Imaging Recommendations for Image-Guided Biopsy in Oncology
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(03): 334-342
DOI: DOI: 10.1055/s-0043-1761265
Abstract
The percutaneous needle biopsy (PNB) is the initial step for obtaining the diagnosis and it helps in treatment. It aids in primary diagnosis, tumor staging, or ruling out infective etiology. It is a safe and successful minimally invasive method compared to open biopsies. PNB is defined as the placement and insertion of a needle into a suspected lesion or organ with the intent of retrieving tissue or cells for diagnosis. It can fine needle aspiration cytology or core needle biopsy. The patient needs to be counseled regarding the procedure and detailed history, including anticoagulant history needs to be taken. The SIR consensus guidelines have divided biopsies into low-risk procedures with a bleeding risk of < 1.5% and high-risk procedures > 1.5%. There are advancements in needle design (e.g., echogenic tip while performing ultrasound-guided needle biopsy) and image-guidance technology (ultrasound quality, multi-slice CT scan) that improved these procedures safety and efficacy. There are different types of needles available such as coaxial, aspiration needles, Murphy's bone biopsy needle, which depends on the tissue which needs to be sampled or the organ to be biopsied. Various different types of biopsy guns, such as semi-automatic, automatic, or manual are available. The newer technology such as fusion and navigation biopsies helps in better characterizing and localization of the lesion, improving the yield of the biopsy. Open and excisional biopsies have a higher mortality and morbidity rate than percutaneous biopsies, they are reserved for cases where the image-guided method has failed to provide the diagnostic yield. Sample collection must be done under a sterile container in formaline or microbiological examination. Regular analysis and rad-path correlation are key to success and improving diagnostic yield. This abstract provides an overview of the existing biopsy literature.
Keywords
diagnostic accuracy - complication - core-needle biopsy (CNB) - oncologic imaging - percutaneous biopsyNote
The article is not under consideration for publication elsewhere. Each author participated sufficiently for the work to be submitted. Publication is approved by all authors.
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The percutaneous needle biopsy (PNB) is the initial step for obtaining the diagnosis and it helps in treatment. It aids in primary diagnosis, tumor staging, or ruling out infective etiology. It is a safe and successful minimally invasive method compared to open biopsies. PNB is defined as the placement and insertion of a needle into a suspected lesion or organ with the intent of retrieving tissue or cells for diagnosis. It can fine needle aspiration cytology or core needle biopsy. The patient needs to be counseled regarding the procedure and detailed history, including anticoagulant history needs to be taken. The SIR consensus guidelines have divided biopsies into low-risk procedures with a bleeding risk of < 1.5%-and high-risk procedures > 1.5%. There are advancements in needle design (e.g., echogenic tip while performing ultrasound-guided needle biopsy) and image-guidance technology (ultrasound quality, multi-slice CT scan) that improved these procedures safety and efficacy. There are different types of needles available such as coaxial, aspiration needles, Murphy's bone biopsy needle, which depends on the tissue which needs to be sampled or the organ to be biopsied. Various different types of biopsy guns, such as semi-automatic, automatic, or manual are available. The newer technology such as fusion and navigation biopsies helps in better characterizing and localization of the lesion, improving the yield of the biopsy. Open and excisional biopsies have a higher mortality and morbidity rate than percutaneous biopsies, they are reserved for cases where the image-guided method has failed to provide the diagnostic yield. Sample collection must be done under a sterile container in formaline or microbiological examination. Regular analysis and rad-path correlation are key to success and improving diagnostic yield. This abstract provides an overview of the existing biopsy literature.
