Immune Checkpoint Blockers and Ovarian Cancer
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(02): 182-189
DOI: DOI: 10.4103/ijmpo.ijmpo_73_16
Abstract
Although the idea, called “cancer immunotherapy,” is very appealing and has previously been shown to work in several mouse models of cancer, it has in general been very difficult to translate cancer immunotherapy approaches to humans. Because of this frustration, by the 1990s, many scientists and biotechnology companies had given up on the idea of cancer immunotherapy. After few years, first detection T-cell suppression of effect of cytotoxic T-lymphocyte antigen-4 (CTLA4) molecule was established. Antibody (Ab) to CTLA4 could increase T-cell starting a completely new age of tumor immunology. Immune checkpoints are new ways in manipulation of immunological control over malignant tumors. It has lent an important measure to manage, especially recurrent and refractory cancers and those cancer where there is an unmet need like recurrent melanoma, renal cell carcinoma, and recurrent ovarian cancer. As a new development, this subject is experiencing rapid progress, and multiple avenues are opening up. Although there are many hurdles to overcome this needs constant updating, especially for students of ovarian cancer who are looking at it with much hope.
Publication History
Article published online:
06 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Although the idea, called “cancer immunotherapy,” is very appealing and has previously been shown to work in several mouse models of cancer, it has in general been very difficult to translate cancer immunotherapy approaches to humans. Because of this frustration, by the 1990s, many scientists and biotechnology companies had given up on the idea of cancer immunotherapy. After few years, first detection T-cell suppression of effect of cytotoxic T-lymphocyte antigen-4 (CTLA4) molecule was established. Antibody (Ab) to CTLA4 could increase T-cell starting a completely new age of tumor immunology. Immune checkpoints are new ways in manipulation of immunological control over malignant tumors. It has lent an important measure to manage, especially recurrent and refractory cancers and those cancer where there is an unmet need like recurrent melanoma, renal cell carcinoma, and recurrent ovarian cancer. As a new development, this subject is experiencing rapid progress, and multiple avenues are opening up. Although there are many hurdles to overcome this needs constant updating, especially for students of ovarian cancer who are looking at it with much hope.
Introduction
Immune checkpoints are many inhibitory pathways connected to the immune system those are crucial for maintaining self-tolerance. They modulate the duration and amplitude of physiological immune responses in peripheral tissues to minimize collateral tissue damage. The blockade of immune checkpoints has become one of the most promising approaches to activating therapeutic anti-tumor immunity.[1] This is T-lymphocyte-mediated unique tumor-specific immunity with complex pathways. Rapidity of the development of drugs acting through these pathways has been spectacular. There is a great expectation from this modality in treatment. Approval has already come from Food and Drug Administration (FDA) for melanomas and squamous and nonsquamous non-small cell lung cancer (SCLC). Ovarian cancer is other major area where immunity was shown to play major role. Hence, trials are on for this indication. The initial euphoria of discovery of drugs of the century is taken over by the despair of hitherto low response. However, a greater challenge waits us and that is to find the reason of such stable and durable response of only about 10%–20%. It has not been possible till date to increase this even using two or more such agents covering broader area of the mechanism of action. Currently, they are combined with other targeted therapies such as poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitor and also with different chemotherapies. While good markers need to be developed to find out the subset of people who will be benefited from such modality, extra effort seems to be needed to pinpoint reason of such truncated effect and more so to detect ways to increase cure rate subverting the weakness involved. Being a significant footstep toward understanding the process of neoplasia and is control, it seems worthwhile to take a close look in this subject of immune checkpoint and its use in ovarian cancer; hence, this review.
Major Pathways
Regulation of T-lymphocyte-mediated tumor-specific immunity is, however, through highly complex pathways which include both inhibitory as well as stimulatory processes.[2] Cancer antigens are either released by cell death or as such present on membrane in a variety of aggressive cancer cells. Dendritic cells (DCs or antigen presenting cell [APC]) recognize, take up, process, and present the tumor antigens from dead cell to the immune system. Major histocompatibility complex (MHC) Class I (short peptides of 8–9 amino acids) expresses them in activated CD8+ cells while MHC Class II (longer peptides of 14–20 amino acids) works by activating CD4+ T-cells. Activated DC induces robust cross-priming of tumor antigen–specific T-cells within the draining lymph node, leading to systemic infiltration of both treated and distant tumor sites. CD8+ T-cells thus migrating back to tumor sites enter the tumor microenvironment (TME). There, CD8+ T-cells recognize tumor cells and lyse them, releasing additional tumor antigens, and perpetuating the cycle.
A variety of positive and negative signals can promote or hinder this process at each step [Figure 1]. The regulation of T-cell activation historically requires two signals. Signal one is transmitted after recognition of antigen in the context of MHC by the T-cell receptor (TCR). However, MHC binding is insufficient for producing a T-cell response by itself. In fact, lack of further stimulatory signals sends the T-cell into anergy. Signal two is a positive signal transmitted by B7 (CD80/86) binding to CD28 balanced by a negative signal transmitted by B7 binding to cytotoxic T-lymphocyte antigen-4 (CTLA4).[3] Thus, CD28 and CTLA4 each interact with both B7-1 and B7-2. B7 is a type of peripheral membrane protein found on activated APCs that, when paired with either a CD28 or CD152 (CTLA4) surface protein on a T-cell, can produce a costimulatory signal or a coinhibitory signal to enhance or decrease the activity of an MHC-TCR signal between the APC and the T-cell, respectively.[4] Blockade of CD28 by B7 binding is effective in stopping T-cell activation, but CTLA4 (CD152) has twenty times greater affinity than CD28 for B7 proteins. Besides being present on activated APCs, B7 is also found on T-cells themselves.[5] Cytokines can also contribute to T-cell activation, called “signal 3,” further research has revealed an array of positive and negative regulatory signals in addition to the basic one as described for T-cell activation.
