Imaging Recommendations for the Diagnosis, Staging, and Management of Adult Brain Tumors
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 026-038
DOI: DOI: 10.1055/s-0042-1759712
Abstract
Neuroimaging plays a pivotal role in the clinical practice of brain tumors aiding in the diagnosis, genotype prediction, preoperative planning, and prognostication. The brain tumors most commonly seen in adults are extra-axial lesions like meningioma, intra-axial lesions like gliomas and lesions of the pituitary gland. Clinical features may be localizing like partial seizures, weakness, and sensory disturbances or nonspecific like a headache. On clinical suspicion of a brain tumor, the primary investigative workup should focus on imaging. Other investigations like fundoscopy and electroencephalography may be performed depending on the clinical presentation. Obtaining a tissue sample after identifying a brain tumor on imaging is crucial for confirming the diagnosis and planning further treatment. Tissue sample may be obtained by techniques such as stereotactic biopsy or upfront surgery. The magnetic resonance (MR) imaging protocol needs to be standardized and includes conventional sequences like T1-weighted (T1W) imaging with and without contrast, T2w imaging, fluid-attenuated axial inversion recovery, diffusion-weighted imaging (DWI), susceptibility-weighted imaging, and advanced imaging sequences like MR perfusion and MR spectroscopy. Various tumor characteristics in each of these sequences can help us narrow down the differential diagnosis and also predict the grade of the tumor. Multidisciplinary co-ordination is needed for proper management and care of brain tumor patients. Treatment protocols need to be adapted and individualized for each patient depending on the age, general condition of the patient, histopathological characteristics, and genotype of the tumor. Treatment options include surgery, radiotherapy, and chemotherapy. Imaging also plays a vital role in post-treatment follow-up. Sequences like DWI, MR perfusion, and MR spectroscopy are useful to distinguish post-treatment effects like radiation necrosis and pseudoprogression from true recurrence. Radiological reporting of brain tumor images should follow a structured format to include all the elements that could have an impact on the treatment decisions in patients.
Publication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Neuroimaging plays a pivotal role in the clinical practice of brain tumors aiding in the diagnosis, genotype prediction, preoperative planning, and prognostication. The brain tumors most commonly seen in adults are extra-axial lesions like meningioma, intra-axial lesions like gliomas and lesions of the pituitary gland. Clinical features may be localizing like partial seizures, weakness, and sensory disturbances or nonspecific like a headache. On clinical suspicion of a brain tumor, the primary investigative workup should focus on imaging. Other investigations like fundoscopy and electroencephalography may be performed depending on the clinical presentation. Obtaining a tissue sample after identifying a brain tumor on imaging is crucial for confirming the diagnosis and planning further treatment. Tissue sample may be obtained by techniques such as stereotactic biopsy or upfront surgery. The magnetic resonance (MR) imaging protocol needs to be standardized and includes conventional sequences like T1-weighted (T1W) imaging with and without contrast, T2w imaging, fluid-attenuated axial inversion recovery, diffusion-weighted imaging (DWI), susceptibility-weighted imaging, and advanced imaging sequences like MR perfusion and MR spectroscopy. Various tumor characteristics in each of these sequences can help us narrow down the differential diagnosis and also predict the grade of the tumor. Multidisciplinary co-ordination is needed for proper management and care of brain tumor patients. Treatment protocols need to be adapted and individualized for each patient depending on the age, general condition of the patient, histopathological characteristics, and genotype of the tumor. Treatment options include surgery, radiotherapy, and chemotherapy. Imaging also plays a vital role in post-treatment follow-up. Sequences like DWI, MR perfusion, and MR spectroscopy are useful to distinguish post-treatment effects like radiation necrosis and pseudoprogression from true recurrence. Radiological reporting of brain tumor images should follow a structured format to include all the elements that could have an impact on the treatment decisions in patients.
Keywords
brain tumors - neuroimaging - radiologyIntroduction
Following the discovery of X-rays, there were a few reports of imaging being utilized for the diagnosis and localization of brain tumors, although of very limited utility.[1] A major leap in the field of neuroimaging came with the invention of computed tomography (CT).[1] In the 1980s, when it was recognized that nuclear magnetic resonance (MR) could be applied to medical imaging, magnetic resonance imaging (MRI) was introduced.[2] MRI has become a cornerstone in neuroimaging, based on its capability to demonstrate soft tissues, with exquisite detail. Advanced MRI sequences, along with modalities like positron emission tomography (PET), can now further help us by demonstrating the pathophysiological underpinnings of brain tumors and be useful for preoperative planning.
Epidemiology, Clinical Presentation in India and Global
The most common brain tumors seen in adults are extra-axial lesions like meningiomas, intra-axial lesions like gliomas, and pituitary gland tumors.[3] A published meta-analysis on primary brain tumors has estimated the worldwide incidence rates to be 10.82 per 1,00,000 person-years.[4] Primary brain tumors are a very heterogeneous group with the incidence and prevalence rates differing across tumor types. According to Porter et al, in the United States, the highest incidence was for gliomas and meningiomas, measuring 6 per 1,00,000 person-years for each.[5] The overall incidence of brain and spinal cord tumors is 5 to 10 per 1,00,000 in India.[6] The majority (38.7%) of these lesions were astrocytomas.[6]
Clinically, presenting symptoms may be related to lesion localization. Examples include focal seizures, sensory impairment, motor weakness, ataxia and cognitive disturbances or personality changes.[7] Symptoms may also be generalized and nonspecific like headaches with vomiting, which may point toward the underlying raised intracranial pressure.[7] Visual disturbances may occur either due to the former or the latter.
Clinical and Diagnostic Workup Excluding Imaging
On clinical suspicion of a brain tumor, the primary investigative workup focuses on imaging.[8] When the patient presents with a headache, a fundoscopy may be performed to look for papilledema, which could be a sign of raised intracranial tension due to a brain tumor. When a seizure is an initial presentation, electroencephalography is usually performed.[8] Further, cerebrospinal fluid (CSF) markers based on the tumoral genetic material and proteins like circulating tumor DNA and microRNA have been shown to aid in brain tumor diagnosis.[9]
Obtaining a tissue sample after the identification of a brain tumor on imaging is crucial for confirming the diagnosis and planning further treatment.[8] This importance is even more in the era of molecular diagnostics, where genotyping is necessary for accurate prognostication.[8] [10] The tissue sample is usually obtained by biopsy or upfront surgery.[8] Stereotactic biopsy has a low risk of morbidity and a good sampling rate with higher chances of an accurate tissue diagnosis.[8] [11] The genetic makeup of glioma may be identified by methods like DNA sequencing or cheaper, more widely available alternatives like immunohistochemistry (IHC).[12] The diagnosis and classification of a brain tumor should be based on the World Health Organization (WHO) 2021 classification system.[13]
Recommendations
The diagnostic modality of choice on clinical suspicion of a brain tumor is contrast-enhanced MRI.[8]
The tissue sample from a diffuse infiltrative glioma is to be routinely subjected to IHC for the detection of Isocitrate Dehydrogenase (IDH) 1 R132H mutant protein and alpha-thalassemia mental retardation X-linked (ATRX) nuclear expression.[8]
When IDH1 mutation is not detected by IHC, DNA sequencing must be performed for the detection of IDH1 or IDH2 mutations in all diffuse astrocytomas and oligodendrogliomas. It should also be done in patients less than 55 years old with glioblastomas.[8] [14]
All IDH-mutant gliomas should be tested for 1p/19q codeletion status when there is ATRX retention.[8] [15]
IDH-mutant astrocytomas must be interrogated for cyclin dependent kinase inhibitor (CDKN) 2A/B homozygous deletions.[8] [15]
To label an IDH-wild diffuse glioma as a glioblastoma, testing should be done for a gain of chromosome 7 with a loss of chromosome 10, epidermal growth factor receptor amplification and telomerase reverse transcriptase promoter mutation when there is an absence of microvascular proliferation and necrosis.[8] [15]
In diffuse midline gliomas, testing should be done for Lys27Met mutations in histone 3 genes (H3K27M) mutation.[8]
Imaging
Current recommendations for brain tumor MRI protocol include the following sequences: 2D T1-weighted imaging (T1WI), fluid-attenuated inversion recovery (FLAIR) 2D axials, axial susceptibility-weighted imaging (SWI), DWI 2D axials, 3D T1 postcontrast. Advanced imaging includes dynamic susceptibility (T2*) and dynamic contrast enhancement (T1) perfusion-weighted imaging (PWI), magnetic resonance spectroscopy (MRS), diffusion tensor imaging (DTI), and functional MRI (fMRI). Similarly, 3D T2WI and 3D postcontrast T1W spoiled gradient recalled imaging are useful for neuronavigation/presurgical biopsy planning.