Keywords
diagnostic accuracy - complication - core-needle biopsy (CNB) - oncologic imaging - percutaneous biopsyIntroduction
Percutaneous needle biopsy is an integral part of cancer diagnosis and treatment. It is the first step in achieving the diagnosis.[1] It helps in tumor staging because it provides pathologic confirmation of metastasis. Percutaneous image biopsy-guided biopsies are minimally invasive, safe, and effective procedures.[2] The safety and efficacy of these treatments have increased due to advancements in needle design (e.g., echogenic tip while doing ultrasound-guided needle biopsy) and image-guidance technologies.[3] Open and excisional biopsies are reserved for selected cases where the image-guided procedure has failed to give the diagnostic yield because it is more mortality and morbidity than percutaneous biopsy. This document provides an overall review of the current literature on biopsy.[4]
Indications
Following are scenarios where biopsy is recommended-[Table 1]
A)Diagnosis of pulmonary nodules-computed tomography (CT) is the preferred image guidance modality. Ideally, the most vertical (perpendicuar) path is selected, which avoids bullae, interlobar fissures, and large vessels[5] ([Fig. 1A–C]).
B)Mediastinal lesions-CT-guided direct mediastinal or extrapleural approach is generally preferred over a transpulmonary approach to avoid pleural transgression and thereby decrease the risk of pneumothorax and pulmonary haemorrhage.[6] Ultrasonographic (USG) guidance can be used for large mediastinal lesions, which are in contact with the chest wall and are easily visualised ([Fig. 1D–F]).
C)Abdominal and pelvic lesions-Soft-tissue masses involving the peritoneum, omentum, retroperitoneal nodes, and mesenteric lymph nodes are amenable to percutaneous biopsies under USG or CT guidance depending on size and location. Liver biopsies are done under USG guidance; however, CT guidance can be preferred when there is difficulty in visualizing lesions such as under the dome of the diaphragm. In certain cases, a combination of modalities can be used where both USG and CT scan can be used for lesions near para cardiac location[7] or navigation/fusion techniques with USG guidance can be done ([Fig. 2]).
D)Musculoskeletal tumors-Soft tissue masses can be easily targeted on USG; however, CT guidance is recommended for lesions limited to bone. The needle path and approach need to be discussed with the orthopaedic oncosurgeon because an inappropriately performed biopsy will lead to contamination of the compartment, wider excision during surgery, a wider area of irradiation, and difficulty in flap reconstruction of the defect.
E)Spinal lesions-The biopsy of vertebral lesions depends on the location of the lesion within the vertebra and the level of vertebra. It can be transpedicular, parapedicular, transforaminal, and para laminar for lumbar and thoracic vertebrae. It can be an anterolateral, posterolateral, posterior, and anterior transoral approach under fluoroscopy or CT guidance for the cervical vertebra, depending on the location of the lesion[8] ([Fig. 3]).
F)Head and neck lesions-Deep seated head and neck biopsies are most commonly performed under CT guidance. Various approaches such as subzygomatic, retromandibular, paramaxillary and submastoid, transoral and posterior approaches are commonly used.[9] In contrast, the biopsies of superficial organs such as lymph node, submental, parotid glands, and thyroid gland lesions are done under USG guidance ([Fig. 4]).
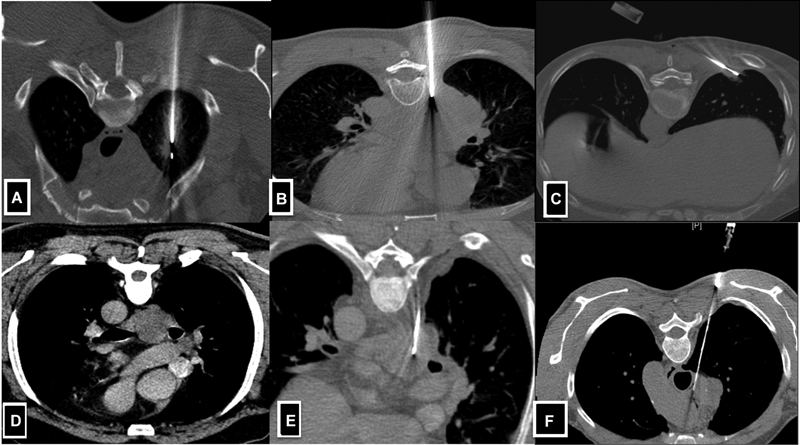
| Fig 1 :(A-F) Axial CT images demonstrate lung and mediastinal lesion biopsy technique. A-Solitary lesion in the right upper lobe posterior approach taken where the needle is perpendicular to the lesion. B-Large lung mass in the right lower lobe posterior approach taken for biopsy. C-Small lesion located in the subpleural location of the right lower lobe, tangential approach taken to obtain the larger length of the core. D & E-CECT reveals subcarinal lymph node in k/c/o carcinoma of the lung. Extrapleural posterior approach was taken with hydro dissection of the parietal and visceral pleural and a biopsy of the mediastinal lymph node was done.
