Importance of early and deeper responses to long-term survival in CML patients: Implications of BCR-ABL testing in management of CML in Indian setting
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2014; 35(01): 10-16
DOI: DOI: 10.4103/0971-5851.133704
Abstract
The prognosis of patients with chronic myeloid leukemia (CML) has changed radically since the advent of imatinib mesylate, a selective inhibitor of BCR-ABL tyrosine kinase. Shortly thereafter, more potent BCR-ABL inhibitors (dasatinib and nilotinib) were introduced for use in patients resistant to or intolerant of imatinib. All three drugs are now approved for initial therapy for chronic phase CML. Response to tyrosine kinase inhibitor (TKI) treatment is assessed with standardized quantitative reverse transcriptase polymerase chain reaction (Q-RTPCR) and/or cytogenetics at 3, 6 and 12 months. Clinical trials have clearly demonstrated that early and deeper cytogenetic and molecular response to TKI therapy is associated with lower rate of disease progression and improved long-term outcomes. In recent times, molecular response as determined by BCR-ABL transcript levels at defined time points is rapidly gaining popularity as a predictive marker for subsequent outcomes in CML. Optimal response is defined as BCR-ABL transcript levels of ≤10% at 3 months, <1>10% at 6 months and >1% from 12 months onward define failure. Patients who do not achieve molecular milestones at 3 or 6 months with 3 months being highly predictive are less likely to achieve cytogenetic responses eventually; early identification of such patients who have a low probability of achieving an adequate response are thus candidates for alternative treatment. Review of literature by electronic search of MEDline, Google Scholar was done using keywords and data was identified and systematically evaluated.
Keywords
BCR ABL - chronic myeloid leukemia - dasatinib - early response - imatinib - nilotinib - polymerase chain reaction - molecular responsePublication History
Article published online:
19 July 2021
© 2014. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The prognosis of patients with chronic myeloid leukemia (CML) has changed radically since the advent of imatinib mesylate, a selective inhibitor of BCR-ABL tyrosine kinase. Shortly thereafter, more potent BCR-ABL inhibitors (dasatinib and nilotinib) were introduced for use in patients resistant to or intolerant of imatinib. All three drugs are now approved for initial therapy for chronic phase CML. Response to tyrosine kinase inhibitor (TKI) treatment is assessed with standardized quantitative reverse transcriptase polymerase chain reaction (Q-RTPCR) and/or cytogenetics at 3, 6 and 12 months. Clinical trials have clearly demonstrated that early and deeper cytogenetic and molecular response to TKI therapy is associated with lower rate of disease progression and improved long-term outcomes. In recent times, molecular response as determined by BCR-ABL transcript levels at defined time points is rapidly gaining popularity as a predictive marker for subsequent outcomes in CML. Optimal response is defined as BCR-ABL transcript levels of ≤10% at 3 months, <1>10% at 6 months and >1% from 12 months onward define failure. Patients who do not achieve molecular milestones at 3 or 6 months with 3 months being highly predictive are less likely to achieve cytogenetic responses eventually; early identification of such patients who have a low probability of achieving an adequate response are thus candidates for alternative treatment. Review of literature by electronic search of MEDline, Google Scholar was done using keywords and data was identified and systematically evaluated.
INTRODUCTION
Cancer registries in India report chronic myeloid leukemia (CML) to be the most common adult leukemia in Indians with an annual incidence ranging from 0.8 to 2.2/100,000 population for men and from 0.6 to 1.6 per 100 000 population for women.[1] Introduction of imatinib mesylate has lead to unprecedented improvements in response and prognosis in CML. Second-generation tyrosine kinase inhibitors (TKIs) (dasatinib and nilotinib) earlier reserved for imatinib resistant or intolerant patients are now recommended for first-line use in chronic phase (CP) CML.[2,3] Early monitoring of patients by assessing cytogenetic and molecular response at defined time points has emerged as a critical success factor for long-term disease management. Patients with cytogenetic or molecular response as early as 3 months have a more favorable prognostic outcome as compared to non-responders. This review article attempts to summarize the importance of achieving early and deeper responses in CML management that predicts long-term clinical outcomes and guiding treatment modifications in Indian setting.
