Importance of Cytohistological Correlation and Diagnostic Utility of Endoscopic Ultrasound in Gastric Glomus Tumor: A Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2019; 40(04): 576-578
DOI: DOI: 10.4103/ijmpo.ijmpo_61_18
Abstract
We present a case of gastric glomus tumor (GGT) in a 60-year-old female patient presented with progressive dysphagia for both solid and liquid diagnosed with the help of endoscopic ultrasound (EUS)-guided fine-needle aspiration cytology with histological correlation and detailed immunohistochemistry evaluation. Till date, only seven cases were correctly diagnosed by EUS-guided aspiration cytology. We report this case to highlight the cytological features and importance of EUS in diagnosing GGT.
Keywords
Endoscopic ultrasound - fine-needle aspiration cytology - gastric glomus tumor - immunohistochemistryPublication History
Received: 15 March 2018
Accepted: 21 June 2018
Article published online:
03 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
We present a case of gastric glomus tumor (GGT) in a 60-year-old female patient presented with progressive dysphagia for both solid and liquid diagnosed with the help of endoscopic ultrasound (EUS)-guided fine-needle aspiration cytology with histological correlation and detailed immunohistochemistry evaluation. Till date, only seven cases were correctly diagnosed by EUS-guided aspiration cytology. We report this case to highlight the cytological features and importance of EUS in diagnosing GGT.
Keywords
Endoscopic ultrasound - fine-needle aspiration cytology - gastric glomus tumor - immunohistochemistryIntroduction
Glomus tumor (GT) is a rare mesenchymal tumor arising from the neuroarterial structure known as glomus body and commonly located in the extremities and soft tissue.[1] Gastric GT (GGT) is a subepithelial tumor first described by de Busscher et al. There rarity and overlapping of clinical and conventional radiological features with the other more common submucosal gastric tumors result into difficult preoperative accurate diagnosis. Endoscopic ultrasound-guided fine-needle aspiration cytology (EUS-FNAC) emerged as one of the best modalities for preoperative diagnosis. However, in smaller lesion, inadequacy of the material may limit to perform immunohistochemistry (IHC) or cell block preparation and hurdle in confirmation of the diagnosis. Till date, to the best of our knowledge, only seven cases were correctly diagnosed by EUS-FNAC, possibly due to adequate material to perform IHC.[2] We describe a case of GGT in a 60-year-old female provisionally diagnosed on cytology with confirmation on histology and IHC panel.
Case Report
A 60-year-old female referred to the outpatient department of our hospital with the chief complaints of progressive dysphagia for both solid and liquid associated with pain for the last 4 months. She observed partial relief from the symptoms on intake of proton pump inhibitors. No history of any other associated symptoms such as vomiting, regurgitation, gastrointestinal (GI) bleed, malena, or jaundice was present. However, significant weight loss of about 5 kg was noted during the period of illness. Her hemogram and liver and kidney function tests were within normal limits. The upper GI endoscopy revealed extensive ulcerations in mid and lower third of the esophagus with a small hiatus hernia at the lower esophageal sphincter. The stomach showed a large submucosal mass lesion in the anterior wall of the body region. D1 and D2 mucosa appeared normal. Contrast-enhanced computed tomography showed a well-defined, rounded endoexophytic lesion with heterogeneously moderate enhancement measuring 26 mm × 25 mm and peripheral calcifications in the anterior wall of stomach [Figure 1a]. An EUS was also done along with FNAC, which showed a large predominantly hypoechoic lesion arising from the second layer of stomach measuring 2.6 cm × 2.2 cm [Figure 1b] and [c]. No penetration into the deeper layer was noted. The FNAC was reported as benign lesion suggestive of GT. A wide local excision of the lesion was planned. Intraoperatively, a 2 cm × 2 cm mass was seen in the body of the stomach which was excised with 1 cm margin [Figure 2]. A frozen section confirmation of the margin was done which was reported negative for malignancy. No lymph nodes were seen. Postoperative course was uneventful, and the patient was discharged after 5 days. Currently, at 1-year follow-up, the patient is fine and symptom-free.