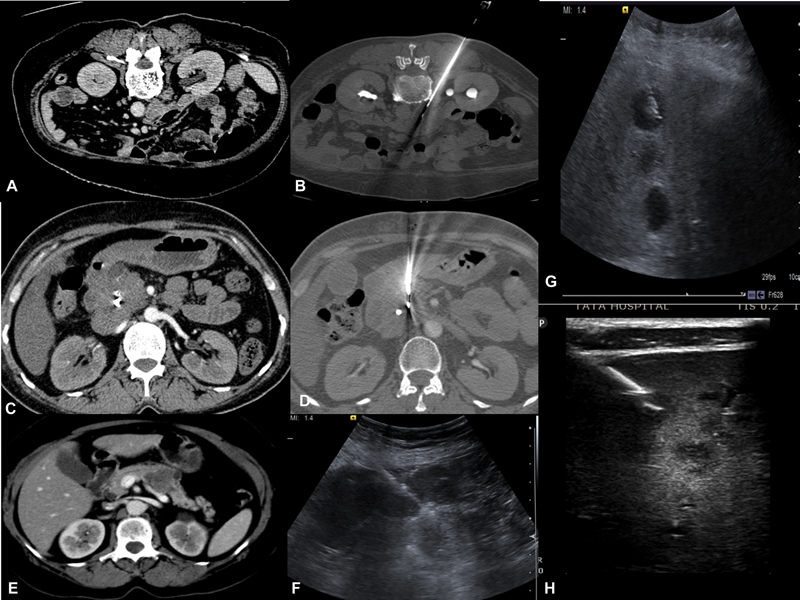
| Figure 2:(A-B) Left Paraspinal approach for biopsy of the aortocaval lymph node. (C-D) CECT reveals pancreatic head mass where biopsy is performed via transgastric approach.(E-F) CECT reveals pancreatic body mass, USG guided biopsy of pancreatic mass with compression of bowel loops. (G-H) USG guided liver lesions biopsy traversing normal liver parenchyma. H-Lesions appreciated by using high-frequency probe.
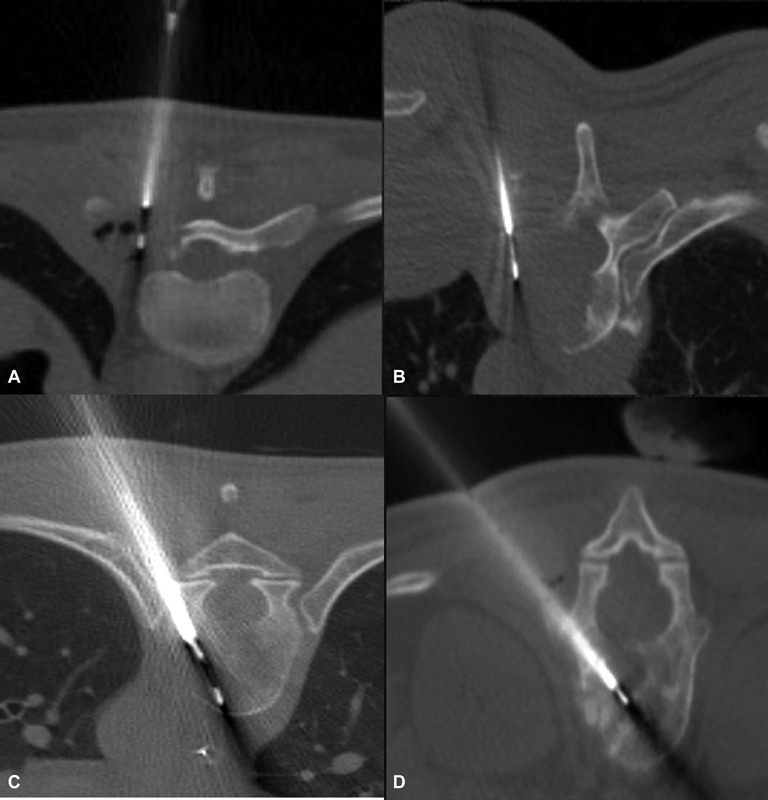
| Figure 3:(A and B) Axial CT images demonstrate vertebral lesion biopsy by parapedicular technique. (C and D) Axial CT images demonstrate vertebral lesion biopsy by transpedicular technique.
