Incidence of bone metastasis in carcinoma buccal mucosa
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(02): 70-73
DOI: DOI: 10.4103/0971-5851.180137
Abstract
Introduction: Head and neck cancer is a leading health problem in India due to the habit of chewing tobacco and bad oral and dental hygiene. Carcinoma buccal mucosa is more common and is 2.5% of all malignancies at our center. Most of the patients present in stage III and IV and the survival in these cases is not very good. Bone metastasis in advanced cases of carcinoma buccal mucosa is rarely reported in the world literature. Materials and Methods: We present here cases developing bone metastasis in carcinoma buccal mucosa in last 5 years. These patients were young with loco-regionally advanced disease where bone metastasis developed within 1-year of definitive treatment. Results: The flat bones and vertebrae were mainly involved and the survival was also short after diagnosis of metastasis despite the treatment with local Radiotherapy and chemotherapy. Conclusion: The exact cause of metastasis cannot be proved, but the probability of subclinical seedling of malignant cells before the eradication of the primary tumor should be considered along with advanced local and nodal disease with high grade of tumor.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Head and neck cancer is a leading health problem in India due to the habit of chewing tobacco and bad oral and dental hygiene. Carcinoma buccal mucosa is more common and is 2.5% of all malignancies at our center. Most of the patients present in stage III and IV and the survival in these cases is not very good. Bone metastasis in advanced cases of carcinoma buccal mucosa is rarely reported in the world literature.
Materials and Methods:
We present here cases developing bone metastasis in carcinoma buccal mucosa in last 5 years. These patients were young with loco-regionally advanced disease where bone metastasis developed within 1-year of definitive treatment.
Results:
The flat bones and vertebrae were mainly involved and the survival was also short after diagnosis of metastasis despite the treatment with local Radiotherapy and chemotherapy.
Conclusion:
The exact cause of metastasis cannot be proved, but the probability of subclinical seedling of malignant cells before the eradication of the primary tumor should be considered along with advanced local and nodal disease with high grade of tumor.
INTRODUCTION
Cancer is a leading health problem in India with approximately 1 million new cases occurring every year. Out of this, 2 lacks occur in head and neck compared to 30,000 new cases in the United States. Carcinoma of the buccal mucosa is the most common oral cavity cancer in India. As per the data available from the National Cancer Registry Programme of the ICMR, the males of Ahmedabad urban showed highest age adjusted rate (AAR) for mouth cancer (12.9) followed by Bhopal (9.9). For females however, Bengaluru showed the highest AAR (6.5) followed by Kamrup urban district (5.8).[1] In the hospital-based cancer registry report, cancer of the mouth is also ranked as the leading site in Mumbai in males and was within the first five leading sites in all registries in males. In the developed countries, carcinoma buccal mucosa is relatively uncommon as compared to the Indian subcontinent. The high incidence of carcinoma of the buccal mucosa in our country is attributable to the oral consumption of tobacco in its various forms, use of lime with betel leaves and nuts, alcohol, smoking habits, and poor socio-economic condition. In India, two-third of the head and neck cancer case present in an advance nodal stage at the time of reporting at a tertiary care center.
About 70% of patients develop bony metastasis primarily from breast and prostate carcinoma[2] and bone metastasis is rarely seen in head and neck cancers. Primary buccal mucosa malignancies rarely metastasize to distant sites. They usually metastasize to lymph nodes or spread locally. With the development of newer radiotherapy techniques and availability of better chemotherapy drugs used concurrently has led to better control in carcinoma buccal mucosa treatment. However, this better control in the local disease has led to increased incidence of the distance of distant metastasis.[3] Distant metastasis affects survival and the treatment plan adversely. Its incidence depends on the primary site of involvement, its T and N stage and post-treatment control of the nodal disease. Ferlito et al.[4] has shown that patients presenting with advance nodal disease shows a higher incidence of distant metastasis, especially when there is extensive soft tissue or jugular vein involvement in the neck.
In this study, we saw a higher incidence of bone metastasis in carcinoma buccal mucosa and mostly in patient who underwent surgery, so we present the incidence and probable cause of the metastasis in this presentation.