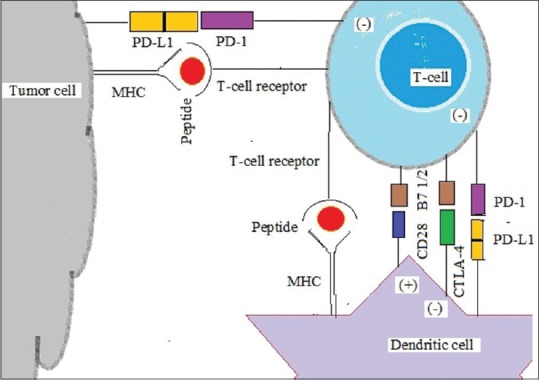
| Figure 1:Immune checkpoints in T cell, dendritic cell and tumour cell
Other Pathways
Other most important pathway is the programmed cell death protein -1 (PD-1) receptor mediated. It is also known as CD279 and is expressed on the surface of activated T-cells whereas its ligands, PD ligand-1 (PD-L1) (B7-H1; CD274) and PD-L2 (B7-DC; CD273) are commonly expressed on the surface of DCs or macrophages. PD-1 and PD-L1/PD-L2 belong to the family of immune checkpoint proteins that act as coinhibitory factors, which can halt or limit the development of the T-cell response. PD-1/PD-L1 interaction ensures that the immune system is activated only at the appropriate time to minimize the possibility of chronic autoimmune inflammation.
When PD-L1 binds to PD-1, an inhibitory signal is transmitted into the T-cell, which reduces cytokine production and suppresses T-cell proliferation. Tumor cells exploit this immune-checkpoint pathway as a mechanism to evade detection and inhibit the immune response.
PD-L1 is commonly overexpressed on tumor cells or on nontransformed cells in the TME (Pardoll, 2012). PD-L1 expressed on the tumor cells binds to PD-1 receptors on the activated T-cells, which leads to the inhibition of the cytotoxic T-cells. These deactivated T-cells remain inhibited in the TME. The PD-1/PD-L1 pathway represents an adaptive immune resistance mechanism that is exerted by tumor cells in response to endogenous antitumor activity.
In regulation of T-cells by immune checkpoint, the net signal shapes the strength and quality of the T-cell response.
While both CTLA4 and PD-1/PD-L1 are coinhibitory pathways, there are a number of costimulatory pathways such as CD28 as well. Five of them are of tumor necrosis factors (TNF) receptor superfamily such as CD27, CD40, OX40, glucocorticoid-induced TNF receptor (GITR), and CD137. Another two stimulatory checkpoint molecules belong to the B7-CD28 superfamily - CD28 itself and inducible costimulator (ICOS). Still, another simulator CD122 is the interleukin (IL)-2 receptor beta subunit. All of them promoted T-cell proliferation. CD28 was the target of the TGN1412 “superagonist” which caused severe inflammatory reactions in the first-in-man study in London in March 2006.[6]
All other costimulatory molecules including antagonists of their antibodies are subject of intense research effort by many pharmaceutical companies. Celldex Therapeutics is working on CDX-1127, an agonistic anti-CD27 monoclonal Ab (mAb)[7] which in animal models has been shown to be effective in the context of TCR stimulation[8] CD40. This molecule found on a variety of immune system cells, including APCs has CD40L. The Swiss pharmaceutical company Roche acquired this project of anti-CD40 agonist mAb when VLST was shut down in 2013.[9]
CD137, also called 4-1BB, when bound by CD137 ligand, the result is T-cell proliferation. The German biotech company Pieris Pharmaceuticals has developed an engineered lipocalin that is bispecific for CD137 and human epidermal growth factor receptor 2 HER2.[10]
Anti-OX40 monoclonal antibodies have been shown to have clinical utility in advanced cancer.[11]
The pharma company AstraZeneca has three drugs in development targeting OX 40. TG Therapeutics is working on anti-GITR antibodies which has shown to promote an anti-tumor response through the loss of regulatory T-cell (Treg) lineage stability.[12]
Nektar Therapeutics is working on NKTR-214, a CD122-based immune-stimulatory cytokine to increase the proliferation of CD8+ effector T-cells. Jounce Therapeutics is developing an ICOS agonist for its role in T-cell effector function.
Among inhibitory checkpoint molecules, most important are CD152 or CTLA4 and PD-1. Others are adenosine 2A receptor, B7-H3, B7-H4, B- and T-lymphocyte attenuator, indoleamine 2,3-dioxygenase (IDO), killer-cell immunoglobulin-like receptor (KIR), lymphocyte activation gene-3, T-cell immunoglobulin (Ig)- and mucin-domain-containing molecule-3, and V-domain Ig suppressor of T-cell activation. While first two are described others, molecules are also used IDO is known to suppress T- and NK-cells, generate and activate Tregs and myeloid-derived suppressor cells (MDSCs), and promote tumor angiogenesis. NewLink Genetics[13] and Incyte.[14]
Drugs or drug candidates that inhibit/block the inhibitory checkpoint molecules (above) are confusingly sometimes known as immune checkpoint inhibitors.
Chemokines promote the trafficking of T-cells to tumors, where selectins and leukocyte function-associated antigen-1 or the vascular endothelial growth factor (VEGF) and the endothelin B receptor can promote or deter T-cell infiltration into the tumor.[15] Within the tumor, a variety of immune suppressive influences inhibits T-cell activity. These include immune suppressive cells (CD4+FOXp3+ Tregs and MDSCs), immune suppressive cytokines (transforming growth factor-β [TGF-β] and IL-10), metabolic enzymes (IDO and arginase), and immune checkpoints PD-L1.[1,16] Tumor cells can co-opt immune checkpoint pathways to evade the T-cell response, expressing PD-L1 on their surface as the result of constitutive oncogenic signaling,[17] epithelial to mesenchymal transition,[18] or adaptive resistance to immune attack.[19] Regardless of the mechanism, tumor cell surface PD-L1 expression provides a means of evading active immunity.[19,20] Developing strategies for abrogating these immune suppressive mechanisms to support CD8+ T-cell activity at the tumor site is critical for the success of immunotherapy.