Standard MRI brain tumor protocol is required to reduce the quantitative and qualitative differences due to different protocols followed at various institutes.
[Table 1] gives an overview of the adult brain tumor protocol followed at our institute.
|
MR sequence |
TE |
TR |
TI |
FOV |
Matrix |
Slice thickness |
Flip angle |
|---|---|---|---|---|---|---|---|
|
2D axial T1 precontrast |
20 milliseconds |
2,200 milliseconds |
NA |
240 mm |
320 × 256 |
3 mm |
10–15 degrees |
|
2D axial and sagittal T2WI |
120 milliseconds |
>2,600 milliseconds |
NA |
240 mm |
480 × 480 |
3 mm |
90 degrees |
|
2D axial FLAIR |
120 milliseconds |
>6,500 milliseconds |
2400 milliseconds |
240 mm |
320 × 256 |
3 mm |
90 degrees |
|
2D axial DWI (b = 0,1000 second mm2) |
70 milliseconds |
>6,000 milliseconds |
NA |
240 mm |
128 × 254 |
3 mm |
90 degrees |
|
3D axial T1 postcontrast |
20 milliseconds |
2,200 milliseconds |
NA |
256 mm |
320 × 256 |
<1> |
10–15 degrees |
|
DSC (T2*) perfusion |
25–23 milliseconds |
2,400 milliseconds |
NA |
256 mm |
320 × 256 |
<1> |
30 degrees |
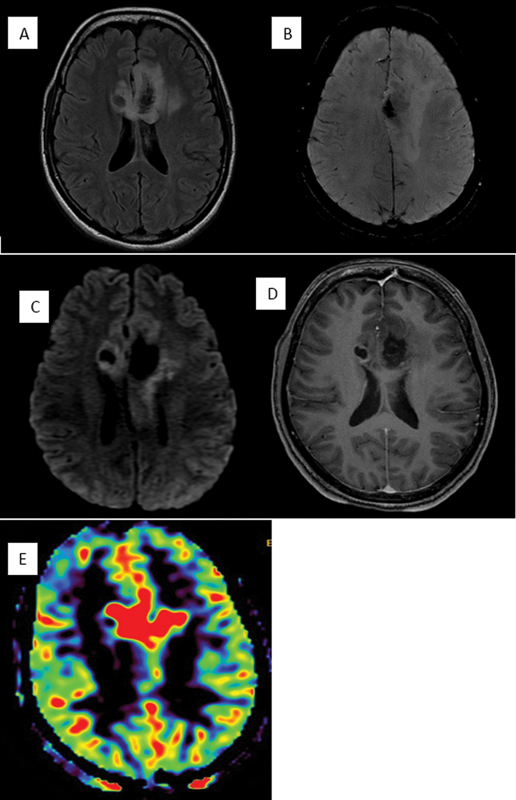
| Figure 1:Characteristic imaging features in a case of glioblastoma. Fluid-attenuated axial inversion recovery mixed signal intensity lesion (A) crossing across the midline via corpus callosum showing central blooming (compatible with bleed) (B) along with diffusion heterogeneity (C). On postcontrast images (D) irregular peripheral enhancement noted and on relative cerebral blood volume maps (E), increased perfusion noted in the corresponding region.
Recommendations for T1W Pre- and Postcontrast Images
Before contrast administration, T1 shortening may be seen due to broken-down blood products, mineralization, fat, and melanin.
The integrity of the blood–brain barrier can be demonstrated by T1 postcontrast imaging.
The degree/pattern of contrast enhancement may not be useful to predict the grade of neoplasm.
Recommendations for T2W image/FLAIR images:
T2/FLAIR hyperintensity may be seen in vasogenic and cytotoxic edema/nonenhancing regions of the tumor/white matter pathology.
T2/FLAIR mismatch is highly specific for IDH mutant 1p/19 q non-co deleted diffuse astrocytoma.[24] This, however, needs to be differentiated from the “bright rim” sign of dysembryoplastic neuroepithelial tumor.
Recommendations for SWI/GRE (Gradient Echo)
For identifying regions of increased MR susceptibility. Blooming can be seen in areas of broken-down blood products, increased tumoral vascularity (higher percent of deoxyhemoglobin), and mineralization.
Recommendations for DWI/ADC map:
The image is weighted by the degree of water molecule diffusion and can be represented as an ADC map.
A bright signal on DWI with a low ADC may be seen in densely packed cellular tumors/cytotoxic edema.
Kitis et al showed that lower-grade gliomas (LGG) have considerably high minimum ADC values than HGGs.[25]
Apart from gliomas, it was found by Bozdağ et al that high-grade meningiomas had a lower mean, minimum, and maximum ADC values than low-grade meningiomas (900, 850, 950 × 10−6 mm2/s). This was in addition to a notable relation with tumor grading and proliferation (Ki-67, mitotic indices).[26]
Differentiating lymphoma from glioblastoma can at times be challenging. ADC values are lower in lymphoma than those in HGG. In a study by Makino et al, with an ADC cutoff of 1.0 × 10 − 3 mm2/s, it was possible to differentiate PCNSL from glioblastoma.[27]
Recommendations for MRS
Lower N-Acetylaspartate (NAA) and creatine levels, higher lactate along with lipid levels, and higher NAA/choline, as well as choline/creatine ratios, were found in HGGs, implying that metabolite measurement can be used to predict tumor grade, although metabolically active non-neoplastic etiologies like demyelination may have a similar pattern.[28]
Recommendations for MR Perfusion—DSC (Dynamic Susceptibility Contrast)/T2*Perfusion
An important perfusion parameter in T2 * perfusion is relative cerebral blood volume (rCBV). It is common to find high-grade tumors to have enhanced perfusion, as shown by elevated rCBV.[28] [29]
In a meta-analysis that compared nine studies, HGGs were shown to have considerably higher absolute and relative tumor blood volumes compared with LGGs.[30]
In comparison to traditional MRI findings, Law et al observed increased rCBV (1.75) in HGGs compared with LGGs; however, oligodendroglioma is an exception among gliomas, where increased rCBV may be linked to fine intratumoral capillaries rather than malignant potential and neo-angiogenesis.[28]
A steady increase in rCBV within a tumor over time may forecast the interval change of a previously low-grade neoplasm to a higher grade.[31]
PWI is less useful for grading in meningiomas as all grades show elevated perfusion due to the common expression of vascular endothelial growth factor (VEGF), an angiogenesis marker.[32]
PCNSL lesions can also be distinguished from metastases and glioblastomas using DSC-MRI. PCNSL has reduced rCBV compared with metastases and glioblastomas. This is because PCNSL lesions are angiocentric rather than angiogenic, meaning that they surround pre-existing blood vessels rather than create new ones.[33] [34] [35] Metastases might have variable rCBV values depending on the primary lesion.
The dynamic passage of contrast through a region can be plotted as a signal intensity to time or a mean curve (MC). Another useful perfusion metric in MC analysis is percentage signal recovery (PSR), which measures the tendency of the MC to return to the baseline. The PSR may be used to evaluate the integrity of the blood–brain barrier.[34] [35] [36] Studies in which preloading has not been utilized for minimizing the T1 impact of gadolinium-based contrast agents in DSC-perfusion images found that lymphoma has the greatest PSR, followed by metastases and glioblastoma.[34] [35] [36] [37] The elevated PSR in PCNSL ([Fig. 2]) is due to T1 effects associated with the accumulation of contrast in the tumor interstitium dominating T2* effects.[35]
PSR normally returns close to baseline in gliomas, while in brain metastases and extra-axial tumors, PSR may not return to baseline due to increased contrast extravasation.
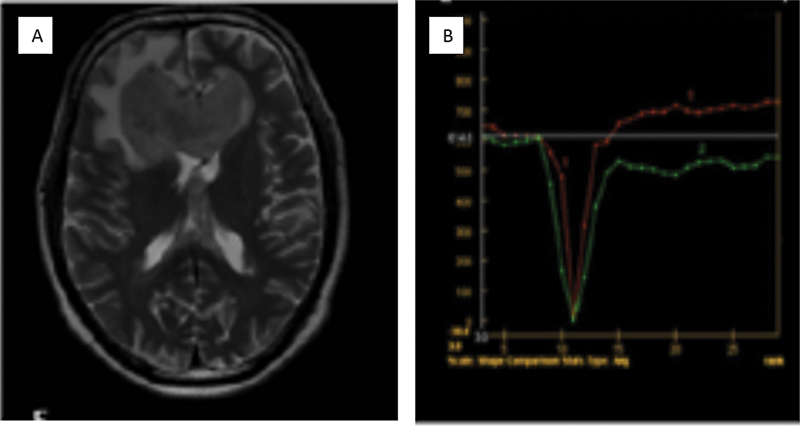
| Figure 2:Case of diffuse large B cell lymphoma. T2-weighted imaging reveals a small area of hyperintensity in the genu of the corpus callosum. (A). Percentage signal recovery is high (red curve) on dynamic susceptibility contrast perfusion imaging in lymphoma with mild elevation in relative cerebral blood volume (B).