MATERIALS AND METHODS
From January 2008 to October 2014 we registered a total of 5791 cases of cancer. Head and neck cancer was 25.75% of all malignancies and carcinoma buccal mucosa was 9.91% of all head and neck cancers and 2.55% of all malignancies. It was more common in males than in females with a ratio of 4:1. Tobacco chewing and bad oral hygiene was the main causative factor. Carcinoma buccal mucosa was more common in younger age group with a median age of 45 years. 87% patients presented in stage III and IV and 75% of patients reported after surgery for adjuvant treatment.
RESULTS
Carcinoma buccal mucosa was reported on 9.91% of all head and neck cancers and most of the patients did not complete the planned treatment. On follow-up, of 148 patients, 24 (16.2%) had a local recurrence within 1-year, one developed second primary after 8 years, two had lung metastasis and four patients developed bone metastasis. The incidence of bone metastasis is 0.2% of all head and neck cancer as compared to 0.1% reported in the literature. The sacrum, pelvis, vertebrae, and phalynx of index finger were the bones involved in these cases. The bone metastasis developed 6-9 months after completion of the primary treatment and all patients had a locally advanced disease with nodal metastasis. All four patients underwent surgery as the primary treatment followed with adjuvant concurrent chemotherapy and radiotherapy. All had grade II to III squamous cell carcinoma. The incidence of bone metastasis is 2.71% of all carcinoma buccal mucosa. We could not get the reported incidence in the literature. Patients complained of severe local bony pain and a X-ray/computed tomography scan showed lytic bony lesion at the site of involvement in all the four cases [Figures [Figures11–3]. Fine needle aspiration cytology of all cases from the bone metastatic site was done and it was metastatic squamous cell carcinoma [Figure 4]. All the patients were given palliative local radiotherapy to involved bone for pain control 30 Gy in 10 fractions in 2 weeks using 6 MeV or 15 MeV photons on linear accelerator followed by chemotherapy with cisplatin and paclitaxel. After radiotherapy, all patients had complete pain relief. Despite treatment, the disease progressed and all the patients died within 6-9 months of development of bony metastasis.