Ovarian Cancer Scenario
More than three decades back, T-cell infiltration was noticed in ovarian cancers.[21] In 2003, Zhang et al. appreciated their role in improved survival. They observed at least 60%-benefit in 5 year-survival in a cohort of 74 patients who were treated well with a complete clinical response after debulking and platinum-based therapy. CD3+ T-cells within their tumor and monokines induced by interferon (IFN)-γ plus macrophage-derived chemokines made the difference.[22] The reason of such heterogeniety is still unclear though subject has grown and gained much strength. While Treg subsets (CD4+) has confusing immune suppressing role as Tregs improved survival is noticed in patients who had higher numbers of intraepithelial CD8+ T-cells compared with patients without intraepithelial CD8+ T-cells (median survival 55 vs. 26 months).[23,24] Strong positive correlation is observed between the levels of CD8+ T-cells and granzyme B within tumors.[25] MHC-related interferon regulatory factor-1 and metastasis-related chemokine receptor (CXCR) 6 are the two genes differentially expressed in tumors with high versus low CD8+ T-cell infiltration. Heterogeneity in the TME among patients with epithelial ovarian cancer (EOC), and various immune cell populations those have been associated positively or negatively with clinical prognosis, includes tumor-infiltrating lymphocytes (TILs),[22,23,26] MDSCs,[27] and tumor-associated macrophages.[28] TILs express the negative regulatory immune receptor PD-1,[29] which is upregulated on T-cell activation and suppresses T-effector functions, whereas several cellular populations, including cancer cells and tumor-associated myeloid cells, express its ligand PD-L1.[30,31,32,33] Expression of PD-L1 by tumors has been associated with decreased intraepithelial TILs and poor overall survival (OS) in EOC.[33]
In an immune-competent murine model of EOC, PD-1 and PD-L1 blockade has led to eradication of tumors through the expected reprograming of the TME,[34] which suggests potential benefit from PD-1/PD-L1 inhibition for patients with EOC. The attenuation of T-cell function by CTLA4 was also noticed in EOC.[35] Although the binding of B7-1 or B7-2 to CD28 provides an important costimulatory signal, the engagement of CTLA4 by these ligands induces cell cycle arrest and diminished cytokine production.[36,37,38]
Documented effort to test ovarian cancer started with Hodi et al.,[39] who gave two patients a single infusion and then nine cases[40] up to 11 infusion of 3 mg/kg ipilimumab after an autologous ovarian tumor cell vaccine transduced with granulocyte-macrophage colony-stimulating factor (GM-CSF) (GVAX). Only three patients had stable disease (SD) of >2 months. A phase II clinical trial of ipilimumab in relapsed platinum-sensitive ovarian cancer with measurable disease is ongoing though not recruiting any more (NCT01611558). Some trials either in posttransplantation or along with other checkpoint molecules such as nivolumab and pembrolizumab are recruiting. There are other two trials which are either suspended or terminated. The PD-1 antagonist nivolumab is tested at 1 or 3 mg/kg every 2 weeks in 18 patients with relapsed platinum-resistant disease regardless of PD-L1 expression; there was a 17%-overall response rate (ORR) and a 44%-disease control rate (DCR = complete response [CR] + partial response [PR] + SD), with 2 CR, 1 PR, and 5 patients with SD.[41] A phase Ib study tested the PD-1 antagonist pembrolizumab at 10 mg/kg every 2 weeks in 26 patients with heavily treated PD-L1+ ovarian cancer chemotherapy.[42] There was a durable ORR of 11.5%, and a DCR of 34.6%, with 1 CR, 2 PR, and 6 patients with SD. Now, pembrolizumab has highest number of clinical trials in ovarian cancer. The PD-L1 antagonist avelumab was given at 10 mg/kg every 2 weeks in a phase Ib study of 75 patients with platinum-resistant or chemotherapy-refractory ovarian cancer regardless of PD-L1 expression[43] with an ORR of 10.7%-and a DCR of 54.7%. A phase I study of another PD-L1 antagonist, BMS-936559, revealed one objective response in 17 ovarian cancer patients.[44] It is evident from discussion that much obstacles are remaining in establishing immunotherapy in ovarian cancer as response rate is low, and no FDA approval is in the offing. Expectedly, Professor Maurie Markman has commented at the 33rd Annual Chemotherapy Foundation Symposium “The checkpoint inhibitors are not ready for prime time yet in ovarian cancer. It's not because there's evidence that they don’t work – it's just that there's no evidence at all.”
New Drugs of Immunotherapy
Ipilimumab
Brunet et al.,[45] of Institut national de la santé et de la recherche médicale, France (Inserm) came across complementary DNA (cDNA) clones defining a sequence, CTLA4, which could encode a 223-amino-acid protein while screening mouse cytolytic-T-cell-derived cDNA libraries. Jim Allison of Berkley with graduate student Max Krummel and postdoctoral fellow Cynthia Chamber were able to provide evidence that CTLA4 actually served to inhibit the activity of T-cells. That was beginning. They showed in 6-week-old golden Syrian hamsters who received five footpad injections of heat-killed Staphylococcus A bacteria coated with CTLA4 Ig and suspended in 0.2 ml phosphate-buffered saline. Three days after the final injection, draining lymph nodes were removed, and lymphocytes were isolated and fused with the P3 × 3. Ag8.653 myeloma line using a standard polyethylene glycol fusion technique. Hybridoma supernatants were tested for reactivity to CTLA4 Ig and for lack of reactivity to CD4 Ig by ELISA thus developing first anti-CTLA4 Ab.[46] Medarex (former NASDAQ symbol: MEDX) an American biopharmaceutical company based in Princeton, New Jersey, developed anti-CTLA4 mAb, MDX-010. In 2009, Medarex was purchased by Bristol-Myers Squibb.
The anti-CTLA4 Ab (MDX-010; provided by Medarex) is a fully human IgG1κ Ab derived from transgenic mice having human genes encoding heavy and light chains to generate a functional human repertoire. This Ab has been shown to bind to CTLA4 expressed on the surface of human T-cells and inhibit binding of CTLA4 to B7 molecules.[47] It is the first anti-CTLA4 agent in clinical development. It was approved by the US FDA in 2011 and the European Medicines Agency for the treatment of metastatic melanoma following research showing improved survival.[48] Ipilimumab antagonizes CTLA4 and prevents ligand binding.[49] It is undergoing clinical trials for the treatment of non-SCLC (NSCLC), SCLC,[50] bladder cancer,[51] and metastatic hormone-refractory prostate cancer.[52]
Tremelimumab
Tremelimumab (formerly CP-675,206) is a human IgG2 mAb specific for CTLA4. In the phase III trial in advanced melanoma, 655 patients were enrolled and randomly assigned to treatment with tremelimumab or chemotherapy. Previously, in the development by Pfizer,[53] it is now in investigation by MedImmune, a wholly owned subsidiary of AstraZeneca.[54] Unlike ipilimumab which is an IgG1 isotype, tremelimumab is an IgG2 isotype[55] and has not attained approval for any.