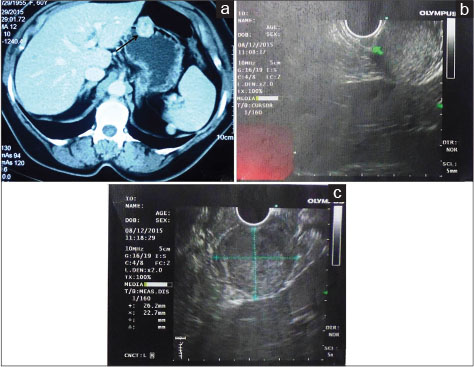
| Fig. 1 (a) Contrast-enhanced computed tomography scan showing a well-defined rounded endoexophytic lesion with heterogeneously moderate enhancement and peripheral calcifications in the anterior wall of stomach, (b and c) endoscopic ultrasound showing a large predominantly hypoechoic lesion arising from the second layer of the stomach. No penetration into the deeper layer was noted
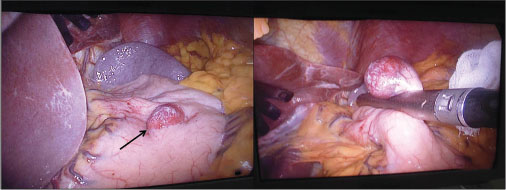
| Fig. 2 Intraoperative image showing a circumscribed globular mass in the body of the stomach
Pathological features
FNAC smears revealed a moderately cellular aspirate with few caught up smooth muscle fibers [Figure 3a] in the background. The tumor was comprised of monomorphic round-to-oval cells arranged in tiny clusters. The cells were displaying hyperchromatic to vesicular chromatin with occasional prominent nucleoli [Figure 3b], arrow] and moderate amount of eosinophilic cytoplasm [Figure 3c]. No significant pleomorphism, spindling, epithelioid appearance, salt and pepper chromatin, mitosis, or necrosis was seen. However, due to limitations of aspirate, further IHC evaluation or cell block making was not feasible. Considering the clinical, radiological, and cytological features together, a provisional diagnosis of GT was made and a histological correlation was advised.
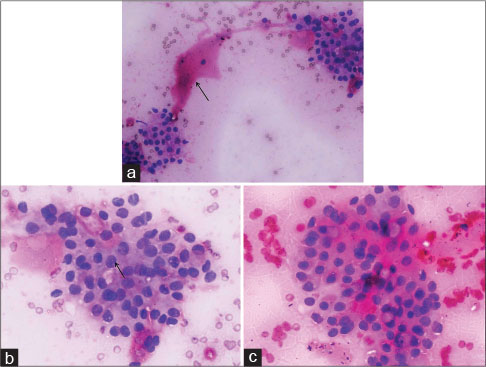
| Fig. 3 Fine-needle aspiration cytology smears displaying (a) a moderately cellular aspirate with few caught up smooth muscle fibers (arrow) in the background (Giemsa, ×200), (b) tumor comprises monomorphic round-to-oval cells showing hyperchromatic to vesicular nuclei with occasional prominent nucleoli (arrow) and moderate amount of eosinophilic cytoplasm (Geimsa, ×400) and (c) (H and E, ×400)
On gross examination, tumor appeared as circumscribed globular mass measuring 25 mm × 20 mm. On microscopy, hematoxylin and eosin-stained sections showed a cellular tumor located in the muscularis propria of the body of the stomach [Figure 4a]. The cells were round-to-oval with vesicular chromatin, prominent nucleoli [Figure 4b], and moderate amount of eosinophilic cytoplasm. No mitosis or necrosis was seen. An IHC panel was applied to differentiate GT from GI stromal tumor and carcinoid. The cells revealed diffuse positivity for smooth muscle actin, HHF-35, caldesmon, and synaptophysin (weak positive). However, CD117, CD34, chromogranin-A, desmin, and S-100 were negative [Figure 4c], [d], [e], [f]. The MIB-1 labeling index was approximately 1%–2%. Based on morphology and IHC, final diagnosis of benign GT was offered.