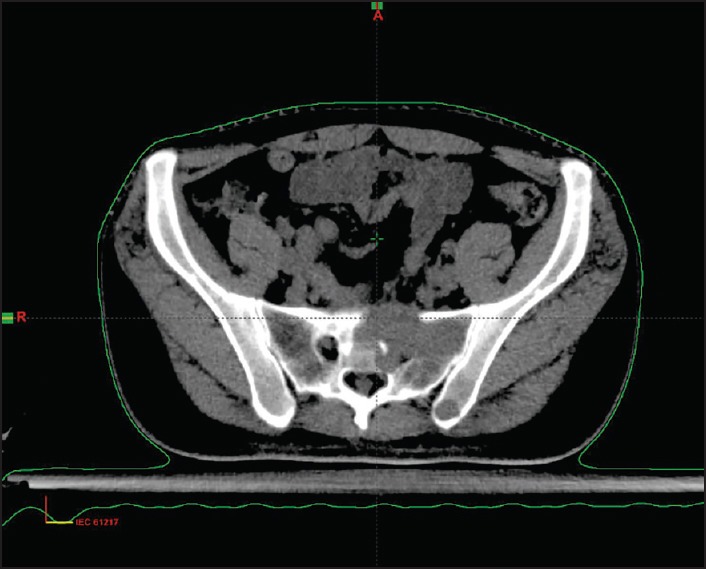
| Fig. 1 Osteolytic lesion with soft tissue in sacrum
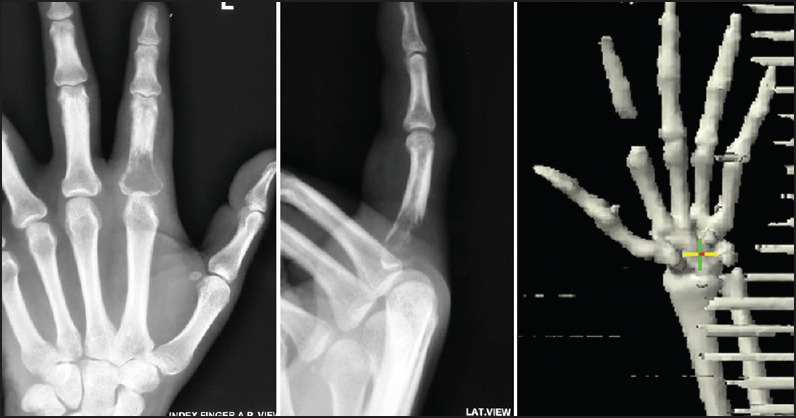
| Fig. 3 Osteolytic lesion in the proximal phalynx of index finger
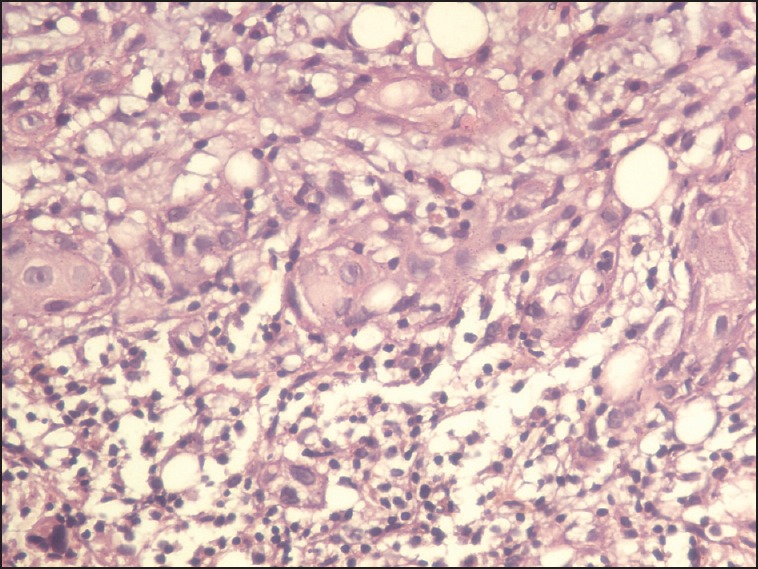
| Fig. 4 View of squamous cell carcinoma metastasis with bone marrow cells (×40)
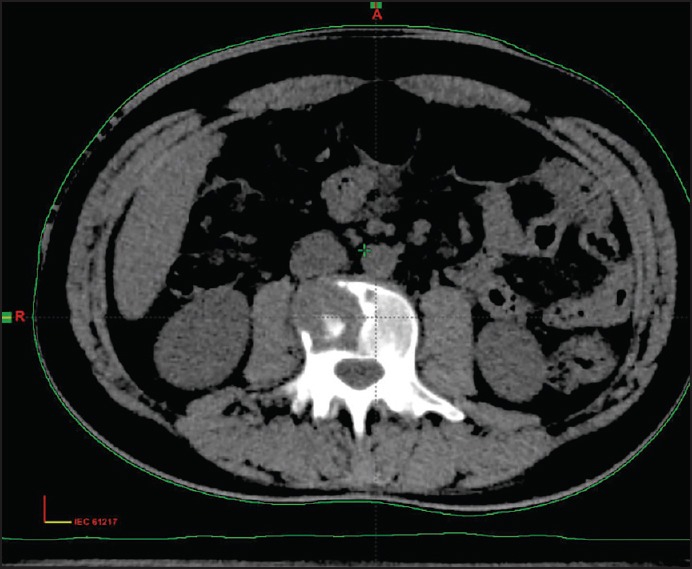
| Fig. 2 Osteolytic lesion in the lumber vertebra
DISCUSSION
Head and neck squamous cell carcinoma most commonly spreads with the lymphatics and has a high propensity for locoregional spread, clinical evidence of non-lymphatic distant spread accounts for approximately 10% of the cases and is typically found in the lungs, brain, bones, and skin.[5] With newer management regimens and improved understanding of squamous cell carcinoma, loco-regional control of cancer above the clavicles has increased.[6] However, the overall disease-free survival rate has not improved accordingly.[7] Distant metastases and second primary tumors are being recognized as a cause for an increasing proportion of failures in patients with head and neck malignancy.[8] Studies have been done on the risk factors for metastasis in head and neck cancer, and consensus has been reached regarding the increased risk with the higher stage of the tumor at the initial presentation, size of the primary lesion (T4), the grade of the tumor, and the site of the lesion. With the incidence being highest in the hypopharynx 60%, followed by the base of the tongue 53%, and the anterior tongue 50%.[9] Metastasis from the buccal mucosa is extremely rare and only one case report on bone metastasis from buccal mucosa had been found in our literature search.[10] In last 5 years, we found four cases of carcinoma buccal mucosa which has metastasized to bones. All the patients were young and had loco-regionally advanced disease and high-grade tumor at presentation.