LESSONS FROM THE INTERNATIONAL RANDOMIZED STUDY OF INTERFERON AND STI571 (IRIS) IN NEWLY DIAGNOSED CHRONIC MYELOID LEUKEMIA
It was first demonstrated by Kantarjian et al. as early as in 2002 that patients who achieved major cytogenetic response (MCyR) by 3 months in the IRIS study had a longer time to disease progression in the subsequent period of 12 months.[4] Similarly, Hughes et al. in 2003 reported significantly better progression free survival (PFS) outcomes in patients who achieved >3 log reduction of BCR-ABL titers by 12 months.[5] These findings were strengthened further with landmark analysis from 5-year follow up of the IRIS study which clearly demonstrated significantly better PFS in patients who achieved complete cytogenetic responses (CCyR) and major molecular responses (MMR) by 12 and 18 months, respectively (98% and 100%).[6] Treatment failure at these time points has been shown to be closely related to poor PFS and overall survival (OS). Acknowledging the evidence that emerged from the IRIS study along with other independent studies, the European LeukemiaNet (ELN) defined ‘treatment milestones’ at 3-, 6-, 12-, 18 months and subsequently thereafter for assessment of optimal response, suboptimal response and treatment failure [Table 1].
Table 1
Definition of Responses-ELN 2013 guidelines[3]
Definition of Responses-ELN 2013 guidelines[3]
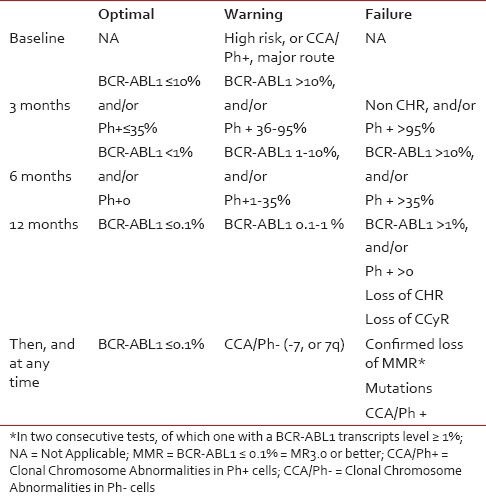
|
OUTCOME OF PATIENTS TREATED FIRST LINE WITH IMATINIB
While long-term results of the Phase III clinical trial of imatinib versus interferon-α (IFN- α) combined with low-dose cytarabine in patients with untreated CP CML showed a superior outcome in the imatinib arm, with an 8 year OS of 85% and PFS of 92%, a substantial fraction of patients do have resistance to therapy with imatinib or develop intolerance. In the IRIS trial 8 year follow-up report, 37% of patients initially treated with imatinib had an unfavorable outcome, with 32%-failing to achieve or losing a CCyR and 5%-developing intolerance to imatinib.[7]
Second-generation TKIs were initially approved as second-line therapy after development of imatinib resistance. Results from a 15-month follow up of a phase 2 dasatinib study (START-C trial; n = 387) in imatinib-resistant/intolerant CP-CML patients showed that dasatinib-induced notable responses, with 91% and 59%- patients achieving complete hematologic response (CHR) and MCyR respectively while PFS and OS were 90% and 96%, respectively.[8] In another phase 2 study where imatinib-resistant CP-CML patients were randomized to receive either dasatinib (n = 101) or high dose imatinib, (800 mg/day, n = 49) after a 2-year follow-up dasatinib demonstrated higher rates of CHR (93% vs 82%; P = 0.034), MCyR (53% vs 33%; P = 0.017), CCyR (44% vs 18%; P = 0.0025) and a better PFS (86% vs. 65%; P = 0.0012) than high dose imatinib.[9] Similarly, in a phase 2 nilotinib study in imatinib-resistant/intolerant CP-CML patients (n = 321), rates of MCyR and CCyR after a minimum follow up of 19 months were 59% and 44%, respectively, and the estimated survival at 24 months was 88%.[10]
The recommended dose of dasatinib in imatinib resistant/intolerant CP-CML is 100 mg once daily (based on the results of the Phase III dose optimization study in CP-CML in second line after imatinib failure, where dasatinib 100 mg once daily regimen was as effective and better tolerated than 70 mg twice daily regimen),[11] while the recommended dose for nilotinib is 400 mg administered twice daily.[10] Patients with inadequate response to imatinib may thus benefit from the second-generation TKIs, nilotinib and dasatinib, in the second-line setting therefore necessitating early identification of these patients before progression to advanced phases.[7]
In addition, recent trials have also demonstrated advantages of these agents over imatinib as initial therapy. Two prospective, randomized, company sponsored studies with second-generation TKIs (DASISION and ENESTnd studies) showed superiority of dasatinib and nilotinib over imatinib, when used as first-line therapy in newly diagnosed patients particularly in the speed and depth of the response.[2,3]
CHANGING SCENARIO: EMERGING EVIDENCE
The initial evidence regarding the importance of a deeper molecular/cytogenetic response by 3 months after initiation of TKI treatment emerged from studies [Table 2] nearly a decade earlier. More recently, molecular response determined by BCR-ABL transcript levels according to the international scale at defined time points has been used as a predictive marker for suboptimal response or failure. Several studies have confirmed the predictive value of BCR-ABL transcript levels measured at 3- or 6 months after starting treatment with TKIs for subsequent cytogenetic and molecular responses as well as long-term survival outcomes[12] [Table 3].