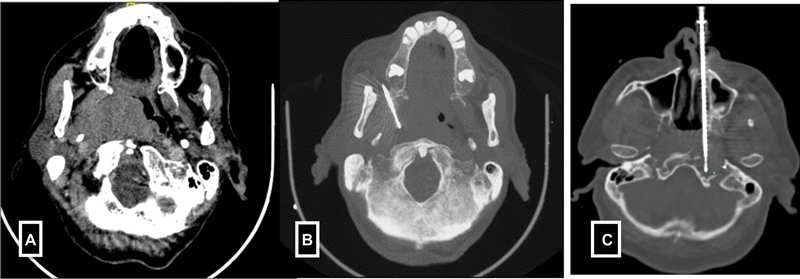
| Figure 4:(A-B) Paramaxiiary approach for parapharyngeal mass biopsy. (C) Trans nasal approach for petrous mass biopsy.
|
1. To establish the diagnosis of the benign or malignant nature of the lesion. 2. To obtain tissue for microbiological analyses in suspected or known infections in the post-treatment setting. 3. To classify a malignancy and perform immunohistochemistry (IHC) on the sample and obtain molecular analysis for the targeted. 4. To stage a patient with suspected malignant tumors elsewhere.[3] Contraindication Absolute- - Difficult location, - Irreversible coagulopathy and - Refusal to Consent. Relative - Unco-operative patient (can be done under general anasthesia), - Significant co-morbidities (e.g., respiratory or hemodynamic instability), - Pregnancy (especially if ionizing radiation is present, can be done ultrasound-guided.)[4] |
Definitions
According to the Cardiovascular and Interventional Radiological Society of Europe (CIRSE), the definition of percutaneous needle biopsy (PNB) is “The placement or insertion of a needle into a suspected lesion or organ for the intent of retrieving tissue or cells for diagnosis.” Imaging techniques used as a guide for PNB are ultrasound, fluoroscopy, computed tomography, positron emission tomography CT (PET-CT) and magnetic resonance imaging (MRI). The imaging technology used is determined by nature, location of the lesion, the patient's compliance, availability of the technique, and preference of the operators. Fine-needle aspiration cytology (FNAC) and core biopsy (CB) are two types of PNB. FNAC is a technique that involves using a thin, hollow needle (18–25 gauge) to collect cells for cytological evaluation by aspiration. FNAC is used to prove metastasis or where the lesion is difficult to biopsy due to the vicinity of a critical structure such as a major blood vessel, bowel or any major organ. CB is the extraction of a portion of tissue for the histological assessment using a hollow needle (9–20 gauge) with a cutting and capturing device.[4] [10]
The technical success of PNB is defined as the procurement of sufficient samples to establish the diagnosis. Clinical success is defined as a patient's outcome depending on the biopsy result. According to the protocol, clinical success guides the clinician for appropriate surgical or medical management.[4]
Patient Information and Consent
Prior to doing a biopsy, the patient's detailed medical history, anticoagulant history, clinical examination, laboratory data such as complete blood count and coagulation profile need to be done except for superficial cases, i.e., low-risk case (Category 1-according to CIRSE). Pre-procedural cross-section images need to be available for procedural planning, such as choosing the best imaging guidance (with or without contrast injection to delineate the lesion better), the patient's position, access routes, needle type and trajectory, and the number of samples to be collected. Following institutional protocols, informed consent should be sought directly by the interventionist doing the procedure. The indications, procedure details, complications, and alternative options must be appropriately discussed with patients. The need for prophylactic antibiotics (transrectal biopsies), peri-procedural drugs, such as anesthesia and pain medication that may be required and post-procedure medications must be explained.[11] [12]
Contraindications and Precautions