Recommendations for MR Perfusion—Dynamic Contrast Enhancement (DCE) / T1 Perfusion
Another PWI technique that can be used for tumor grading is DCE, a T1-based approach to estimate tumor capillary permeability ([Fig. 3]). Higher K-trans values suggest a high grade/recurrent/progressive tumor and are extremely useful to discriminate tumor recurrence from radiation necrosis. However, the complexity of postprocessing, poor technique standardization, and lack of clinical validation are the drawbacks to widespread use.[38]

| Figure 3:Case of IDH wild glioblastoma in the right temporal lobe. The lesion represents heterogeneous hyperintensity on T2-weighted imaging (A) as well as elevated K trans value of 0.322 on dynamic contrast enhancement perfusion imaging (B).
Recommendations for MR Perfusion—Arterial Spin Labeling (ASL)
The parameter that is obtained is cerebral blood flow (CBF) without contrast administration. Increased CBF indicates a higher grade/recurrent lesion.
eASL has an additional advantage over ASL in terms of more accurate quantification of various metrics of tissue perfusion.
Recommendations for Diffusion Tensor Imaging (DTI)
Imaging of white matter fiber arrangement using the property of anisotropic diffusion of water molecules.
Fractional anisotropy (FA), an important DTI metric is a measure of the integrity of white matter tracts. Other metrics include mean diffusivity, and planar anisotropy.
Tractograms are used to visualize the white matter tract directionality and predict displacement/ infiltration by the tumor. This can be used for preoperative planning.
In addition, within brain neoplasms, disrupted tissue architecture results in altered FA that correlates with tumor cellularity.[39] Glioblastoma patients who show higher DTI abnormality in preoperative images have a long tumor progression-free survival and increased overall survival.[40] [41]
The limitations of tractography are the ability to resolve only a single fiber direction for a given voxel, while most voxels in the image contain multiple fiber directions[42] [43] and the crossing fiber problem that can be overcome with higher-order models like high angular resolution diffusion imaging that are more time-consuming.
Recommendations for Functional Magnetic Resonance Imaging (fMRI)
Areas of functional activation can be detected from the local fluctuations in the blood-oxygen levels in the brain.
This property is exploited by task-based fMRI for preoperative identification of functional areas, useful in surgical planning.
Resting-state fMRI at present is used as a research tool.
Recommendations for the Screening of the Entire Neuraxis
The primary intracerebral malignancies that may need entire neuraxial screening for drop metastases or multicentricity include medulloblastoma, ependymoma, pineal gland malignancies, germinoma, choroid plexus carcinoma.[44]
[Flowcharts 1] and [2] show an algorithm for the interpretation of MR images in patients with a suspected brain tumor.[45] Postchemoradiotherapy effects may be classified as early and late effects. Early effects may occur in the first 3 to 4 months after treatment, while delayed effects can occur from 6 months to years after treatment.[46] Chemotherapy further exacerbates these effects by increasing the disruption of the blood-brain barrier.
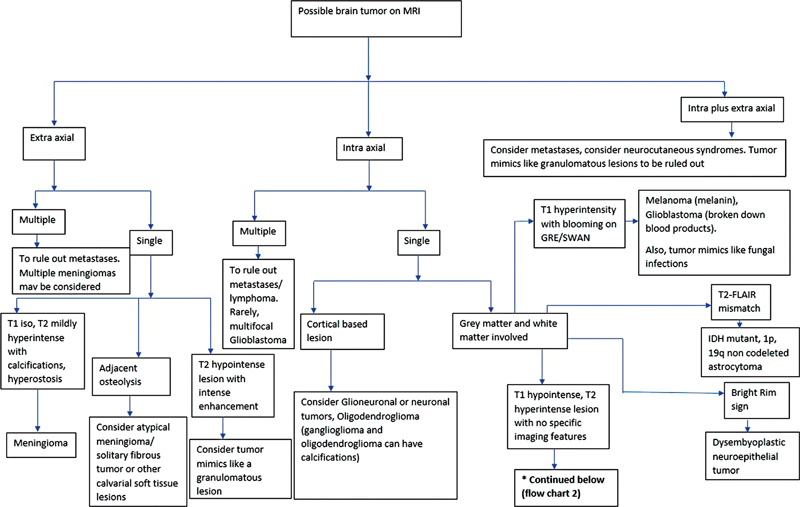
| Flowchart 1:Image interpretation algorithm 1. FLAIR, fluid-attenuated axial inversion recovery; MRI, magnetic resonance imaging.
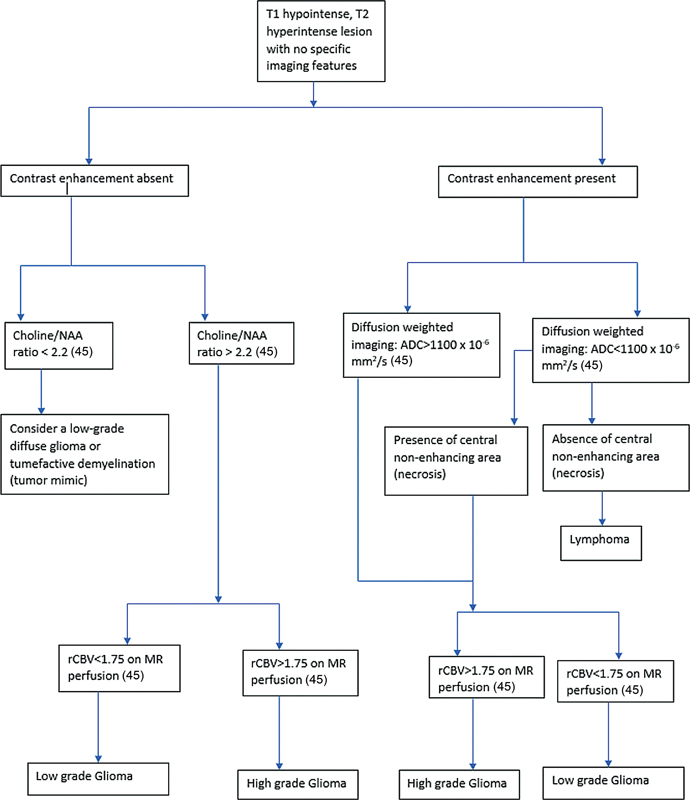
| Flowchart 2:Image interpretation algorithm 2. ADC, apparent diffusion coefficient; MR, magnetic resonance; NAA,—; rCBV, relative cerebral blood volume.