Table 2
Demonstrating correlation between early and deeper response and disease outcome
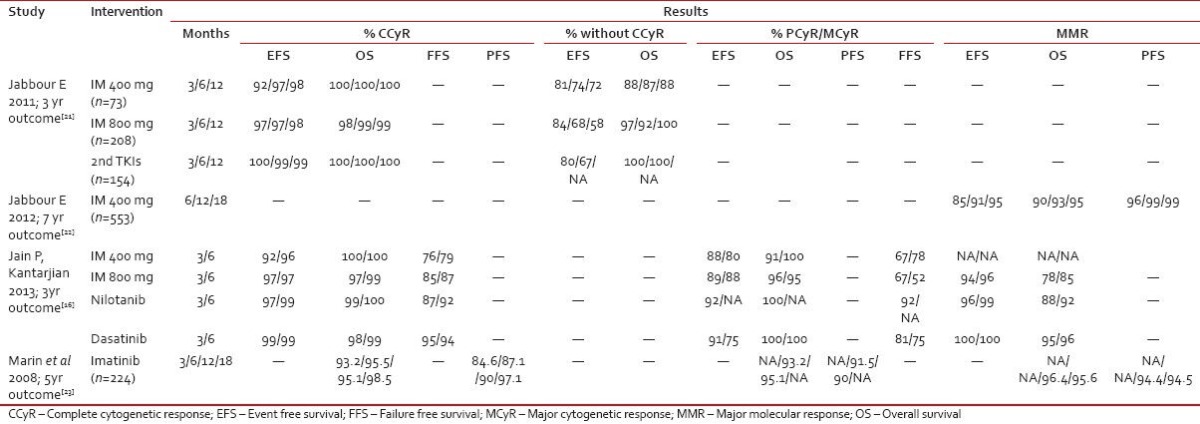
|
Table 3
Correlation between early molecular response and outcomes
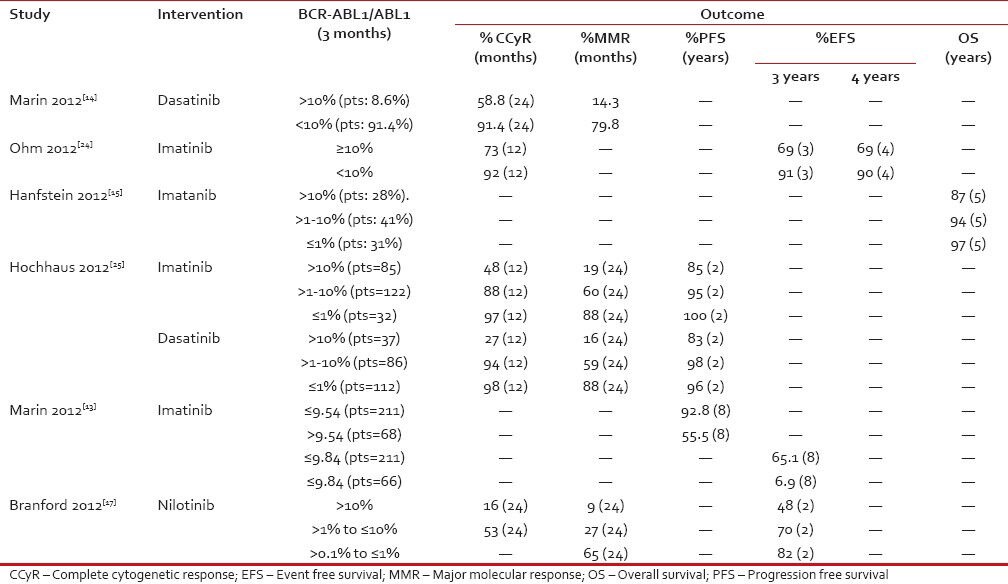
|
In a retrospective analysis of 282 CP CML patients who received imatinib first line in a real world clinical setting by the Hammersmith group, it was identified that patients with BCR-ABL transcript levels less than 9.84% at 3 months had significantly higher 8 year OS (93% vs 57%) than patients with higher transcript levels. Similar predictive values for 8 year OS (94% vs 75%) were observed with patients whose transcript levels were less than 1.67% at 6 months and 0.53% at 12 months. However, transcript levels at 3 months emerged to be strongly predictive for the various outcomes including PFS, event free survival (EFS) and cumulative incidence of CCyRs.[13] Patients with BCR-ABL transcripts >10% at 3 months had a significantly worse 2-year cumulative incidence of CCyR (58.8% vs 96.6%, P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4080644/#ref14" rid="ref14" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_387067901" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>14]
In another retrospective analysis done on 1440 newly diagnosed CP CML patients who were randomized in the CML study IV to receive imatinib 400 mg/d, imatinib 400 mg/d+IFN-α, imatinib 400 mg/d+low-dose cytarabine, imatinib 400 mg/d after failure of IFN-α, imatinib 800 mg/d; similar findings of improved OS rates at 5 years were seen in groups of patients who had transcript levels >1-10% (94% OS) and ≤1% (97% OS) at 3 months in comparison to OS rate of 87% in patients with BCR-ABL transcript levels >10% at 3 months. BCR-ABL transcript levels >1% at 6 months was associated with inferior survival rates at 5 years compared to those <1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4080644/#ref15" rid="ref15" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_387067897" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>15]