Programed cell death 1-targeting agents
Nivolumab (ONO-4538, BMS-936558, or MDX1106), marketed as OPDIVO, is a human IgG4 anti-PD-1 mAb developed by Ono Pharmaceutical and Medarex (later acquired by Bristol-Myers Squibb) for the treatment of cancer. It is a fully human IgG4 mAb targeting PD-1. It is approved by the FDA for the treatment of patients with unresectable or metastatic melanoma who no longer respond to other drugs (December 2014). In addition, it is approved for the treatment of squamous NSCLC (March 2015). Patients on the trial had advanced melanoma, NSCLC, castration-resistant prostate cancer, renal cell cancer (RCC), or colorectal cancer. Patients received nivolumab at doses of 0.1–10.0 mg/kg of body weight every 2 weeks for up to 12 cycles until disease progression or a CR occurred.
Pembrolizumab
On September 4, 2014, the US FDA approved pembrolizumab under the FDA Fast Track Development Program.[56] It is approved for use following treatment with ipilimumab or after treatment with ipilimumab and a BRAF inhibitor in advanced melanoma patients who carry a BRAF mutation.[57] It is marketed by Merck. Pembrolizumab (MK-3475, formerly lambrolizumab) is a pembrolizumab has been very successful in treating melanoma and NSCLC, similar to nivolumab. Significant differences cannot be assessed in the absence of a randomized trial comparing the two agents. However, binding affinities of the agents are different. In phase I trials, neither agent has been found to have a maximally tolerated dose. That said, more time and energy have been spent on searching for an appropriate dose for pembrolizumab. Pembrolizumab was invented by Gregory Carven, Hans van Eenennaam, and John Dulos at Organon BioSciences which later became Schering-Plough Research Institute and then Merck and Co.[58] MRC Technology humanized the Ab pembrolizumab for Organon in 2016. On October 2, 2015, the US FDA approved pembrolizumab for the treatment of metastatic NSCLC in patients whose tumors express PD-L1 and who have failed treatment with other chemotherapeutic agents.
Pidilizumab
Pidilizumab is a humanized IgG1 Ab targeting PD-1. The agent was initially evaluated in a phase I trial targeting hematologic malignancies. At present, there are a number of clinical trials underway in both hematologic and solid tumors.[59]
The results of two pidilizumab clinical trials were recently published in peer-reviewed journals. In a single-center, single-arm, phase II trial, 32 patients with relapsed follicular lymphoma received pidilizumab at a dose of 3 mg/kg every 4 weeks for four infusions with up to eight additional infusions administered. In addition, rituximab was given at a dose of 375 mg/m2 of body surface area every week for 4 weeks. Investigators reported that 19 of 29 evaluable patients achieved an objective response, with CRs in 15 patients (51.7%).[60] An additional phase II trial involved patients with diffuse large B-cell lymphoma following autologous hematologic stem cell transplantation (AHSCT). Sixty-six patients were treated with three doses of pidilizumab in the first 1–3 months after AHSCT. The PFS rate was 72% at 6 months after AHSCT (90% confidence interval, 60%–82%), meeting the primary end-point. Thirty-five patients had measurable disease following AHSCT, and the response rate in those patients was 51%.[61]
Programed cell death ligand 1-targeting agents
PD-L1 inhibitors are currently undergoing clinical trials for the treatment of various types of cancer; however, no PD-L1 inhibitors have since been approved by the FDA.
Atezolizumab (MPDL3280A)
MPDL3280A is an engineered human IgG1 mAb that targets PD-L1. Combining unique property to eliminate Ab-dependent cell-mediated cytotoxicity (ADCC) effector function, MPDL3280A, unlike some other anti-PD-L1 antibodies, does not deplete cells expressing PD-L1. It appears to have significant activity in a subset of patients with NSCLC. This was demonstrated in phase I trial of atezolizumab, in which 53 patients with NSCLC had a 23% response rate where PD-L1 expression is shown; thus PD-L1 might even be a immunohistochemical biomarker with some degree of predictive capability. The POPLAR trial compared atezolizumab with docetaxel in the second- or third-line setting in patients with NSCLC, regardless of histology where OS benefit with atezolizumab compared with docetaxel in patients with a high level of PD-L1 expression.
Durvalumab (MEDI4736)
MEDI4736 is a human IgG1 mAb recognizing human PD-L1. It is similar to MPDL3280A in eliminating complement-mediated cytotoxicity and ADCC due to mutations in the Fc receptor. A phase I dose of 10 mg/kg every 2 weeks is currently being evaluated in several histologies in an expansion phase.[62] Brahmer et al. treated 13 NSCLC patients with MEDI4736, with three PRs[63] as presented in 2014 American Society of Clinical Oncology Annual Meeting. Several large combination trials in lung cancers are ongoing. At present, five trials are aimed at ovarian cancer.
Avelumab
Avelumab (MSB0010718C) is a fully human monoclonal PD-L1 Ab of isotype IgG1, currently in development by Merck KGaA, Darmstadt, Germany, and Pfizer for NSCLC.[64] MSB0010718C is IgG1 targeting PD-L1. It is a native Fc receptor allowing for ADCC.
Avelumab binds to the PD-L1 and inhibits binding (PD-1). Formation of a PD-1/PD-L1 receptor/ligand complex is prevented leading to increased CD8+ T-cell-mediated immune response.[65]
BMS-936559
BMS-936559 or MDX-1105 is a high-affinity, fully human IgG4 mAb that binds PD-L1, and that blocks PD-L1 from binding its two known receptors PD-1 and CD8.[44,66] It was safe in a phase I trial that included 17 ovarian cancer patients in escalating doses of 0.3–10 mg/kg intravenous every 14 days in 6-week cycles for up to 16 cycles and achieved objective responses: 1 (6%) with a PR and 3 (18%) with SD lasting more than 24 weeks. Common side effects included fatigue, infusion reactions, diarrhea, arthralgia, pruritus, rash, nausea, and headache.