Pseudoprogression is a type of early post-treatment effect that can occur in the first 3 to 6 months. Pseudoresponse is again an early type of reaction seen when HGGs are treated with anti-VEGF agents.[47]
Radiation necrosis ([Fig. 4]) is due to severe local tissue response to radiation. Although it usually occurs 3 to 12 months after radiotherapy, it can even occur several years or decades later.[47] It has been described as having a Swiss cheese enhancement pattern.[46] However, the identification of radiation necrosis based on the pattern of enhancement alone can prove to be difficult.[46] DWI and advanced MRI sequences like PWI and MRS can help with the diagnosis in these cases. Studies on post-treatment DWI and PWI have shown that recurrent tumors have reduced ADC and increased rCBV values compared with post-treatment effects ([Fig. 5]).[46] Chemical exchange saturation transfer MRI and PET using amino acid radiotracers (rather than fluorodeoxyglucose) have demonstrated the capability to differentiate tumor recurrence/residue and post-therapy changes.[48] [49]
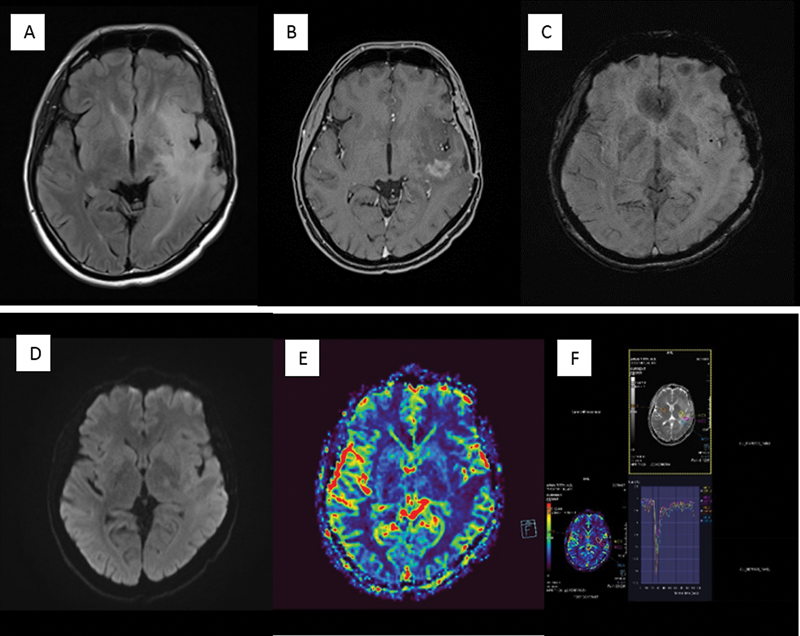
| Figure 4 :Post-treatment change in the form of radiation necrosis in a patient after resection of a higher-grade glioma followed by postoperative radiotherapy. (A) There is fluid-attenuated axial inversion recovery (FLAIR) hyperintensity surrounding the operative cavity. (B) There is heterogeneous “swiss-cheese” enhancement within. (C) On susceptibility-weighted imaging, there are multiple foci of blooming in the hyperintensity that most likely represent postradiotherapy cavernomas. (D) No restriction on diffusion-weighted imaging and (E, F) no elevated relative cerebral blood volume on perfusion imaging within the FLAIR hyperintensity and the enhancing area as compared with the contralateral side on perfusion color map and dynamic
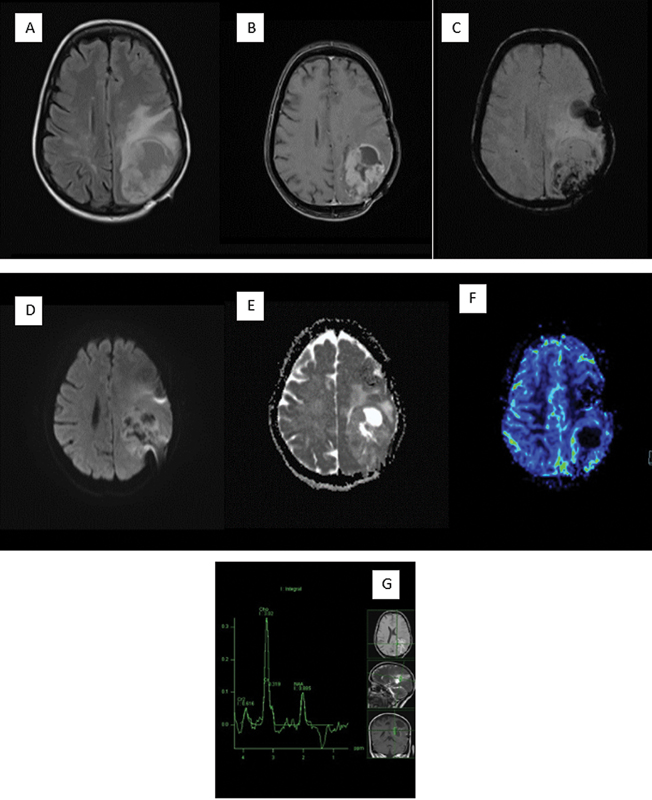
| Figure 5:Recurrence in the postoperative higher grade glioma patient. (A) Fluid-attenuated axial inversion recovery image is noted to show a heterogeneous hyperintensity in the left parietal lobe. (B) Heterogeneous enhancement on postcontrast scan has been seen. (C) Susceptibility-weighted imaging shows blood products that are most likely postoperative. (D, E) There is heterogeneous diffusion on diffusion-weighted imaging and apparent diffusion coefficient with a few areas of restriction. (F) Dynamic susceptibility contrast-perfusion image shows elevated relative cerebral blood volume within the enhancing region. (G) Spectroscopy with intermediate echo time shows significantly elevated choline with a lactate peak.
Recommendations in the Imaging of Treated Brain Tumors
Identification of post-treatment effects is of importance as these can be followed up with or without medical management while the recurrence warrants treatment.
Advanced MRI sequences like DWI, PWI, MRS, and PET-CT may be used to differentiate post-treatment changes from recurrence or residual lesions.[46]
Radiographic response assessment: Given the wide range of interpretations, it is not unexpected that tumor response reporting in radiology varies as well. An evaluation of post-therapeutic response in gliomas is done using modified Response Assessment in Neuro-Oncology (mRANO) criteria.[50] [51]
Recommendations for mRANO Criteria
Response of the tumor is determined in comparison to baseline images (pretreatment image for recurrent glioblastoma and postradiation scan for newly detected glioblastoma).
Lesions can be measurable and/or nonmeasurable. Measurable lesions demonstrate contrast enhancement with clearly defined margins, on 2 or more axial sections (preferably 5 mm axial slices with no interslice gap). The lesion should be at least 10 mm in size (maximum diameter) if the slice thickness is less than 5 mm and twice the slice thickness if the slice thickness is more than 5 mm. Nonmeasurable lesions show poorly defined margins that cannot be measured, lack contrast enhancement, and/or are too small to measure (<10>
It divides the radiographic/clinical response into four types: (1) Complete response (CR), (2) partial response (PR), (3) progressive disease (PD), (4) stable disease (SD).
CR: Disappearance of all measurable enhancement and nonmeasurable disease, with scans done at least 4 weeks apart. CR seen on the first scan is preliminary and on the second scan is durable. If the second scan shows enhancing lesion compared with the preliminary CR, then it is a pseudoresponse and preliminary PD. Clinically with CR, the patient should improve/be stable with no corticosteroid usage (except for physiological dose).
PR: Measurable enhancement showing more than or equal to50%-reduction in the sum of perpendicular diameter and/or more than or equal to 65%- reduction in total lesion volume with scans done at least 4 weeks apart. The first scan showing PR compared with baseline is preliminary PR. If the second scan shows progression compared with preliminary PR, then it is pseudoresponse and preliminary PD and for a confirmed PD, at least two sequential scans must show an increase in tumor size. If the second scan shows a PR, then it is a durable PR. Clinically, with PR the patient should improve/be stable with no corticosteroid usage (except for physiological dose).
PD: Measurable enhancement showing more than or equal to 25%-increase in the sum of perpendicular diameter or more than or equal to 40%-increase in lesion volume in 2 sequential scans done more than or equal to 4 weeks apart. The first scan showing PD is preliminary PD and if the second scan also shows PD, then it is confirmed PD. If a second scan done 4 weeks later shows SD or PR/CR, then it is pseudoprogression and is labeled as preliminary PD. Clinically with PD, the patient should have significant worsening not attributable to any other illness/steroid dose.
SD: The patient not qualifying for CR/PR/PD is clinically stable with reduced/stable steroid dose intake compared with baseline.
mRANO criteria are yet to be widely adopted in clinical radiology practice, owing in part to the strict guidelines that must be followed, and limitations such as high interobserver variability in interpretation, dependence on clinical status and treatment history, and consistency in taking multiple measurements over time. Relying on gadolinium enhancement for assessing therapeutic response was a fundamental shortcoming of the RANO criteria as both tumor progression and post-treatment change can show enhancement.
After the advent of immunotherapy for glial neoplasms, immunotherapy response assessment for neuro-oncology has been introduced to overcome the challenges in radiology reporting which is a slight modification of the mRANO criteria. The main differences are 1. Patients on immunotherapy with new contrast-enhancing lesions outside the radiation field will not fall under the PD category, 2. Unlike RANO, a follow-up scan after 3 months is required to confirm disease progression as the therapeutic effect of immunotherapy may be delayed.