Similar evidence was published from a single-center experience involving treatment of 483 newly diagnosed CP CML patients who received 400- or 800 mg imatinib, nilotinib, or dasatinib as a first-line treatment by Jain et al. in 2013. In the landmark analysis at 3 months by molecular responses the cumulative proportions of 3 year failure free survival (FFS) for 3 month BCR-ABL levels was 85% for those with ≤1%, 73% for >1% to 10%, and 61% for those with >10% (P = 0.016). The corresponding 3 year FFS values for patients with 6 month BCR-ABL levels was 89% for those with ≤1%, 56% for >1% to 10%, and 49% for those with >10% (P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4080644/#ref16" rid="ref16" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_387067914" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>16] Failure to achieve response at set times as defined by ELN 2009 recommendations, loss of CCyR, intolerance, or treatment discontinuation for any reason were included to estimate the FFS. The response at the early time points predicted for long-term outcome, regardless of the choice of TKI.
An exploratory analysis of DASISION and ENESTnd studies demonstrated a predictive value of initial and early molecular response for survival (PFS and OS by 36- and 48 months, respectively). The ENESTnd study comparing nilotinib 300 mg twice daily and imatinib 400 mg once daily in newly diagnosed CP CML patients reported correlation between BCR-ABL transcript levels at 3 months and PFS/OS by 4 years. Patients with BCR ABL of >1% to ≤10% at 3 months with nilotinib had higher cumulative incidence of CCyR by 24 months than patients with BCR ABL of >10% (53% vs 16%). Similarly, cumulative incidence of MMR by 24 months was 65%, 27%, and 9% in patients with BCR ABL of >0.1% to ≤1%, >1% to ≤10%, and >10%, respectively. Estimated EFS rates at 24 months decreased with higher transcript levels at 3 months (82% in patients with BCR ABL of ≤1%, 70% in BCR ABL of >1% to ≤10% and 48% in BCR ABL of > 10%).