Enoblituzumab
Enoblituzumab is a mAb designed for the treatment of cancer. Formerly known as MGA271, the drug is a humanized IgG1κ mAb recognizing human B7-H3.
Varlilumab
Varlilumab is fully human monoclonal agonist anti-CD27 mAb that has been shown to activate human T-cells in the context of TCR stimulation. Potent antitumor responses, it may be particularly effective in combination with other immunotherapies. In addition to the immune enhancing properties of varlilumab, the mAb may also provide direct therapeutic effects against tumors with CD27 expression. Human B- and T-cell lymphomas often express CD27 at high levels, and varlilumab has shown potent antitumor activity against these types of tumors in preclinical models. Therefore, in patients with lymphomas/leukemia that express CD27, varlilumab may function through two-independent mechanisms.
Lirilumab
Lirilumab (INN) is a human mAb designed to binds to KIR 2DL1/2L3.[67] This drug was developed by Innate Pharma and is licensed to Bristol-Myers Squibb.
Epacadostat
Epacadostat is an orally available hydroxyamidine. It inhibits IDO an enzyme responsible for the oxidation of tryptophan into kynurenine with potential immunomodulating and antineoplastic activities. Epacadostat targets and binds to IDO. Thus, it increases and restores the proliferation and activation of various immune cells, including DCs, NK cells, and T-lymphocytes, as well as IFN production, and a reduction in tumor-associated Tregs.
Combinations of Immunotherapeutic Agents
Combinations can be of three types where either (a) two even three immune checkpoint inhibitors are used, (b) other targeted therapy is used, or (c) chemotherapy and other standard treatments are used. Other than the promising single-agent indications mentioned above, immune checkpoint inhibition is not effective in a majority of patients. Newer combination strategies are planned and executed. In one such trial, tremelimumab and interferon were administered concurrently in standard doses, followed by maintenance interferon. Of the 33 evaluable patients, there were three CRs and seven PRs. The median OS was 15.9 months.[68] One area of remarkable success involved the use of nivolumab and ipilimumab in patients with metastatic melanoma. A randomized phase II trial of ipilumumab investigated the potential synergy of adding GM-CSF (or sargramostim) with ipilumumab.[69] The response rate was 53%. The combination of ipilimumab and nivolumab has subsequently been evaluated in a variety of malignancies. In RCC, two variations of the combination were tested. The same regimen was evaluated in NSCLC, with disappointing results. Carefully tailor regimens to specific patient populations and lower doses is planned now (1 mg/kg of ipilimumab and 1 mg/kg of nivolumab.[70]
Among recent combinations used in advanced and refractory ovarian cancer, safety study has started for iIpilimumab in combination with MGA271 (enoblituzumab). A phase I/II, open-label study is recruiting where nivolumab monotherapy or nivolumab combined with Ipilimumab are used in subjects with advanced or metastatic solid tumors. Tremelimumab is combined with PARP-inhibitor in BRCA-deficient ovarian cancer and also with either durvalumab or durvalumab plus first-line chemotherapy in advanced solid tumors, including ovarian cancer.
A study of the safety, tolerability, and efficacy of epacadostat administered in combination with nivolumab and a dose escalation and cohort expansion study of anti-CD27 (varlilumab) and anti-PD-1 (nivolumab) in select advanced cancers includes ovarian cancer patients. Pembrolizumab is becoming very popular as at least seventeen trial are planned or recruiting in combination with MGA271, epacadostat, niraparib, acalabrutinib (ACP-196), CSF-1 receptor inhibitor PLX3397 (pexidartinib), VEGF inhibitor Ziv-aflibercept, pegylated recombinant human IL-10 (AM0010), and standard chemotherapy in ovarian cancer.
Avelumab in combination with pegylated liposomal doxorubicin is starting. Durvalumab and pembrolizumab with tremelumumab or VTX-2337 (a novel Toll-like receptor 8 agonist), with olaparib or cediranib is being tried. Recently, atezolizumab plus bevacizumab with or without acetylsalicylic acid are tried in a recurrent platinum-resistant ovarian cancer trial.
Conclusions
Handling immunological therapy has never been very smooth. The glaring mechanism of action and efficacy was a breakthrough of the year in 2013 which was true at least for melanoma.[71] The subject being very interesting work hard is needed to find out what more is needed to get even better clinical effect. However, before that, translational scientists and clinical investigators should address efficiently a variety of important clinical and scientific questions regarding tumor microenvironment and interactions of tumor biology with human immunology. As combinations are already been tried and had shown hope experience and experimentation with these drug may bring out a bigger breakthrough if not a medical breakthrough of the century.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64.
- Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013;39:1-10.
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996;14:233-58.
- Richard C, Sunshine G, Benjamini E. Immunology: A Short Course. London: Wiley-Liss; 2003. p. 131.
- Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections]. J Immunol 2004;172:34-9.
- Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010;161:512-26.
- CDX-1127 – Monoclonal antibody targeting CD27. Celldex Therapeutics; Available from: http://www.celldex.com/pipeline/cdx-1127.php. [Last accessed on 2017 Apr 24].
- He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol 2013;191:4174-83.
- Zimm A. Cancer Miracle Patients Studied Anew for Disease Clues; 12 April, 2014. Bloomberg. Available from: https://www.bloomberg.com/news/articles/2014-04-11/cancer-miracle-patients-studied-anew-for-disease-clues. [Last retrieved on 2015 Jun 25].
- Pieris Pharmaceuticals to Present Data on Novel Anti-CD137 and HER2 Bispecificimmuno-Oncology program at UBS Global Healthcare Conference. Pieris Pharmaceuticals; 19 May, 2015. Available from: http://www.pieris.com/news-and-events/press-releases/detail/500/pieris-pharmaceuticals-to-present-data-on-novel-anti-cd137. [Last retrieved on 2015 Jun 05].
- Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013;73:7189-98.
- Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res 2013;1:320-31.