Brain tumor reporting and data system (BT-RADS) was created to make MRI reporting of post-treatment brain tumors easier and more consistent by assigning scores from 0 to 4 to the patients' images.[51] [52] Score 0—new baseline study, score 1—improvement in imaging findings, score 2—stable imaging findings, score 3—worsening imaging findings and is divided into three subgroups depending on the underlying cause (post-therapy effect= 3a, the combined effect of treatment changes and tumor progression= 3b, tumor progression favored= 3c). Score 4—worsening of imaging findings likely indicating tumor progression. T2* perfusion and DWI can help categorize patients better, into individual subgroups in BT-RADS score 3. Each category in BT-RADS is linked to a specific management decision leading to better communication between the referring physician and the radiologist.[53] [54]
Principles of Management
General management should precede definitive treatment. When the presentation is in the emergency setting with a depressed sensorium or status epilepticus, the initial measures should focus on the maintenance of the airway, respiratory efforts, blood pressure, heart rate, and seizure control.[53] [54] [55] Dexamethasone may be used to treat peritumoral edema.[56]
Recommendations
In a diffuse infiltrating glioma seen on imaging, the treatment of choice is the maximum possible safe resection followed by radiotherapy and chemotherapy. If resection is not feasible due to the eloquence of adjacent areas or the poor general condition of the patient, a stereotactic biopsy may be considered for deciding on further management.[8] [11] [57] Management decisions without histological diagnosis should be stringently avoided and may only be taken in unusual situations like difficulty in biopsy as in elderly patients with comorbidities or where imaging very apparently shows a large HGG.[8]
For IDH-mutant astrocytoma, WHO grade 2 the treatment is the maximum possible resection. This is followed by radiotherapy and chemotherapy with the PCV (lomustine, procarbazine, and vincristine) regimen in patients with subtotal resection and/or when the patient is over 40 years old.[8] [57] [58]
For IDH-mutant astrocytoma, WHO grade 3 and grade 4, maximum possible resection, followed by radiotherapy and chemotherapy with temozolomide.[8] [57] [58]
For IDH-mutant, 1p/19q-co deleted oligodendroglioma, WHO grade 2 and 3, treatment is the maximum possible resection. If further treatment is needed, it is radiotherapy, followed by chemotherapy with the PCV regimen.[8] [57] [58]
For IDH-wild glioblastoma, aged less than 70 years, the standard of care is maximum possible resection, followed by concomitant radiotherapy and daily chemotherapy with temozolomide plus 6 cycles of maintenance temozolomide. Elderly patients who are not suitable candidates for combined radio and chemotherapy are to be managed based on O6-methylguanine-DNA methyl-transferase (MGMT) methylation status.[8] [57] [58] [59]
For incidentally detected, asymptomatic meningiomas, conservative management with follow-up is recommended. Surgery, with Simpson grade 1 resection, is indicated for meningiomas that grow or become symptomatic. Radiosurgery may be offered in patients who cannot undergo surgery when the tumor is relatively small with no significant mass effect. Patients with subtotally resected WHO grade 1 meningiomas or WHO grade 2 meningiomas with Simpsons grade 1 to 3 resection may be followed up or given adjuvant radiotherapy. WHO grade 2 meningiomas with Simpsons grade 4 or 5 resections should be given adjuvant radiotherapy. Maximal possible resection, followed by radiotherapy, is recommended in WHO grade 3 meningiomas.[59] [60]
Transsphenoidal resection is recommended for enlarging, symptomatic, nonfunctioning pituitary adenomas with mass effect and for functional adenomas. Radiotherapy may be used as adjuvant therapy for residual lesions. Follow-up is recommended for pituitary incidentalomas that are asymptomatic and surgery, if there is significant tumor growth on follow-up. Prolactinomas are generally treated with dopamine agonists. Surgery is reserved for cases with inadequate response. Radiotherapy may be used for residue after dopamine agonists or surgery.[61]
For primary CNS lymphoma, systemic chemotherapy is the first line of management.[62] [63] Surgery may be considered in a superficially located large lesion that is causing a significant mass effect. For induction chemotherapy, high-dose intravenous methotrexate is the agent of choice and it may be combined with other chemotherapeutic agents. For consolidation, high-dose chemotherapy with autologous stem cell transplantation is as efficacious as whole-brain radiotherapy (WBRT).[63]
Brain metastases are to be treated with the best combination of multimodality treatment. Surgery may be considered in solitary brain metastases or when there is a significant mass effect with raised intracranial pressure. Surgery may also be considered in patients with multiple brain metastases, especially when the prognosis is favorable. Stereotactic radiosurgery, WBRT, or systemic chemotherapy with agents based on the primary tumor may also be considered.[64]
Follow-Up Imaging and Management of Recurrent Disease Including Specific Interventional and Palliative Measures
The imaging appearance after tumor resection, chemotherapy, and radiotherapy is very complex and is influenced by multiple factors. These include blood products after the surgery, inflammatory response, and edema caused by the surgery or chemoradiation.[46] Apart from post-treatment changes like radiation necrosis, pseudoprogression, and pseudoresponse, there may be residual or a recurrent tumor ([Fig. 5]) at the margins of the resection cavity that is of particular concern.
Recommendations
In diffuse infiltrating gliomas, a postoperative scan within 24 to 48 hours is recommended, preferably with contrast-enhanced MRI to assess the extent of resection.[8] [65] A baseline MRI is done after 3 to 4 weeks after the completion of radiotherapy.[8] After the completion of therapy, imaging may be performed in 2- to 6-month intervals. But, the duration of intervals may be prolonged or shortened, depending on the extent of residual disease, and histological and genetic characteristics of the tumors. For example, IDH mutant gliomas may be followed up at 3- to 6-month intervals while IDH-wild gliomas at 2- to 3-month intervals. However, in the event of suspected disease progression, a shorter interval of 4 to 8 weeks may be adopted to confirm the same. In the rare event of a possible glioma being followed up without proper histological diagnosis, a shorter interval of approximately 2 to 3 months between imaging sessions is recommended.[8] When there is a recurrence, the prior treatment and the patient's functional status usually are considered for decision-making. A second surgery may be considered.[8]
Patients with incidentally detected, suspected meningiomas that are asymptomatic and patients with treated WHO grade 1 meningiomas are to be followed up with annual MR examinations for 5 years. Thereafter, follow-ups may be farther apart depending on the age, and the clinical condition of the patient. For treated WHO grade 2, biannual follow-up is recommended for 5 years as they have an increased risk of recurrence. Following this, yearly follow-up is recommended.[60] Fractionated radiotherapy may be used in patients with recurrent meningiomas.[60]
For pituitary incidentalomas that are microadenomas, an annual scan for 2 to 3 years is recommended. After this, the follow-up intervals may be prolonged if there is no increase in size. For incidentally detected pituitary macroadenomas, annual imaging follow-up with visual field charting every 6 to 12 months is recommended.[61] Aggressive pituitary lesions, after the completion of treatment, are to be followed up every 3 to 12 months by imaging depending on the rate of tumor growth and proximity to important anatomical structures. This may be coupled with endocrine evaluation depending on the clinical scenario.[66]
Brain metastases are to be followed up at intervals of 3 months. If there is any recurrence or progression during follow-up, the treatment decision depends on the patient's general condition, degree of progression, and the initial treatment. Treatment options include surgery, radiotherapy, and systemic chemotherapy.[64]
Synoptic Reporting Formats
Structured reporting better conveys the anatomical and pathophysiological information from the radiologist to the referring physician. A CT or an MR scan report for a brain tumor should have a detailed description of the number of tumors, their specific location, and their distance from eloquent structures as this plays a very important role in preoperative decision-making. The dimensions of the tumor along three different axes are to be mentioned; more specifically the sizes of the T2, FLAIR abnormality, and the enhancing area on the postcontrast T1W image are to be mentioned separately when the tumor is partially enhancing.[67]
Information about the extent of T2, FLAIR white matter hyperintensity adjacent to the tumor which may represent edema, the mass effect due to the tumor visible as effacement of ventricles, CSF spaces and midline shift are also to be conveyed in the report. Tumoral characteristics on DWI, SWI, PWI, and the effect of the tumor on adjacent vascular structures are also very important.
The presence of additional pathology in the neuroparenchyma like white matter disease or diffuse neuroparenchymal volume loss and calvarial pathology are all significant clinically and need to be mentioned. Finally, the radiological impression should include a possible list of differentials based on the imaging findings following the recent WHO 2021 nomenclature of brain tumors.
No conflict of interest has been declared by the author(s).
References
- Castillo M. History and evolution of brain tumor imaging: insights through radiology. Radiology 2014; 273 (2, Suppl) S111-S125
- Geva T. Magnetic resonance imaging: historical perspective. J Cardiovasc Magn Reson 2006; 8 (04) 573-580
- McNeill KA. Epidemiology of brain tumors. Neurol Clin 2016; 34 (04) 981-998
- de Robles P, Fiest KM, Frolkis AD. et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-oncol 2015; 17 (06) 776-783
- Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-oncol 2010; 12 (06) 520-527
- Dasgupta A, Gupta T, Jalali R. Indian data on central nervous tumors: a summary of published work. South Asian J Cancer 2016; 5 (03) 147-153
- Alther B, Mylius V, Weller M, Gantenbein A. From first symptoms to diagnosis: Initial clinical presentation of primary brain tumors. Clinical and Translational Neuroscience. 2020; 4 (02) DOI: 10.1177/2514183 × 20968368.