Antiplatelet/anticoagulation drugs should be stopped before the procedure, especially for biopsies with a moderate or high risk of bleeding. Evaluation of coagulation status and discontinuing anticoagulation medications before the procedure as per the Society of Interventional Radiology (SIR) guidelines. The SIR consensus guidelines have divided biopsies into low-risk procedures with a bleeding risk of < 1.5%-and high-risk procedures >1.5%. Superficial biopsies and transjugular liver biopsies are categorized as low-risk, while solid organ and deep non-organ biopsies are categorized as high risk for bleeding. The SIR guidelines recommend the correction of the International Normalized Ratio (INR) to 2.0 to 3.0 for low-bleeding-risk procedures and 1.5 to 1.8 for high-bleeding risk procedures. Platelet transfusion should be considered if the platelet count is < 20 × 109/L or < 50 109/L for low and high bleeding risk procedures, respectively. It is recommended to withhold the antiplatelet or anticoagulant medications for 3 to 5 days before high-bleeding risk biopsies ([Table 2]).[13]
Technique/Protocol/Image Acquisition
A careful review of the cross-sectional/functional images needs to be done. The preferred imaging modality is chosen, and the biopsy trajectory is planned. Patients should be fasting for 4 to 6 hours before the procedure when sedation or deep anesthesia is needed, especially for children (below 15 years) and individuals who are apprehensive or have lesions in a critical location. Peripheral venous access (18–20 Gauge) should be obtained in all patients before the procedure and vitals need to be monitored pre-procedure, intra procedure, and at least 2 hours after the procedure. On the biopsy day, the checklist must be filled before the biopsy.[4]
Patient Positioning and Preparation
The patient is positioned depending on the lesion location, modality used, and easy access route to the lesion avoiding the major neurovascular structures, lung fissure and bullae, bowel etc. The patient should be in a comfortable and stable position for the procedure. The target route must be as short as possible; however, in certain specific situations, a longer route is advised, such as in subcapsular liver or lung lesions. A longer track with intervening normal liver parenchyma in the liver reduces the risk of hemoperitoneum due to the tamponade effect; similarly, in subpleural lesions, a longer oblique intraparenchymal needle path helps in the easy maneuvering of the needle and increases technical success.[14] In some situations, intravenous contrast media injection (including the contrast-enhanced US, e.g., for isoechoic liver or renal tumors) is required to visualize the target lesion before biopsy and visualize the vital structures. All biopsies are done in free-breathing techniques, rarely in a difficult location such as lung bases; it should be done in the end-expiratory phase as the lesion position will be relatively more stable.[15]
Techniques
Coaxial technique-A guide needle, larger than the biopsy needle (typically 9–19G), is advanced under image guidance, reaching the edge of the target. The inner stylet is removed, the biopsy gun is introduced into the lesion, and multiple specimens are taken with a single puncture. This may prevent tumor cells from seeding along the needle tract by re-inserting the inner stylet of the coaxial needle before the removal compared to the non-coaxial method. The coaxial technique reduces procedural time and procedure-related pain (by reducing repeated punctures) and also decreases the rate of complications ([Fig. 5]).