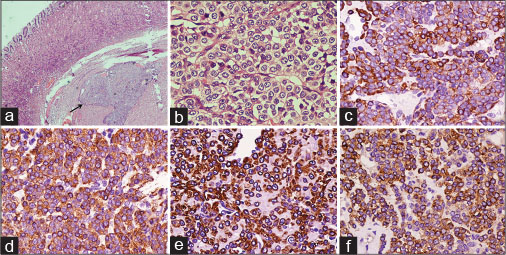
| Fig. 4 Photomicrographs showing (a) a cellular tumor located in the muscularis propria of the body of the stomach (H and E, ×40), (b) cells appear round-to-oval with vesicular chromatin, prominent nucleoli, and moderate amount of eosinophilic cytoplasm (H and E, ×400), and immunopositivity for (c) HHF-35, (d) smooth muscle actin, (e) caldesmon, and (f) synaptophysin (IHC, ×400)
Discussion
GGTs are very rare which account for 1% of mesenchymal tumors of the GI tract.[3] GGT usually occurs in the fifth to sixth decade of life and shows a female preponderance. They may be detected incidentally or presents with nonspecific symptoms such as upper abdominal pain, vomiting, or upper GI bleeding. These are mostly solitary, circumscribed, arise from the submucosa or muscularis propria and show a predilection for antrum. On computed tomography, they show a strong enhancement in the arterial phase which persists in the portal venous and delayed phases, thus reflecting their hypervascular nature.[4] However, this radiological feature is not typical of GT and shared by more common carcinoid as well as GI stromal tumors. Similarly, on EUS imaging, they appear as hypoechoic well-defined mass which is common to other mesenchymal tumor. Therefore, imaging modalities are not very useful for certain diagnosis of these lesions. However, incorporation of FNAC in EUS mostly achieved a preoperative diagnosis and overcame the limitations of imaging which is necessary for the optimal surgical interventions.
On cytology smear, GGT appears as sheets of round-to-oval monomorphic cells with hyperchromatic nuclei, inconspicuous nucleoli, and moderate amount of cytoplasm. Few naked nuclei may also be seen. However, salt and pepper chromatin typical characteristic of neuroendocrine tumors is absent which is an important clue to differentiate the two most logical and common differentials. In our case, based on cytological features, we made a provisional diagnosis of GT, due to inadequacy of material to perform immunocytochemistry or cell block preparation. The diagnosis was later confirmed on histological examination and IHC of the resected specimen. GGT shows immunopositivity for vimentin, smooth muscle actin, HHF-35, and caldesmon. Sometimes, a weak nonspecific positivity for synaptophysin may be noted which was also seen in the present case.[5] However, other neuroendocrine markers such as chromogranin-A, CD56, or PGP9.5 do not reveal positivity.
GGTs are mostly benign neoplasms classified into three types based on different components as solid GT, glomangioma, and glomangiomyoma.[6] Folpe et al.[7] proposed the criteria for malignant GT which includes deep location, size ≥2 cm, moderate-to-high nuclear grade, atypical mitotic figures, and mitotic figures ≥5/50 high-power fields. GGTs have a different clinical course in comparison to peripherally located soft-tissue GTs. Thambi et al.[8] suggested that size >5 cm is more important than cellular atypia and mitotic activity in GGTs. A wide local resection, mostly laparoscopic with negative margins, is the treatment of choice. However, large tumor size or features suggesting a malignant potential may lead to a subtotal gastrectomy.
To conclude, GGT is usually a benign mesenchymal tumor which can be correctly diagnosed by EUS-FNAC, provided adequacy of the aspirate in addition to localizing the layer of its origin. Correct preoperative diagnosis is required to perform an optimum conservative wide local excision and avoid any major surgical intervention.
Conflict of Interest
There are no conflicts of interest.