- Disrupting Tumor Evasion of the Immune System. Newlink Genetics. Available from: http://www.newlinkgenetics.com/platforms/ido-pathway-inhibitors/. [Last retrieved on 2015 Jun 05].
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015;27:39-46.
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008;14:28-36.
- Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet 2001;357:539-45.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63.
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014;5:5241.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54.
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-7.
- Haskill S, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer 1982;45:728-36.
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13.
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538-43.
- Leffers N, Vermeij R, Hoogeboom BN, Schulze UR, Wolf R, Hamming IE, et al. Long-term clinical and immunological effects of p53-SLP® vaccine in patients with ovarian cancer. Int J Cancer 2012;130:105-12.
- Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009;4:e6412.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9.
- Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 2009;58:15-23.
- Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. Ahigh M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014;7:19.
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010;107:7875-80.
- Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res 2013;19:1363-74.
- Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin Immunol 2008;129:471-81.
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562-7.
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-5.
- Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013;73:6900-12.
- Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy 2011;3:539-56.
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity 1997;7:445-50.
- Doyle AM, Mullen AC, Villarino AV, Hutchins AS, High FA, Lee HW, et al. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med 2001;194:893-902.
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001;19:225-52.
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003;100:4712-7.
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005-10.
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Matsumura N, et al. Efficacy and safety of anti-PD-1 antibody (nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. JClinOncol 2014;32 5 Suppl; abstr 5511). [Abstr #5511].
- Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. JClin Oncol 2015;33 (Suppl; abstr 5509). [Abstr #5510].
- Disis ML, Patel MR, Pant S, Infante JR, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: A phase Ib, open-label expansion trial. J ClinOncol 2015;33 (Suppl; abstr 5509). [Abstr #5509].
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65.
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. Anew member of the immunoglobulin superfamily – CTLA-4. Nature 1987;328:267-70.
- ;Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65.
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003;100:8372-7.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23.
- Tarhini AA, Thalanayar PM. Melanoma adjuvant therapy. Hematol Oncol Clin North Am 2014;28:471-89.
- Clinical trial number NCT00527735. Phase II Study for Previously Untreated Subjects with Non Small Cell Lung Cancer (NSCLC) or Small Cell Lung Cancer (SCLC). Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- First-Line Gemcitabine, Cisplatin+Ipilimumab for Metastatic Urothelial Carcinoma Clinical trial number NCT01524991. Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- ;Clinical trial number NCT00323882. Phase I/II Study of MDX-010 in Patients with Metastatic Hormone-Refractory Prostate Cancer (MDX010-21) (COMPLETED. Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- Available from: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_discontinuation_of_phase_iii_clinical_trial_for_patients_with_advanced_melanoma. [Last accessed on 2016 Apr 10].
- ;Mechanism of Pathway: CTLA-4 Inhibition. Available from: https://www.azimmuno-oncology.com/ctla-4-inhibition# Tremelimumab. [Last accessed on 2017 Apr 24].
- Poust J. Targeting metastatic melanoma. Am J Health Syst Pharm 2008;65 24 Suppl 9:S9-15.
- ;U.S. Food and Drug Administration. FDA Approves Keytruda for Advanced Melanoma; 04 September, 2014. Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm465444.htm. [Last retrieved on 2015 Dec 24].
- Approves Anti-PD-1 Drug for Advanced Melanoma. Available from: http://www.cancernetwork.com/melanoma/fda-approves-pembrolizumab-keytruda-advanced-melanoma. [Last accessed on 2016 Apr 12].
- US 8952136 Antibodies to Human Programmed Death Receptor PD-1. Available from: http://www.google.co.in/patents/US8952136. [Last accessed on 2016 Apr 12].
- Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014;588:368-76.
- ;Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77.
- Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol 2013;31:4199-206.
- Fairman D, Narwal R, Liang M, et al. Pharmacokinetics of MEDI4736, a fully human anti-PDL1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol 2014;32 (suppl; abstr 2602). [Abstr 2602].
- Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li, X, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32 (Suppl; abstr 8021^). [Abstr 8021].
- Merck-Pfizer Alliance. Merck-Pfizer Alliance Avelumab Fact Sheet. Available from: https://www.pfizer.com/files/news/asco/Merck-PfizerAlliance_AvelumabFactSheet_19May2015US.pdf. [Last retrieved on 2015 Dec 02].
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44.
- Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014;11:24-37.
- Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009;114:2667-77.
- Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 2010;16:1042-8.
- Hodi FS, Lee SJ, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (IPI) versus IPI alone in metastatic melanoma: E1608. J Clin Oncol 2013;31 (Suppl; abstr CRA9007). [Abstr CRA9007].
- Antonia SJ, Gettinger SN, Chow LQ, Juergens RA, Borghae H, Shen Y, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 2014;32 (Suppl; abstr 8023). [Abstr 8023].
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3.

| Figure 1:Immune checkpoints in T cell, dendritic cell and tumour cell
References
- Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252-64.
- Chen DS, Mellman I. Oncology meets immunology: The cancer-immunity cycle. Immunity 2013;39:1-10.
- Lenschow DJ, Walunas TL, Bluestone JA. CD28/B7 system of T cell costimulation. Annu Rev Immunol 1996;14:233-58.
- Richard C, Sunshine G, Benjamini E. Immunology: A Short Course. London: Wiley-Liss; 2003. p. 131.
- Taylor PA, Lees CJ, Fournier S, Allison JP, Sharpe AH, Blazar BR. B7 expression on T cells down-regulates immune responses through CTLA-4 ligation via T-T interactions [corrections]. J Immunol 2004;172:34-9.
- Eastwood D, Findlay L, Poole S, Bird C, Wadhwa M, Moore M, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. Br J Pharmacol 2010;161:512-26.
- CDX-1127 – Monoclonal antibody targeting CD27. Celldex Therapeutics; Available from: http://www.celldex.com/pipeline/cdx-1127.php. [Last accessed on 2017 Apr 24].
- He LZ, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol 2013;191:4174-83.
- Zimm A. Cancer Miracle Patients Studied Anew for Disease Clues; 12 April, 2014. Bloomberg. Available from: https://www.bloomberg.com/news/articles/2014-04-11/cancer-miracle-patients-studied-anew-for-disease-clues. [Last retrieved on 2015 Jun 25].