- Weller M, van den Bent M, Preusser M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021; 18 (03) 170-186 DOI: 10.1038/s41571-020-00447-z. Erratum in: Nat Rev Clin Oncol. 2022 May;19(5):357–358. PMID: 33293629; PMCID: PMC7904519
- Xiao F, Lv S, Zong Z. et al. Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res 2020; 12 (04) 1379-1396
- Merve A, Millner TO, Marino S. Integrated phenotype-genotype approach in diagnosis and classification of common central nervous system tumours. Histopathology 2019; 75 (03) 299-311
- Akshulakov SK, Kerimbayev TT, Biryuchkov MY, Urunbayev YA, Farhadi DS, Byvaltsev VA. Current trends for improving safety of stereotactic brain biopsies: advanced optical methods for vessel avoidance and tumor detection. Front Oncol 2019; 9: 947 DOI: 10.3389/fonc.2019.00947.
- Tanboon J, Williams EA, Louis DN. The diagnostic use of immunohistochemical surrogates for signature molecular genetic alterations in gliomas. J Neuropathol Exp Neurol 2016; 75 (01) 4-18
- Louis DN, Perry A, Wesseling P. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncol 2021; 23 (08) 1231-1251
- Barresi V, Eccher A, Simbolo M. et al. Diffuse gliomas in patients aged 55 years or over: a suggestion for IDH mutation testing. Neuropathology 2020; 40 (01) 68-74
- Whitfield BT, Huse JT. Classification of adult-type diffuse gliomas: impact of the World Health Organization 2021 update. Brain Pathol 2022; 32 (04) e13062 DOI: 10.1111/bpa.13062.
- Iv M, Yoon BC, Heit JJ, Fischbein N, Wintermark M. Current clinical state of advanced magnetic resonance imaging for brain tumor diagnosis and follow up. Semin Roentgenol 2018; 53 (01) 45-61
- Blumenthal DT, Aisenstein O, Ben-Horin I. et al. Calcification in high grade gliomas treated with bevacizumab. J Neurooncol 2015; 123 (02) 283-288
- Lyndon D, Lansley JA, Evanson J, Krishnan AS. Dural masses: meningiomas and their mimics. Insights Imaging 2019; 10 (01) 11
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011; 32 (06) 984-992
- Zhang D, Hu LB, Henning TD. et al. MRI findings of primary CNS lymphoma in 26 immunocompetent patients. Korean J Radiol 2010; 11 (03) 269-277
- Yamasaki F, Kurisu K, Satoh K. et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005; 235 (03) 985-991
- Ellingson BM, Malkin MG, Rand SD. et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 2010; 31 (03) 538-548
- Kono K, Inoue Y, Nakayama K. et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001; 22 (06) 1081-1088
- Park SI, Suh CH, Guenette JP, Huang RY, Kim HS. The T2-FLAIR mismatch sign as a predictor of IDH-mutant, 1p/19q-noncodeleted lower-grade gliomas: a systematic review and diagnostic meta-analysis. Eur Radiol 2021; 31 (07) 5289-5299
- Kitis O, Altay H, Calli C, Yunten N, Akalin T, Yurtseven T. Minimum apparent diffusion coefficients in the evaluation of brain tumors. Eur J Radiol 2005; 55 (03) 393-400
- Bozdağ M, Er A, Ekmekçi S. Association of apparent diffusion coefficient with Ki-67 proliferation index, progesterone-receptor status and various histopathological parameters, and its utility in predicting the high grade in meningiomas. Acta Radiol 2021; 62 (03) 401-413
- Makino K, Hirai T, Nakamura H. et al. Differentiating between primary central nervous system lymphomas and glioblastomas: combined use of perfusion-weighted and diffusion-weighted magnetic resonance imaging. World Neurosurg 2018; 112: e1-e6
- Law M, Yang S, Wang H. et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003; 24 (10) 1989-1998
- Law M, Young RJ, Babb JS. et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008; 247 (02) 490-498
- Kong L, Chen H, Yang Y, Chen L. A meta-analysis of arterial spin labelling perfusion values for the prediction of glioma grade. Clin Radiol 2017; 72 (03) 255-261
- Danchaivijitr N, Waldman AD, Tozer DJ. et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation?. Radiology 2008; 247 (01) 170-178
- Winter RC, Antunes ACM, de Oliveira FH. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. Surg Neurol Int 2020; 11: 328
- Xing Z, You RX, Li J, Liu Y, Cao DR. Differentiation of primary central nervous system lymphomas from high-grade gliomas by rCBV and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Clin Neuroradiol 2014; 24 (04) 329-336
- Lee MD, Baird GL, Bell LC, Quarles CC, Boxerman JL. Utility of percentage signal recovery and baseline signal in DSC-MRI optimized for relative CBV measurement for differentiating glioblastoma, lymphoma, metastasis, and meningioma. AJNR Am J Neuroradiol 2019; 40 (09) 1445-1450
- Jiang S, Eberhart CG, Lim M. et al. Identifying recurrent malignant glioma after treatment using amide proton transfer-weighted MR imaging: a validation study with image-guided stereotactic biopsy. Clin Cancer Res 2019; 25 (02) 552-561
- Cha S, Lupo JM, Chen MH. et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2007; 28 (06) 1078-1084
- Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol 2011; 32 (06) 1004-1010
- Roberts HC, Roberts TP, Bollen AW, Ley S, Brasch RC, Dillon WP. Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol 2001; 8 (05) 384-391
- Fudaba H, Shimomura T, Abe T. et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 2014; 35 (11) 2091-2098
- Byrnes TJD, Barrick TR, Bell BA, Clark CA. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed 2011; 24 (01) 54-60
- Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 2004; 232 (01) 221-228
- Farquharson S, Tournier JD, Calamante F. et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg 2013; 118 (06) 1367-1377
- Mandelli ML, Berger MS, Bucci M, Berman JI, Amirbekian B, Henry RG. Quantifying accuracy and precision of diffusion MR tractography of the corticospinal tract in brain tumors. J Neurosurg 2014; 121 (02) 349-358
- Dahnert, Wolfgang. (2011). Radiology Review Manual (7th ed.). Philadelphia: Wolter Kluwer Health
- Al-Okaili RN, Krejza J, Woo JH. et al. Intraaxial brain masses: MR imaging-based diagnostic strategy–initial experience. Radiology 2007; 243 (02) 539-550 [PubMed: 17456876]
- Iv M, Bisdas S. Neuroimaging in the era of the evolving WHO classification of brain tumors, from the AJR special series on cancer staging. AJR Am J Roentgenol 2021; 217 (01) 3-15
- Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging 2018; 2018: 6828396 DOI: 10.1155/2018/6828396.
- Sagiyama K, Mashimo T, Togao O. et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A 2014; 111 (12) 4542-4547
- Wen PY, Macdonald DR, Reardon DA. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28 (11) 1963-1972
- Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 2017; 14 (02) 307-320
- Kim S, Hoch MJ, Cooper ME, Gore A, Weinberg BD. Using a website to teach a structured reporting system, the brain tumor reporting and data system. Curr Probl Diagn Radiol 2020 [published online]
- BT-RADS website. Brain tumor reporting and data system (BT-RADS). Accessed November 18, 2022, at: www.btrads.com
- Singhal V. Clinical approach to acute decline in sensorium. Indian J Crit Care Med 2019; 23 (Suppl 2): S120-S123
- Shorvon S. The management of status epilepticus. J Neurol Neurosurg Psychiatry 2001; 70 (Suppl 2): II22-II27
- Maschio M, Aguglia U, Avanzini G. et al; Brain Tumor-related Epilepsy study group of Italian League Against Epilepsy (LICE). Management of epilepsy in brain tumors. Neurol Sci 2019; 40 (10) 2217-2234
- ;Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 2011; 4 (02) 233-242
- Jiang T, Nam DH, Ram Z. et al; Chinese Glioma Cooperative Group (CGCG), Society for Neuro-Oncology of China (SNO-China), Chinese Brain Cancer Association (CBCA), Chinese Glioma Genome Atlas (CGGA), Asian Glioma Genome Atlas (AGGA) network. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett 2021; 499: 60-72
- , Ed. (2020). Management of Gliomas: Individualized Treatment Options, Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 18(7.5), 985–988. Accessed November 18, 2022, at: https://jnccn.org/view/journals/jnccn/18/7.5/article-p985.xml
- McFaline-Figueroa JR, Lee EQ. Brain tumors. Am J Med 2018; 131 (08) 874-882
- Goldbrunner R, Stavrinou P, Jenkinson MD. et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-oncol 2021; 23 (11) 1821-1834
- Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA 2017; 317 (05) 516-524
- von Baumgarten L, Illerhaus G, Korfel A, Schlegel U, Deckert M, Dreyling M. The diagnosis and treatment of primary CNS lymphoma. Dtsch Arztebl Int 2018; 115 (25) 419-426
- Hoang-Xuan K, Deckert M, Ferreri AJM. et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro-oncol 2022; •••: noac196 ; Epub ahead of print DOI: 10.1093/neuonc/noac196.