References
- 1 Tsuneyoshi M, Enjoji M. Glomus tumor: A clinicopathologic and electron microscopic study. Cancer 1982; 50: 1601-7
- 2 Kato S, Kikuchi K, Chinen K, Murakami T, Kunishima F. Diagnostic utility of endoscopic ultrasound-guided fine-needle aspiration biopsy for glomus tumor of the stomach. World J Gastroenterol 2015; 21: 7052-8
- 3 Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol 2002; 26: 301-11
- 4 Hu SD, Hu DM, Huang W, Chen KM, Song Q. Computed tomography and clinical characteristics of gastric glomus tumors. J Dig Dis 2014; 15: 477-82
- 5 Wang ZB, Yuan J, Shi HY. Features of gastric glomus tumor: A clinicopathologic, immunohistochemical and molecular retrospective study. Int J Clin Exp Pathol 2014; 7: 1438-48
- 6 Folpe AL. Glomus tumor. In: Fletcher CD, Unni KK, Mertens F. editors Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002: 133-4
- 7 Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol 2001; 25: 1-2
- 8 Thambi R, Sheeja S, Joesph CP, Poothiode U. Gastric glomus tumor: A brief report. Indian J Pathol Microbiol 2014; 57: 509-10
Address for correspondence
Publication History
Received: 15 March 2018
Accepted: 21 June 2018
Article published online:
03 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig. 1 (a) Contrast-enhanced computed tomography scan showing a well-defined rounded endoexophytic lesion with heterogeneously moderate enhancement and peripheral calcifications in the anterior wall of stomach, (b and c) endoscopic ultrasound showing a large predominantly hypoechoic lesion arising from the second layer of the stomach. No penetration into the deeper layer was noted

| Fig. 2 Intraoperative image showing a circumscribed globular mass in the body of the stomach

| Fig. 3 Fine-needle aspiration cytology smears displaying (a) a moderately cellular aspirate with few caught up smooth muscle fibers (arrow) in the background (Giemsa, ×200), (b) tumor comprises monomorphic round-to-oval cells showing hyperchromatic to vesicular nuclei with occasional prominent nucleoli (arrow) and moderate amount of eosinophilic cytoplasm (Geimsa, ×400) and (c) (H and E, ×400)

| Fig. 4 Photomicrographs showing (a) a cellular tumor located in the muscularis propria of the body of the stomach (H and E, ×40), (b) cells appear round-to-oval with vesicular chromatin, prominent nucleoli, and moderate amount of eosinophilic cytoplasm (H and E, ×400), and immunopositivity for (c) HHF-35, (d) smooth muscle actin, (e) caldesmon, and (f) synaptophysin (IHC, ×400)
References
- 1 Tsuneyoshi M, Enjoji M. Glomus tumor: A clinicopathologic and electron microscopic study. Cancer 1982; 50: 1601-7
- 2 Kato S, Kikuchi K, Chinen K, Murakami T, Kunishima F. Diagnostic utility of endoscopic ultrasound-guided fine-needle aspiration biopsy for glomus tumor of the stomach. World J Gastroenterol 2015; 21: 7052-8
- 3 Miettinen M, Paal E, Lasota J, Sobin LH. Gastrointestinal glomus tumors: A clinicopathologic, immunohistochemical, and molecular genetic study of 32 cases. Am J Surg Pathol 2002; 26: 301-11
- 4 Hu SD, Hu DM, Huang W, Chen KM, Song Q. Computed tomography and clinical characteristics of gastric glomus tumors. J Dig Dis 2014; 15: 477-82
- 5 Wang ZB, Yuan J, Shi HY. Features of gastric glomus tumor: A clinicopathologic, immunohistochemical and molecular retrospective study. Int J Clin Exp Pathol 2014; 7: 1438-48
- 6 Folpe AL. Glomus tumor. In: Fletcher CD, Unni KK, Mertens F. editors Pathology and Genetics of Tumours of Soft Tissue and Bone. Lyon, France: IARC Press; 2002: 133-4
- 7 Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: Analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol 2001; 25: 1-2
- 8 Thambi R, Sheeja S, Joesph CP, Poothiode U. Gastric glomus tumor: A brief report. Indian J Pathol Microbiol 2014; 57: 509-10


 PDF
PDF  Views
Views  Share
Share