Similar results were seen in a landmark analysis of DASISION comparing dasatinib 100 mg once daily vs imatinib 400 mg once daily in 516 newly diagnosed CP CML patients. Significantly higher PFS (93% vs 68%; P = 0.0003) and OS (96% vs 86%; P = 0.03) at the end of 3 years were observed in patients with 3 month BCR-ABL levels ≤10% vs >10%. Similarly, results were observed in patients who were randomized to imatinib arm in this study with significantly higher 3 year PFS (96% vs 75%; P < 0 xss=removed>P = 0.0036) in patients with 3 month BCR-ABL levels ≤10% vs >10%.[17,18]
In a recent analysis from the Hammersmith group which consisted of 510 newly diagnosed CP CML patients treated with imatinib (n = 368) and dasatinib (n = 142) Neelkantan et al. aimed to assess if the prognostic accuracy could be further improved by combining the 3- and 6 month transcript results. It emerged that patients who met the 3 month transcript landmark but failed the 6 month transcript landmark had outcomes identical to those who met both landmarks, whereas the patients who failed the 3 month transcript landmark but met the 6 months had prognosis similar to those who failed both landmarks. In summary, the prognosis of patients starting TKI can be established accurately by assessing only the 3 month transcript levels and early intervention strategies can be based robustly on the transcript level at 3 months.[12]
Following the evidence as discussed above and from various other studies [Table 3] both the National Comprehensive Cancer Network (NCCN) panel and the ELN panel updated guidelines to include BCR ABL ≤10% by reverse transcriptase polymerase chain reaction (Q-RTPCR) as a treatment response milestone at 3 months replacing the complete hematological response at the same time point.[2,3] The ELN panel has further updated their target treatment milestones beyond 3 months i.e. BCR-ABL transcript levels <1>
Regarding the definitions of treatment failure and switch to alternative TKI, the ELN panel defines BCR-ABL transcript levels >10% at 6 months and >1% at 12 months (and beyond) as treatment failure, mandating a change of treatment.[3] However, the NCCN panel recommends a switch to alternate TKIs in patients whose BCR-ABL levels are >10% at 3 months, or Ph+ve >0 at 12 months (by cytogenetic bone marrow analysis).[2]
STANDARDIZATION OF Q-RTPCR TECHNIQUES
The prognostic significance of molecular response in CML is being well-recognized lately. However, the main limitation of the Q-RTPCR technique is extensive inter-laboratory variability in Q-RTPCR procedures which makes it difficult to compare results for the same patient measured in different laboratories and also to compare results with those observed in the IRIS trial. Standardization of Q-RTPCR methodology and reporting helps overcome these difficulties. Experts at CML meeting at the National Institutes of Health in Bethesda (October 2005) recommended conversion of local laboratory BCR-ABL values to an International Scale (IS) that is essentially identical to IRIS scale, with 100% IS defined as the standardized baseline and 0.1% IS corresponding to MMR (3-log reduction relative to the standardized baseline). Though the original standards used in the IRIS trial are now lost, Adelaide laboratory (IRIS reference laboratory) has generated extensive quality control data over several years which provides traceability to the IRIS scale and is trying to derive and validate laboratory-specific conversion factors (CFs) that can be used to convert local laboratory values to IS values. Laboratories who have established a validated CF with Adelaide are considered national or regional reference laboratories and are now propagating traceable CFs to other local centres.[19] Many centers in India are attempting to establish validated CF with Adelaide; CMC, Vellore has already established a validated CF with Adelaide.[20]
INDIAN SCENARIO: MY RECOMMENDATIONS
Imatinib has been available as first-line treatment in India for almost a decade now with many generic versions of the drug now available. There are a few single center and observational trials [Table 4] assessing treatment response with imatinib in India. However, studies evaluating early and deeper response with imatinib and its correlation with long-term outcomes are sparse in the Indian clinical practice setting. CHR response rates observed in these studies were similar to data from worldwide randomized clinical trials, while the cytogenetic and molecular response was approximately 15-20%-less in the Indian setting.[28] The exact reasons why reported responses to imatinib in the Indian clinical setting are lower than data from worldwide clinical trials is unknown. Possible explanations could be delayed diagnosis or initiation of imatinib therapy and non-adherence, resistance or intolerance to treatment. With the availability of second-generation TKIs, early prediction of suboptimal response or failure would benefit patients with inadequate response who require appropriate intervention to prevent further progression to advanced phases. The observation that prognostic value of BCR-ABL transcript levels at 3-, 6-, or 12 months on survival outcomes is evident with the 3 month assessment being highly predictive than at 6- and 12 months.[12] The decline of mature precursors may account for the early response within 3 months; however, as the treatment continues, other factors such as adherence to therapy and treatment adjustments due to toxicity, begin to influence the response and interfere with the prognostic power of the 6 month transcript measurement. Centers performing molecular tests in India are few and far between; also, standardization of the test across various laboratories is a major issue. The high cost of these tests further precludes its use in India. Despite these constraints, monitoring BCR-ABL transcript levels at 3 months is strongly recommended to identify patients who respond poorly to first line TKI therapy thereby allowing early treatment optimzation with second-generation TKIs.