- Le Rhun E, Guckenberger M, Smits M. et al; EANO Executive Board and ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021; 32 (11) 1332-1347
- Bette S, Gempt J, Huber T. et al. Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg 2016; 90: 440-447
- Raverot G, Burman P, McCormack A. et al; European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol 2018; 178 (01) G1-G24
- Bink A, Benner J, Reinhardt J. et al. Structured reporting in neuroradiology: intracranial tumors. Front Neurol 2018; 9: 32 DOI: 10.3389/fneur.2018.00032.
Address for correspondence
Publication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Characteristic imaging features in a case of glioblastoma. Fluid-attenuated axial inversion recovery mixed signal intensity lesion (A) crossing across the midline via corpus callosum showing central blooming (compatible with bleed) (B) along with diffusion heterogeneity (C). On postcontrast images (D) irregular peripheral enhancement noted and on relative cerebral blood volume maps (E), increased perfusion noted in the corresponding region.

| Figure 2:Case of diffuse large B cell lymphoma. T2-weighted imaging reveals a small area of hyperintensity in the genu of the corpus callosum. (A). Percentage signal recovery is high (red curve) on dynamic susceptibility contrast perfusion imaging in lymphoma with mild elevation in relative cerebral blood volume (B).

| Figure 3:Case of IDH wild glioblastoma in the right temporal lobe. The lesion represents heterogeneous hyperintensity on T2-weighted imaging (A) as well as elevated K trans value of 0.322 on dynamic contrast enhancement perfusion imaging (B).

| Flowchart 1:Image interpretation algorithm 1. FLAIR, fluid-attenuated axial inversion recovery; MRI, magnetic resonance imaging.

| Flowchart 2:Image interpretation algorithm 2. ADC, apparent diffusion coefficient; MR, magnetic resonance; NAA,—; rCBV, relative cerebral blood volume.

| Figure 4 :Post-treatment change in the form of radiation necrosis in a patient after resection of a higher-grade glioma followed by postoperative radiotherapy. (A) There is fluid-attenuated axial inversion recovery (FLAIR) hyperintensity surrounding the operative cavity. (B) There is heterogeneous “swiss-cheese” enhancement within. (C) On susceptibility-weighted imaging, there are multiple foci of blooming in the hyperintensity that most likely represent postradiotherapy cavernomas. (D) No restriction on diffusion-weighted imaging and (E, F) no elevated relative cerebral blood volume on perfusion imaging within the FLAIR hyperintensity and the enhancing area as compared with the contralateral side on perfusion color map and dynamic

| Figure 5:Recurrence in the postoperative higher grade glioma patient. (A) Fluid-attenuated axial inversion recovery image is noted to show a heterogeneous hyperintensity in the left parietal lobe. (B) Heterogeneous enhancement on postcontrast scan has been seen. (C) Susceptibility-weighted imaging shows blood products that are most likely postoperative. (D, E) There is heterogeneous diffusion on diffusion-weighted imaging and apparent diffusion coefficient with a few areas of restriction. (F) Dynamic susceptibility contrast-perfusion image shows elevated relative cerebral blood volume within the enhancing region. (G) Spectroscopy with intermediate echo time shows significantly elevated choline with a lactate peak.
References
- Castillo M. History and evolution of brain tumor imaging: insights through radiology. Radiology 2014; 273 (2, Suppl) S111-S125
- Geva T. Magnetic resonance imaging: historical perspective. J Cardiovasc Magn Reson 2006; 8 (04) 573-580
- McNeill KA. Epidemiology of brain tumors. Neurol Clin 2016; 34 (04) 981-998
- de Robles P, Fiest KM, Frolkis AD. et al. The worldwide incidence and prevalence of primary brain tumors: a systematic review and meta-analysis. Neuro-oncol 2015; 17 (06) 776-783
- Porter KR, McCarthy BJ, Freels S, Kim Y, Davis FG. Prevalence estimates for primary brain tumors in the United States by age, gender, behavior, and histology. Neuro-oncol 2010; 12 (06) 520-527
- Dasgupta A, Gupta T, Jalali R. Indian data on central nervous tumors: a summary of published work. South Asian J Cancer 2016; 5 (03) 147-153
- Alther B, Mylius V, Weller M, Gantenbein A. From first symptoms to diagnosis: Initial clinical presentation of primary brain tumors. Clinical and Translational Neuroscience. 2020; 4 (02) DOI: 10.1177/2514183 × 20968368.
- Weller M, van den Bent M, Preusser M. et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol 2021; 18 (03) 170-186 DOI: 10.1038/s41571-020-00447-z. Erratum in: Nat Rev Clin Oncol. 2022 May;19(5):357–358. PMID: 33293629; PMCID: PMC7904519
- Xiao F, Lv S, Zong Z. et al. Cerebrospinal fluid biomarkers for brain tumor detection: clinical roles and current progress. Am J Transl Res 2020; 12 (04) 1379-1396
- Merve A, Millner TO, Marino S. Integrated phenotype-genotype approach in diagnosis and classification of common central nervous system tumours. Histopathology 2019; 75 (03) 299-311
- Akshulakov SK, Kerimbayev TT, Biryuchkov MY, Urunbayev YA, Farhadi DS, Byvaltsev VA. Current trends for improving safety of stereotactic brain biopsies: advanced optical methods for vessel avoidance and tumor detection. Front Oncol 2019; 9: 947 DOI: 10.3389/fonc.2019.00947.
- Tanboon J, Williams EA, Louis DN. The diagnostic use of immunohistochemical surrogates for signature molecular genetic alterations in gliomas. J Neuropathol Exp Neurol 2016; 75 (01) 4-18
- Louis DN, Perry A, Wesseling P. et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro-oncol 2021; 23 (08) 1231-1251
- Barresi V, Eccher A, Simbolo M. et al. Diffuse gliomas in patients aged 55 years or over: a suggestion for IDH mutation testing. Neuropathology 2020; 40 (01) 68-74
- Whitfield BT, Huse JT. Classification of adult-type diffuse gliomas: impact of the World Health Organization 2021 update. Brain Pathol 2022; 32 (04) e13062 DOI: 10.1111/bpa.13062.