- Pieris Pharmaceuticals to Present Data on Novel Anti-CD137 and HER2 Bispecificimmuno-Oncology program at UBS Global Healthcare Conference. Pieris Pharmaceuticals; 19 May, 2015. Available from: http://www.pieris.com/news-and-events/press-releases/detail/500/pieris-pharmaceuticals-to-present-data-on-novel-anti-cd137. [Last retrieved on 2015 Jun 05].
- Curti BD, Kovacsovics-Bankowski M, Morris N, Walker E, Chisholm L, Floyd K, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res 2013;73:7189-98.
- Schaer DA, Budhu S, Liu C, Bryson C, Malandro N, Cohen A, et al. GITR pathway activation abrogates tumor immune suppression through loss of regulatory T cell lineage stability. Cancer Immunol Res 2013;1:320-31.
- Disrupting Tumor Evasion of the Immune System. Newlink Genetics. Available from: http://www.newlinkgenetics.com/platforms/ido-pathway-inhibitors/. [Last retrieved on 2015 Jun 05].
- Philips GK, Atkins M. Therapeutic uses of anti-PD-1 and anti-PD-L1 antibodies. Int Immunol 2015;27:39-46.
- Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med 2008;14:28-36.
- Balkwill F, Mantovani A. Inflammation and cancer: Back to virchow? Lancet 2001;357:539-45.
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 2013;3:1355-63.
- Chen L, Gibbons DL, Goswami S, Cortez MA, Ahn YH, Byers LA, et al. Metastasis is regulated via microRNA-200/ZEB1 axis control of tumour cell PD-L1 expression and intratumoral immunosuppression. Nat Commun 2014;5:5241.
- Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54.
- Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 2006;439:682-7.
- Haskill S, Becker S, Fowler W, Walton L. Mononuclear-cell infiltration in ovarian cancer. I. Inflammatory-cell infiltrates from tumour and ascites material. Br J Cancer 1982;45:728-36.
- Zhang L, Conejo-Garcia JR, Katsaros D, Gimotty PA, Massobrio M, Regnani G, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med 2003;348:203-13.
- Sato E, Olson SH, Ahn J, Bundy B, Nishikawa H, Qian F, et al. Intraepithelial CD8+tumor-infiltrating lymphocytes and a high CD8+/regulatory T cell ratio are associated with favorable prognosis in ovarian cancer. Proc Natl Acad Sci U S A 2005;102:18538-43.
- Leffers N, Vermeij R, Hoogeboom BN, Schulze UR, Wolf R, Hamming IE, et al. Long-term clinical and immunological effects of p53-SLP® vaccine in patients with ovarian cancer. Int J Cancer 2012;130:105-12.
- Milne K, Köbel M, Kalloger SE, Barnes RO, Gao D, Gilks CB, et al. Systematic analysis of immune infiltrates in high-grade serous ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic factors. PLoS One 2009;4:e6412.
- Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med 2004;10:942-9.
- Cho H, Hur HW, Kim SW, Kim SH, Kim JH, Kim YT, et al. Pre-treatment neutrophil to lymphocyte ratio is elevated in epithelial ovarian cancer and predicts survival after treatment. Cancer Immunol Immunother 2009;58:15-23.
- Zhang M, He Y, Sun X, Li Q, Wang W, Zhao A, et al. Ahigh M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J Ovarian Res 2014;7:19.
- Matsuzaki J, Gnjatic S, Mhawech-Fauceglia P, Beck A, Miller A, Tsuji T, et al. Tumor-infiltrating NY-ESO-1-specific CD8+ T cells are negatively regulated by LAG-3 and PD-1 in human ovarian cancer. Proc Natl Acad Sci U S A 2010;107:7875-80.
- Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res 2013;19:1363-74.
- Liu Y, Zeng B, Zhang Z, Zhang Y, Yang R. B7-H1 on myeloid-derived suppressor cells in immune suppression by a mouse model of ovarian cancer. Clin Immunol 2008;129:471-81.
- Curiel TJ, Wei S, Dong H, Alvarez X, Cheng P, Mottram P, et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat Med 2003;9:562-7.
- Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8 T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A 2007;104:3360-5.
- Duraiswamy J, Freeman GJ, Coukos G. Therapeutic PD-1 pathway blockade augments with other modalities of immunotherapy T-cell function to prevent immune decline in ovarian cancer. Cancer Res 2013;73:6900-12.
- Preston CC, Goode EL, Hartmann LC, Kalli KR, Knutson KL. Immunity and immune suppression in human ovarian cancer. Immunotherapy 2011;3:539-56.
- Thompson CB, Allison JP. The emerging role of CTLA-4 as an immune attenuator. Immunity 1997;7:445-50.
- Doyle AM, Mullen AC, Villarino AV, Hutchins AS, High FA, Lee HW, et al. Induction of cytotoxic T lymphocyte antigen 4 (CTLA-4) restricts clonal expansion of helper T cells. J Exp Med 2001;194:893-902.
- Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol 2001;19:225-52.
- Hodi FS, Mihm MC, Soiffer RJ, Haluska FG, Butler M, Seiden MV, et al. Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci U S A 2003;100:4712-7.
- Hodi FS, Butler M, Oble DA, Seiden MV, Haluska FG, Kruse A, et al. Immunologic and clinical effects of antibody blockade of cytotoxic T lymphocyte-associated antigen 4 in previously vaccinated cancer patients. Proc Natl Acad Sci U S A 2008;105:3005-10.
- Hamanishi J, Mandai M, Ikeda T, Minami M, Kawaguchi A, Matsumura N, et al. Efficacy and safety of anti-PD-1 antibody (nivolumab: BMS-936558, ONO-4538) in patients with platinum-resistant ovarian cancer. JClinOncol 2014;32 5 Suppl; abstr 5511). [Abstr #5511].
- Varga A, Piha-Paul SA, Ott PA, Mehnert JM, Berton-Rigaud D, Johnson EA, et al. Antitumor activity and safety of pembrolizumab in patients (pts) with PD-L1 positive advanced ovarian cancer: Interim results from a phase Ib study. JClin Oncol 2015;33 (Suppl; abstr 5509). [Abstr #5510].