Table 4
Summary of studies evaluating efficacy and safety of imatinib in CML patients in India
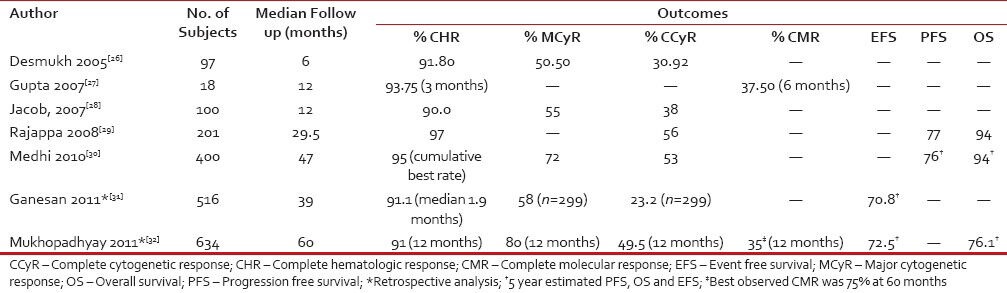
|
CONCLUSION AND SUMMARY/TAKE HOME MESSAGES
The treatment milestones for CML therapy are evolving with an increased focus on early molecular responses and their predictive value on patient outcomes. BCR-ABL transcripts at 3 months provide useful clinical guidance to decide if alternative therapies are warranted for patients with minimal initial molecular response.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- Subramanian PG. Cytogenetic study in CML. Indian J Med Res 2012;135:12-3.
- O′Brien S, Abboud CN, Akhtari M, Altman JK, Berman E, Cohen AD, et al. NCCN Clinical Practice Guidelines in Oncology: Chronic Myelogenous Leukemia. Version 4, 2013. Available at: JNCCN.org. Accessed June 11, 2013.
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al European LeukemiaNet recommendations for the management of chronic myeloid leukemia 2013. Blood 2013;122:872-84.
- Kantarjian HM, O′Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 2003;97:1033-41.
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003;349:1423-32.
- Druker BJ, Guilhot F, O′Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med 2006;355:2408-11.
- Deininger M, O′Brien GS, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs sti571 (iris) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [oral and poster abstract]. 51 st ASH Annual Meeting and Exposition 2009. Available from: http://ash.confex.com/ash/2009/webprogram/Paper23968.html. [Last updated on Dec 2009; Last accessed on Oct 2013]
- Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 2008;22:1200-6.
- Kantarjian H, Pasquini R, Le´vy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily. Cancer 2009; 115:4136-47.
- Jabbour E, Kantarjian H, Cortes J. Chronic myeloid leukemia and second-generation tyrosine kinase inhibitors: When, how, and which one? Semin Hematol 2010;47:344-53.
- Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010;95:232-40.
- Neelakantan P, Gerrard G, Lucas C, Milojkovic D, May P, Wang L, et al. Combining BCR ABL transcript levels at 3 and 6 months in chronic myeloid leukemia: Implications for early intervention strategies. Blood 2013;121:2739-42.
- Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012;30:232-8.
- Marin D, Hedgley C, Clark RE, Apperley J, Foroni L, Milojkovic D, et al. Predictive value of early molecular response in patients with chronic myeloid treated with first-line dasatinib. Blood 2012;120:291-4.
- Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012;26: 2096-102.
- Jain P, Kantarjian H, Nazha A, O′Brien S, Jabbour E, Romo CG, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: Results with four tyrosine kinase inhibitor modalities. Blood 2013;121:4867-74.
- Branford S, Kim DW, Soverini S, Haque A, Shou Y, Woodman RC, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib [abstract]. J Clin Oncol 2012;30:4323-9.
- Saglio G, Kantarjian HM, Shah N, Jabbour EJ, Quintas-Cardama A, Steegmann JL, et al. Early response (Molecular and Cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): Exploratory analysis of DASISION 3-Year Data [oral and poster abstract]. 54 th ASH Annual Meeting and Exposition 2012. Available from: http://ash.confex.com/ash/2012/webprogram/Paper47060.html [Last updated on Dec 2012; Last accessed on Oct 2013].
- White HE, Matejtschuk P, Rigsby P, Gabert J, Lin F, Wang LY, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood 2010; 116:e111-7.
- Balasubramanian P, Chendamarai E, Markose P, Fletcher L, Branford S, George B, et al. International reporting scale of BCR-ABL1 fusion transcript in chronic myeloid leukemia: First report from India. Acta Haematol 2012;127:135-42.
- Jabbour E, Kantarjian HM, O′Brien S, Shan J, Quintas-Cardama A, Faderl S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood 2011;118:4541-6.
- Jabbour E, Saglio G, Hughes TP, Kantarjian H. Suboptimal responses in chronic myeloid leukemia. Cancer 2012; 118:1181-91.
- Marin D, Milojkovic D, Olavarria E, Khorashad JS, Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CMLin early chronic phase treated with imatinib whose eventual outcome is poor. Blood 2008;112:4437-44.
- Ohm L, Arvidsson I, Barbany G, Hast R, Stenke L. Early landmark analysis of imatinib treatment in CML chronic phase:Less than 10
References
- Subramanian PG. Cytogenetic study in CML. Indian J Med Res 2012;135:12-3.
- O′Brien S, Abboud CN, Akhtari M, Altman JK, Berman E, Cohen AD, et al. NCCN Clinical Practice Guidelines in Oncology: Chronic Myelogenous Leukemia. Version 4, 2013. Available at: JNCCN.org. Accessed June 11, 2013.
- Baccarani M, Deininger MW, Rosti G, Hochhaus A, Soverini S, Apperley JF, et al European LeukemiaNet recommendations for the management of chronic myeloid leukemia 2013. Blood 2013;122:872-84.
- Kantarjian HM, O′Brien S, Cortes JE, Shan J, Giles FJ, Rios MB, et al. Complete cytogenetic and molecular responses to interferon-α-based therapy for chronic myelogenous leukemia are associated with excellent long-term prognosis. Cancer 2003;97:1033-41.
- Hughes TP, Kaeda J, Branford S, Rudzki Z, Hochhaus A, Hensley ML, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med 2003;349:1423-32.
- Druker BJ, Guilhot F, O′Brien SG, Gathmann I, Kantarjian H, Gattermann N, et al. Five-Year Follow-up of Patients Receiving Imatinib for Chronic Myeloid Leukemia. N Engl J Med 2006;355:2408-11.
- Deininger M, O′Brien GS, Guilhot F, Goldman JM, Hochhaus A, Hughes TP, et al. International randomized study of interferon vs sti571 (iris) 8-year follow up: Sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP) treated with imatinib [oral and poster abstract]. 51 st ASH Annual Meeting and Exposition 2009. Available from: http://ash.confex.com/ash/2009/webprogram/Paper23968.html. [Last updated on Dec 2009; Last accessed on Oct 2013]
- Hochhaus A, Baccarani M, Deininger M, Apperley JF, Lipton JH, Goldberg SL, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia 2008;22:1200-6.
- Kantarjian H, Pasquini R, Le´vy V, Jootar S, Holowiecki J, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia resistant to imatinib at a dose of 400 to 600 milligrams daily. Cancer 2009; 115:4136-47.
