Fiore M, Miselli F, Lagonigro MS, Coco P, Messina A, et al. Surgery of residual disease following molecular-targeted therapy with imatinib mesylate in advanced/metastatic GIST. Ann Surg 2007;245:341-6.
- Demetri GD, von Mehren M, Blanke CD, Van den Abbeele AD, Eisenberg B, Roberts PJ, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med 2002;347:472-80.
- Blanke CD, Rankin C, Demetri GD, Ryan CW, von Mehren M, Benjamin RS, et al. Phase III randomized, intergroup trial assessing imatinib mesylate at two dose levels in patients with unresectable or metastatic gastrointestinal stromal tumors expressing the kit receptor tyrosine kinase: S0033. J Clin Oncol 2008;26:626-32.
- George S, Blay JY, Casali PG, Le Cesne A, Stephenson P, Deprimo SE, et al. Clinical evaluation of continuous daily dosing of sunitinib malate in patients with advanced gastrointestinal stromal tumour after imatinib failure. Eur J Cancer 2009;45:1959-68.
- Demetri GD, van Oosterom AT, Garrett CR, Blackstein ME, Shah MH, Verweij J, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet 2006;368:1329-38.
- Singh A, Chatterjee P, Pai MC, Chacko RT. Gastrointestinal stromal tumours: A clinico-radiologic review from a single centre in South India. J Med Imaging Radiat Oncol 2009;53:522-9


 PDF
PDF  Views
Views  Share
Share