- Disis ML, Patel MR, Pant S, Infante JR, Lockhart AC, Kelly K, et al. Avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with previously treated, recurrent or refractory ovarian cancer: A phase Ib, open-label expansion trial. J ClinOncol 2015;33 (Suppl; abstr 5509). [Abstr #5509].
- Brahmer JR, Tykodi SS, Chow LQ, Hwu WJ, Topalian SL, Hwu P, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65.
- Brunet JF, Denizot F, Luciani MF, Roux-Dosseto M, Suzan M, Mattei MG, et al. Anew member of the immunoglobulin superfamily – CTLA-4. Nature 1987;328:267-70.
- ;Krummel MF, Allison JP. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J Exp Med 1995;182:459-65.
- Phan GQ, Yang JC, Sherry RM, Hwu P, Topalian SL, Schwartzentruber DJ, et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc Natl Acad Sci U S A 2003;100:8372-7.
- Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23.
- Tarhini AA, Thalanayar PM. Melanoma adjuvant therapy. Hematol Oncol Clin North Am 2014;28:471-89.
- Clinical trial number NCT00527735. Phase II Study for Previously Untreated Subjects with Non Small Cell Lung Cancer (NSCLC) or Small Cell Lung Cancer (SCLC). Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- First-Line Gemcitabine, Cisplatin+Ipilimumab for Metastatic Urothelial Carcinoma Clinical trial number NCT01524991. Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- ;Clinical trial number NCT00323882. Phase I/II Study of MDX-010 in Patients with Metastatic Hormone-Refractory Prostate Cancer (MDX010-21) (COMPLETED. Available from: http://www. ClinicalTrials.gov. [Last accessed on 2017 Apr 24].
- Available from: http://www.pfizer.com/news/press-release/press-release-detail/pfizer_announces_discontinuation_of_phase_iii_clinical_trial_for_patients_with_advanced_melanoma. [Last accessed on 2016 Apr 10].
- ;Mechanism of Pathway: CTLA-4 Inhibition. Available from: https://www.azimmuno-oncology.com/ctla-4-inhibition# Tremelimumab. [Last accessed on 2017 Apr 24].
- Poust J. Targeting metastatic melanoma. Am J Health Syst Pharm 2008;65 24 Suppl 9:S9-15.
- ;U.S. Food and Drug Administration. FDA Approves Keytruda for Advanced Melanoma; 04 September, 2014. Available from: https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm465444.htm. [Last retrieved on 2015 Dec 24].
- Approves Anti-PD-1 Drug for Advanced Melanoma. Available from: http://www.cancernetwork.com/melanoma/fda-approves-pembrolizumab-keytruda-advanced-melanoma. [Last accessed on 2016 Apr 12].
- US 8952136 Antibodies to Human Programmed Death Receptor PD-1. Available from: http://www.google.co.in/patents/US8952136. [Last accessed on 2016 Apr 12].
- Kyi C, Postow MA. Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett 2014;588:368-76.
- ;Westin JR, Chu F, Zhang M, Fayad LE, Kwak LW, Fowler N, et al. Safety and activity of PD1 blockade by pidilizumab in combination with rituximab in patients with relapsed follicular lymphoma: A single group, open-label, phase 2 trial. Lancet Oncol 2014;15:69-77.
- Armand P, Nagler A, Weller EA, Devine SM, Avigan DE, Chen YB, et al. Disabling immune tolerance by programmed death-1 blockade with pidilizumab after autologous hematopoietic stem-cell transplantation for diffuse large B-cell lymphoma: Results of an international phase II trial. J Clin Oncol 2013;31:4199-206.
- Fairman D, Narwal R, Liang M, et al. Pharmacokinetics of MEDI4736, a fully human anti-PDL1 monoclonal antibody, in patients with advanced solid tumors. J Clin Oncol 2014;32 (suppl; abstr 2602). [Abstr 2602].
- Brahmer JR, Rizvi NA, Lutzky J, Khleif S, Blake-Haskins A, Li, X, et al. Clinical activity and biomarkers of MEDI4736, an anti-PD-L1 antibody, in patients with NSCLC. J Clin Oncol 2014;32 (Suppl; abstr 8021^). [Abstr 8021].
- Merck-Pfizer Alliance. Merck-Pfizer Alliance Avelumab Fact Sheet. Available from: https://www.pfizer.com/files/news/asco/Merck-PfizerAlliance_AvelumabFactSheet_19May2015US.pdf. [Last retrieved on 2015 Dec 02].
- Hamid O, Robert C, Daud A, Hodi FS, Hwu WJ, Kefford R, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 2013;369:134-44.
- Drake CG, Lipson EJ, Brahmer JR. Breathing new life into immunotherapy: Review of melanoma, lung and kidney cancer. Nat Rev Clin Oncol 2014;11:24-37.
- Romagné F, André P, Spee P, Zahn S, Anfossi N, Gauthier L, et al. Preclinical characterization of 1-7F9, a novel human anti-KIR receptor therapeutic antibody that augments natural killer-mediated killing of tumor cells. Blood 2009;114:2667-77.
- Kirkwood JM, Lorigan P, Hersey P, Hauschild A, Robert C, McDermott D, et al. Phase II trial of tremelimumab (CP-675,206) in patients with advanced refractory or relapsed melanoma. Clin Cancer Res 2010;16:1042-8.
- Hodi FS, Lee SJ, McDermott DF, Rao UN, Butterfield LH, Tarhini AA, et al. Multicenter, randomized phase II trial of GM-CSF (GM) plus ipilimumab (IPI) versus IPI alone in metastatic melanoma: E1608. J Clin Oncol 2013;31 (Suppl; abstr CRA9007). [Abstr CRA9007].
- Antonia SJ, Gettinger SN, Chow LQ, Juergens RA, Borghae H, Shen Y, et al. Nivolumab (anti-PD-1; BMS-936558, ONO-4538) and ipilimumab in first-line NSCLC: Interim phase I results. J Clin Oncol 2014;32 (Suppl; abstr 8023). [Abstr 8023].
- Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science 2013;342:1432-3.


 PDF
PDF  Views
Views  Share
Share