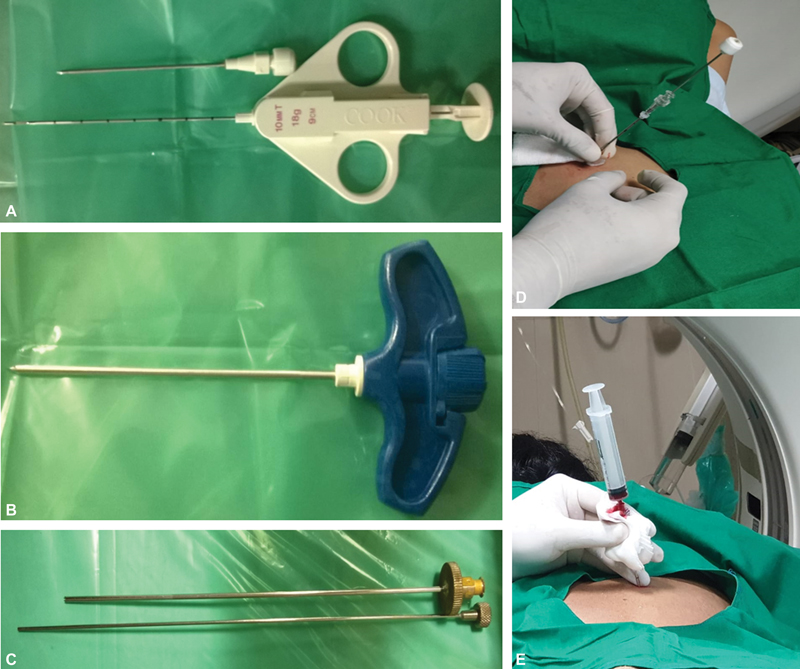
| Figure 5:(A) Coaxial biopsy system showing biopsy needle and gun. (B-C) Murphy's needle and ackerman's used for bone biopsy. (D-E) Post biopsy clot embolisation of biopsy tract in lung biopsy.
Hydro-dissection is a technique where 0.9%-saline solution facilitates image-guided biopsies by displacing important mobile structures (such as bowel, neurovascular bundle, etc.) away from the needle trajectory[16] ([Table 3]).
Image Analysis and Interpretation
A percutaneous needle biopsy can be performed under the US, CT, fluoroscopy, MR, fluoro-CT, cone-beam CT or PET-CT guidance. The advantages and disadvantages of the most frequently used image guidance techniques are summarized in [Table 4].
The selected imaging modality should allow the following:
Proper visualization of the target lesion and all the adjacent relevant anatomy.
Adequate visualization of biopsy equipment used during the procedure.
Comfortable patient positioning and operator's maneuvers.
Prompt and adequate evaluation and management of possible complications (e.g., pneumothorax).
Limited ionizing radiation exposure (particularly in children and young patients).
The new software has become available to facilitate needle insertion and target visualization during a biopsy under challenging lesions that allows fusing images obtained from different modalities, such as CT, MR or PET-CT with real-time USG or fluoroscopy. Special optical or electromagnetic navigation systems are required for performing biopsies using this technique.[17]
USG guidance-The needle can be inserted parallel or perpendicular to the transducer with the freehand or hand-guided technique. The tip needs to be visualized during needle insertion as an echogenic complex. Multiple punctures of the liver/renal capsule should be avoided to avoid the risk of bleeding. TRUS (trans-rectal)/TVUS (trans-vaginal) route can be used to biopsy prostate, vaginal/cervical vault lesions, and other deep-seated pelvic lesions ([Fig. 6]).[18]
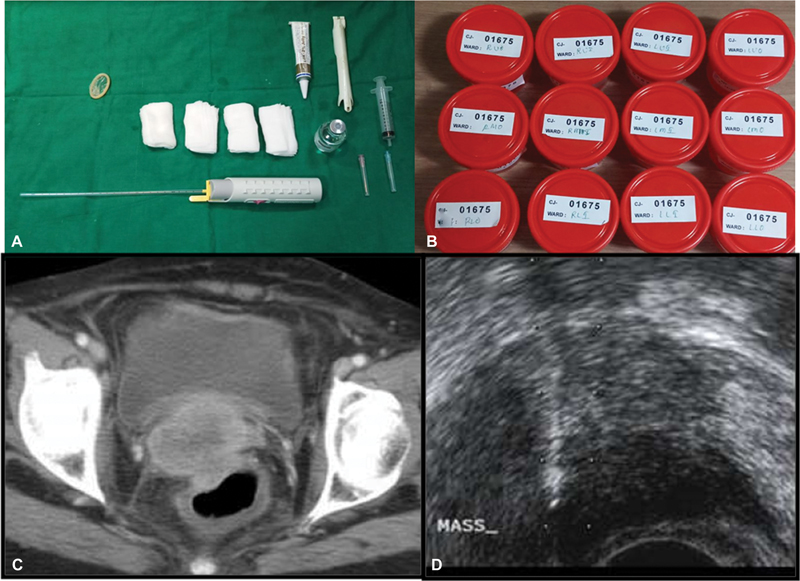
| Figure 6:(A-B) Instrumentation used in TRUS guided biopsy and 12 containers for 12 core biopsy of the prostate. (C-D) Axial CT shows an ill-defined cervical mass sampled through TRUS guided approach.