- Jabbour E, Kantarjian H, Cortes J. Chronic myeloid leukemia and second-generation tyrosine kinase inhibitors: When, how, and which one? Semin Hematol 2010;47:344-53.
- Shah NP, Kim DW, Kantarjian H, Rousselot P, Llacer PE, Enrico A, et al. Potent, transient inhibition of BCR-ABL with dasatinib 100 mg daily achieves rapid and durable cytogenetic responses and high transformation-free survival rates in chronic phase chronic myeloid leukemia patients with resistance, suboptimal response or intolerance to imatinib. Haematologica 2010;95:232-40.
- Neelakantan P, Gerrard G, Lucas C, Milojkovic D, May P, Wang L, et al. Combining BCR ABL transcript levels at 3 and 6 months in chronic myeloid leukemia: Implications for early intervention strategies. Blood 2013;121:2739-42.
- Marin D, Ibrahim AR, Lucas C, Gerrard G, Wang L, Szydlo RM, et al. Assessment of BCR-ABL1 transcript levels at 3 months is the only requirement for predicting outcome for patients with chronic myeloid leukemia treated with tyrosine kinase inhibitors. J Clin Oncol 2012;30:232-8.
- Marin D, Hedgley C, Clark RE, Apperley J, Foroni L, Milojkovic D, et al. Predictive value of early molecular response in patients with chronic myeloid treated with first-line dasatinib. Blood 2012;120:291-4.
- Hanfstein B, Muller MC, Hehlmann R, Erben P, Lauseker M, Fabarius A, et al. Early molecular and cytogenetic response is predictive for long-term progression-free and overall survival in chronic myeloid leukemia (CML). Leukemia 2012;26: 2096-102.
- Jain P, Kantarjian H, Nazha A, O′Brien S, Jabbour E, Romo CG, et al. Early responses predict better outcomes in patients with newly diagnosed chronic myeloid leukemia: Results with four tyrosine kinase inhibitor modalities. Blood 2013;121:4867-74.
- Branford S, Kim DW, Soverini S, Haque A, Shou Y, Woodman RC, et al. Initial molecular response at 3 months may predict both response and event-free survival at 24 months in imatinib-resistant or -intolerant patients with Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase treated with nilotinib [abstract]. J Clin Oncol 2012;30:4323-9.
- Saglio G, Kantarjian HM, Shah N, Jabbour EJ, Quintas-Cardama A, Steegmann JL, et al. Early response (Molecular and Cytogenetic) and long-term outcomes in newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): Exploratory analysis of DASISION 3-Year Data [oral and poster abstract]. 54 th ASH Annual Meeting and Exposition 2012. Available from: http://ash.confex.com/ash/2012/webprogram/Paper47060.html [Last updated on Dec 2012; Last accessed on Oct 2013].
- White HE, Matejtschuk P, Rigsby P, Gabert J, Lin F, Wang LY, et al. Establishment of the first World Health Organization International Genetic Reference Panel for quantitation of BCR-ABL mRNA. Blood 2010; 116:e111-7.
- Balasubramanian P, Chendamarai E, Markose P, Fletcher L, Branford S, George B, et al. International reporting scale of BCR-ABL1 fusion transcript in chronic myeloid leukemia: First report from India. Acta Haematol 2012;127:135-42.
- Jabbour E, Kantarjian HM, O′Brien S, Shan J, Quintas-Cardama A, Faderl S, et al. The achievement of an early complete cytogenetic response is a major determinant for outcome in patients with early chronic phase chronic myeloid leukemia treated with tyrosine kinase inhibitors. Blood 2011;118:4541-6.
- Jabbour E, Saglio G, Hughes TP, Kantarjian H. Suboptimal responses in chronic myeloid leukemia. Cancer 2012; 118:1181-91.
- Marin D, Milojkovic D, Olavarria E, Khorashad JS, Lavallade H, Reid AG, et al. European LeukemiaNet criteria for failure or suboptimal response reliably identify patients with CMLin early chronic phase treated with imatinib whose eventual outcome is poor. Blood 2008;112:4437-44.
- Ohm L, Arvidsson I, Barbany G, Hast R, Stenke L. Early landmark analysis of imatinib treatment in CML chronic phase:Less than 10


 PDF
PDF  Views
Views  Share
Share