- Iv M, Yoon BC, Heit JJ, Fischbein N, Wintermark M. Current clinical state of advanced magnetic resonance imaging for brain tumor diagnosis and follow up. Semin Roentgenol 2018; 53 (01) 45-61
- Blumenthal DT, Aisenstein O, Ben-Horin I. et al. Calcification in high grade gliomas treated with bevacizumab. J Neurooncol 2015; 123 (02) 283-288
- Lyndon D, Lansley JA, Evanson J, Krishnan AS. Dural masses: meningiomas and their mimics. Insights Imaging 2019; 10 (01) 11
- Haldorsen IS, Espeland A, Larsson EM. Central nervous system lymphoma: characteristic findings on traditional and advanced imaging. AJNR Am J Neuroradiol 2011; 32 (06) 984-992
- Zhang D, Hu LB, Henning TD. et al. MRI findings of primary CNS lymphoma in 26 immunocompetent patients. Korean J Radiol 2010; 11 (03) 269-277
- Yamasaki F, Kurisu K, Satoh K. et al. Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 2005; 235 (03) 985-991
- Ellingson BM, Malkin MG, Rand SD. et al. Validation of functional diffusion maps (fDMs) as a biomarker for human glioma cellularity. J Magn Reson Imaging 2010; 31 (03) 538-548
- Kono K, Inoue Y, Nakayama K. et al. The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 2001; 22 (06) 1081-1088
- Park SI, Suh CH, Guenette JP, Huang RY, Kim HS. The T2-FLAIR mismatch sign as a predictor of IDH-mutant, 1p/19q-noncodeleted lower-grade gliomas: a systematic review and diagnostic meta-analysis. Eur Radiol 2021; 31 (07) 5289-5299
- Kitis O, Altay H, Calli C, Yunten N, Akalin T, Yurtseven T. Minimum apparent diffusion coefficients in the evaluation of brain tumors. Eur J Radiol 2005; 55 (03) 393-400
- Bozdağ M, Er A, Ekmekçi S. Association of apparent diffusion coefficient with Ki-67 proliferation index, progesterone-receptor status and various histopathological parameters, and its utility in predicting the high grade in meningiomas. Acta Radiol 2021; 62 (03) 401-413
- Makino K, Hirai T, Nakamura H. et al. Differentiating between primary central nervous system lymphomas and glioblastomas: combined use of perfusion-weighted and diffusion-weighted magnetic resonance imaging. World Neurosurg 2018; 112: e1-e6
- Law M, Yang S, Wang H. et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003; 24 (10) 1989-1998
- Law M, Young RJ, Babb JS. et al. Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 2008; 247 (02) 490-498
- Kong L, Chen H, Yang Y, Chen L. A meta-analysis of arterial spin labelling perfusion values for the prediction of glioma grade. Clin Radiol 2017; 72 (03) 255-261
- Danchaivijitr N, Waldman AD, Tozer DJ. et al. Low-grade gliomas: do changes in rCBV measurements at longitudinal perfusion-weighted MR imaging predict malignant transformation?. Radiology 2008; 247 (01) 170-178
- Winter RC, Antunes ACM, de Oliveira FH. The relationship between vascular endothelial growth factor and histological grade in intracranial meningioma. Surg Neurol Int 2020; 11: 328
- Xing Z, You RX, Li J, Liu Y, Cao DR. Differentiation of primary central nervous system lymphomas from high-grade gliomas by rCBV and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Clin Neuroradiol 2014; 24 (04) 329-336
- Lee MD, Baird GL, Bell LC, Quarles CC, Boxerman JL. Utility of percentage signal recovery and baseline signal in DSC-MRI optimized for relative CBV measurement for differentiating glioblastoma, lymphoma, metastasis, and meningioma. AJNR Am J Neuroradiol 2019; 40 (09) 1445-1450
- Jiang S, Eberhart CG, Lim M. et al. Identifying recurrent malignant glioma after treatment using amide proton transfer-weighted MR imaging: a validation study with image-guided stereotactic biopsy. Clin Cancer Res 2019; 25 (02) 552-561
- Cha S, Lupo JM, Chen MH. et al. Differentiation of glioblastoma multiforme and single brain metastasis by peak height and percentage of signal intensity recovery derived from dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 2007; 28 (06) 1078-1084
- Mangla R, Kolar B, Zhu T, Zhong J, Almast J, Ekholm S. Percentage signal recovery derived from MR dynamic susceptibility contrast imaging is useful to differentiate common enhancing malignant lesions of the brain. AJNR Am J Neuroradiol 2011; 32 (06) 1004-1010
- Roberts HC, Roberts TP, Bollen AW, Ley S, Brasch RC, Dillon WP. Correlation of microvascular permeability derived from dynamic contrast-enhanced MR imaging with histologic grade and tumor labeling index: a study in human brain tumors. Acad Radiol 2001; 8 (05) 384-391
- Fudaba H, Shimomura T, Abe T. et al. Comparison of multiple parameters obtained on 3T pulsed arterial spin-labeling, diffusion tensor imaging, and MRS and the Ki-67 labeling index in evaluating glioma grading. AJNR Am J Neuroradiol 2014; 35 (11) 2091-2098
- Byrnes TJD, Barrick TR, Bell BA, Clark CA. Diffusion tensor imaging discriminates between glioblastoma and cerebral metastases in vivo. NMR Biomed 2011; 24 (01) 54-60
- Lu S, Ahn D, Johnson G, Law M, Zagzag D, Grossman RI. Diffusion-tensor MR imaging of intracranial neoplasia and associated peritumoral edema: introduction of the tumor infiltration index. Radiology 2004; 232 (01) 221-228
- Farquharson S, Tournier JD, Calamante F. et al. White matter fiber tractography: why we need to move beyond DTI. J Neurosurg 2013; 118 (06) 1367-1377
- Mandelli ML, Berger MS, Bucci M, Berman JI, Amirbekian B, Henry RG. Quantifying accuracy and precision of diffusion MR tractography of the corticospinal tract in brain tumors. J Neurosurg 2014; 121 (02) 349-358
- Dahnert, Wolfgang. (2011). Radiology Review Manual (7th ed.). Philadelphia: Wolter Kluwer Health
- Al-Okaili RN, Krejza J, Woo JH. et al. Intraaxial brain masses: MR imaging-based diagnostic strategy–initial experience. Radiology 2007; 243 (02) 539-550 [PubMed: 17456876]
- Iv M, Bisdas S. Neuroimaging in the era of the evolving WHO classification of brain tumors, from the AJR special series on cancer staging. AJR Am J Roentgenol 2021; 217 (01) 3-15
- Zikou A, Sioka C, Alexiou GA, Fotopoulos A, Voulgaris S, Argyropoulou MI. Radiation necrosis, pseudoprogression, pseudoresponse, and tumor recurrence: imaging challenges for the evaluation of treated gliomas. Contrast Media Mol Imaging 2018; 2018: 6828396 DOI: 10.1155/2018/6828396.
- Sagiyama K, Mashimo T, Togao O. et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc Natl Acad Sci U S A 2014; 111 (12) 4542-4547
- Wen PY, Macdonald DR, Reardon DA. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 2010; 28 (11) 1963-1972
- Ellingson BM, Wen PY, Cloughesy TF. Modified criteria for radiographic response assessment in glioblastoma clinical trials. Neurotherapeutics 2017; 14 (02) 307-320
- Kim S, Hoch MJ, Cooper ME, Gore A, Weinberg BD. Using a website to teach a structured reporting system, the brain tumor reporting and data system. Curr Probl Diagn Radiol 2020 [published online]
- BT-RADS website. Brain tumor reporting and data system (BT-RADS). Accessed November 18, 2022, at: www.btrads.com
- Singhal V. Clinical approach to acute decline in sensorium. Indian J Crit Care Med 2019; 23 (Suppl 2): S120-S123
- Shorvon S. The management of status epilepticus. J Neurol Neurosurg Psychiatry 2001; 70 (Suppl 2): II22-II27
- Maschio M, Aguglia U, Avanzini G. et al; Brain Tumor-related Epilepsy study group of Italian League Against Epilepsy (LICE). Management of epilepsy in brain tumors. Neurol Sci 2019; 40 (10) 2217-2234
- ;Dietrich J, Rao K, Pastorino S, Kesari S. Corticosteroids in brain cancer patients: benefits and pitfalls. Expert Rev Clin Pharmacol 2011; 4 (02) 233-242
- Jiang T, Nam DH, Ram Z. et al; Chinese Glioma Cooperative Group (CGCG), Society for Neuro-Oncology of China (SNO-China), Chinese Brain Cancer Association (CBCA), Chinese Glioma Genome Atlas (CGGA), Asian Glioma Genome Atlas (AGGA) network. Clinical practice guidelines for the management of adult diffuse gliomas. Cancer Lett 2021; 499: 60-72
- , Ed. (2020). Management of Gliomas: Individualized Treatment Options, Journal of the National Comprehensive Cancer Network J Natl Compr Canc Netw, 18(7.5), 985–988. Accessed November 18, 2022, at: https://jnccn.org/view/journals/jnccn/18/7.5/article-p985.xml
- McFaline-Figueroa JR, Lee EQ. Brain tumors. Am J Med 2018; 131 (08) 874-882
- Goldbrunner R, Stavrinou P, Jenkinson MD. et al. EANO guideline on the diagnosis and management of meningiomas. Neuro-oncol 2021; 23 (11) 1821-1834
- Molitch ME. Diagnosis and treatment of pituitary adenomas: a review. JAMA 2017; 317 (05) 516-524
- von Baumgarten L, Illerhaus G, Korfel A, Schlegel U, Deckert M, Dreyling M. The diagnosis and treatment of primary CNS lymphoma. Dtsch Arztebl Int 2018; 115 (25) 419-426
- Hoang-Xuan K, Deckert M, Ferreri AJM. et al. European Association of Neuro-Oncology (EANO) guidelines for treatment of primary central nervous system lymphoma (PCNSL). Neuro-oncol 2022; •••: noac196 ; Epub ahead of print DOI: 10.1093/neuonc/noac196.
- Le Rhun E, Guckenberger M, Smits M. et al; EANO Executive Board and ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with brain metastasis from solid tumours. Ann Oncol 2021; 32 (11) 1332-1347
- Bette S, Gempt J, Huber T. et al. Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg 2016; 90: 440-447
- Raverot G, Burman P, McCormack A. et al; European Society of Endocrinology. European Society of Endocrinology Clinical Practice Guidelines for the management of aggressive pituitary tumours and carcinomas. Eur J Endocrinol 2018; 178 (01) G1-G24
- Bink A, Benner J, Reinhardt J. et al. Structured reporting in neuroradiology: intracranial tumors. Front Neurol 2018; 9: 32 DOI: 10.3389/fneur.2018.00032.


 PDF
PDF  Views
Views  Share
Share