Most studies found that distant metastases are diagnosed within a year of presentation, so there is probably subclinical seeding of malignant cells before the eradication of the primary tumor. The average survival with distant metastasis ranges between 4.3 months and 7.3 months.[11,12] In this series also bony metastasis occurred within an average period of 9 months of diagnosis and the survival was also 6-9 months only after the development of bony metastasis.
Bhandari and Jain[13] in his study saw that patients with advanced local and nodal head and neck cancers at presentation developed metastasis. The usual primary sites involved in his presentation were the base of tongue and tonsil with solitary bone metastasis and postoperative buccal mucosa where multiple bones showed osteolytic lesions in the same patient. The exact cause of distant metastasis after local control is not known although all patients who developed bony metastasis had the advanced nodal disease and one patient also had extra capsular spread. All patients in this study had undergone surgery and had advanced local and nodal disease at presentation.
A strong correlation was seen between clinical nodal disease and pathologically involved lymph nodal status in the patient. The patients who presented with clinically palpable lymph nodal (N1-N3) disease were operated and histologically who had three or more lymph nodes showing metastasis with extra capsular spread, lymphovascular invasion (LVI) were more prone to develop distant metastasis. In the present study also the patients who developed bony metastasis had a nodal disease with LVI, so the theory of hematogenous spread through lymph nodes can be true.
Most common site of metastasis is the axial skeleton involving the spine, pelvis, and ribs. Lumbar spine being the single most frequent site of bone metastasis.[14,15] In the appendicular skeleton, the proximal femur and humerus are mainly involved. Patients in this series has involvement of the flat and appendicular bones which are the usual sites involved.
Pietropaoli et al.[16] reviewed radiographs and nuclear medicine studies of 363 patients of head and neck cancers retrospectively. He saw that 1% of these cases had developed bone metastasis mainly involving the pelvic bones, femur, humerus, ribs, and thoracic vertebra. These bony lesions were mainly osteolytic in nature with moth-eaten or permeative borders. In this series also the main bones which showed metastasis are a flat parietal bone of skull, ribs and sacrum, and long bones like shaft of femur and radius. Same bones have been seen involved in the other series by Pietropaoli. The lesion in all the bones was lytic or moth eaten appearance which appeared 3-12 months after the primary treatment was over. The observation regarding the site and duration in this study remains the same as other studies.
Time from primary tumor diagnosis to identification of metastatic disease ranged from being present at diagnosis to a maximum 3.5 years later. The ultimate prognosis for patients with bone metastasis is poor with a median survival of few months only. Time from identification of metastatic disease to patient death was no greater than 8 months.[16] We also saw that the bone metastasis occurred with an average period of 9 months after achieving a complete response to the primary treatment.
Most studies found that distant metastases are diagnosed within a year of presentation, so there is probably subclinical seeding of malignant cells before the eradication of the primary tumor. The average survival with distant metastasis ranges between 4.3 months and 7.3 months. All the four patients operated for carcinoma buccal mucosa in this series that were young and developed metastasis to multiple bones within 1-year of surgery and could not survive longer despite all treatment.
CONCLUSION
Squamous cell carcinoma of the buccal mucosa tends to spread by direct extension and lymphatic metastasis, with hematogenous dissemination occurring late in the natural history of the disease. A probability of subclinical seeding of malignant cells before the eradication of the primary tumor should be considered. In young patients with advanced disease, the distant metastases can affect different organ systems including the bones and almost invariably herald a poor prognosis and treatment is usually palliative and survival remains less than 1-year. In loco-regionally advanced cases of all head and neck carcinoma cases a bone scan should be done prior to definitive treatment in order to avoid the morbidity and start the treatment earlier to improve the survival.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1 Osteolytic lesion with soft tissue in sacrum


 PDF
PDF  Views
Views  Share
Share