CT guidance-The biopsy planning is done on the axial scans and needs to be confirmed on the multiplanar reformatted images. The path length and angle are measured on the CT scan. The skin access point is marked using a radiopaque marker or a radiopaque grid positioned on the patient before the preliminary CT. A low-dose CT scan (reduced kVp and mAs) can be performed to reduce the overall dose of radiation[19] ([Table 4]).
|
USG |
CT/Fluoroscopy |
MRI |
|
|---|---|---|---|
|
Advantages |
Real-time imaging is available. Portability present. Availability. No radiation exposure, feasible for pregnant patients. Less procedure time. Less cost as no need to give contrast. |
Visualization of deep lesions is limited in obese patients. |
Near real time with specific sequences Excellent visualization of deep lesions. No radiation exposure. |
|
Disadvantages |
Visualization of deep lesions is limited in obese patients, however it can be overcome with the upcoming fusion biopsy technique. |
Real-time imaging only possible with CT fluoroscopy. Portability is an issue. Availability is an issue since the cost is more. Radiation exposure is an issue. More procedure time. More cost |
Portability is an issue. Availability is an issue since the cost is more. More procedure time. Maximum cost. |
References
- Gupta S, Madoff DC. Image-guided percutaneous needle biopsy in cancer diagnosis and staging. Tech Vasc Interv Radiol 2007; 10 (02) 88-101
- Cardella JF, Bakal CW, Bertino RE. et al; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for image-guided percutaneous biopsy in adults. J Vasc Interv Radiol 2003; 14 (9 Pt 2): S227-S230
- Schiavon LHO, Tyng CJ, Travesso DJ, Rocha RD, Schiavon ACSA, Bitencourt AGV. Computed tomography-guided percutaneous biopsy of abdominal lesions: indications, techniques, results, and complications. Radiol Bras 2018; 51 (03) 141-146
- Veltri A, Bargellini I, Giorgi L, Almeida PA, Akhan O. CIRSE guidelines on percutaneous needle biopsy (PNB). Cardiovascular and Interventional Radiology 2017; 40 (10) 1501-1513
- Gutierrez A, Abtin F, Suh RD. Percutaneous transthoracic lung biopsy. In percutaneous image-guided biopsy. New York, NY: Springer; 2014. :141–166
- Gupta S, Seaberg K, Wallace MJ. et al. Imaging-guided percutaneous biopsy of mediastinal lesions: different approaches and anatomic considerations. Radiographics 2005; 25 (03) 763-786 , discussion 786–788
- Shankar S, van Sonnenberg E, Silverman SG, Tuncali K. Interventional radiology procedures in the liver. Biopsy, drainage, and ablation. Clin Liver Dis 2002; 6 (01) 91-118
- Heyer CM, Al-Hadari A, Mueller KM, Stachon A, Nicolas V. Effectiveness of CT-guided percutaneous biopsies of the spine: an analysis of 202 examinations. Acad Radiol 2008; 15 (07) 901-911
- Wu EH, Chen YL, Wu YM, Huang YT, Wong HF, Ng SH. CT-guided core needle biopsy of deep suprahyoid head and neck lesions. Korean J Radiol 2013; 14 (02) 299-306
- Tam AL, Lim HJ, Wistuba II. et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol 2016; 27 (01) 8-19
- Lorentzen T, Nolsøe CP, Ewertsen C. et al; EFSUMB. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part I–general aspects (long version). Ultraschall Med 2015; 36 (05) E1-E14
- Sato S, Mishiro T, Miyake T. et al. Prophylactic administration of antibiotics unnecessary following ultrasound-guided biopsy and ablation therapy for liver tumors: open-labeled randomized prospective study. Hepatol Res 2009; 39 (01) 40-46
- Patel IJ, Rahim S, Davidson JC. et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol 2019; 30 (08) 1168-1184.e1 DOI: 10.1016/j.jvir.2019.04.017.
- Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010; 21 (07) 969-975
- Wu CC, Maher MM, Shepard JA. CT-guided percutaneous needle biopsy of the chest: preprocedural evaluation and technique. Am J Roentgenol 2011; 196 (05) W511-4
- Tyng CJ, Bitencourt AG, Martins EB, Pinto PN, Chojniak R. Technical note: CT-guided paravertebral adrenal biopsy using hydrodissection–a safe and technically easy approach. Br J Radiol 2012; 85 (1015): e339-e342
- Grasso RF, Faiella E, Luppi G. et al. Percutaneous lung biopsy: comparison between an augmented reality CT navigation system and standard CT-guided technique. Int J CARS 2013; 8 (05) 837-848
- Rosati A, Gueli Alletti S, Capozzi VA. et al. Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature. Gland Surg 2020; 9 (04) 1092-1101
- Sodickson A, Weiss M. Effects of patient size on radiation dose reduction and image quality in low-kVp CT pulmonary angiography performed with reduced IV contrast dose. Emerg Radiol 2012; 19 (05) 437-445
- Du J, Huang Z, Luo Q. et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci 2015; 20 (01) 26-31
- Atwell TD, Smith RL, Hesley GK. et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. Am J Roentgenol 2010; 194 (03) 784-789
- Loeb S, Vellekoop A, Ahmed HU. et al. Systematic review of complications of prostate biopsy. Eur Urol 2013; 64 (06) 876-892
- Lang EK, Ghavami R, Schreiner VC, Archibald S, Ramirez J. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 2000; 216 (01) 93-96
- Burke DR. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol 2003; 14: 243-246
- Adiga S, Athreya S. Safety, efficacy, and feasibility of an ultra-low dose radiation protocol for CT-guided percutaneous needle biopsy of pulmonary lesions: initial experience. Clin Radiol 2014; 69 (07) 709-714
Address for correspondence
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :(A-F) Axial CT images demonstrate lung and mediastinal lesion biopsy technique. A-Solitary lesion in the right upper lobe posterior approach taken where the needle is perpendicular to the lesion. B-Large lung mass in the right lower lobe posterior approach taken for biopsy. C-Small lesion located in the subpleural location of the right lower lobe, tangential approach taken to obtain the larger length of the core. D & E-CECT reveals subcarinal lymph node in k/c/o carcinoma of the lung. Extrapleural posterior approach was taken with hydro dissection of the parietal and visceral pleural and a biopsy of the mediastinal lymph node was done.

| Figure 2:(A-B) Left Paraspinal approach for biopsy of the aortocaval lymph node. (C-D) CECT reveals pancreatic head mass where biopsy is performed via transgastric approach.(E-F) CECT reveals pancreatic body mass, USG guided biopsy of pancreatic mass with compression of bowel loops. (G-H) USG guided liver lesions biopsy traversing normal liver parenchyma. H-Lesions appreciated by using high-frequency probe.

| Figure 3:(A and B) Axial CT images demonstrate vertebral lesion biopsy by parapedicular technique. (C and D) Axial CT images demonstrate vertebral lesion biopsy by transpedicular technique.

| Figure 4:(A-B) Paramaxiiary approach for parapharyngeal mass biopsy. (C) Trans nasal approach for petrous mass biopsy.

| Figure 5:(A) Coaxial biopsy system showing biopsy needle and gun. (B-C) Murphy's needle and ackerman's used for bone biopsy. (D-E) Post biopsy clot embolisation of biopsy tract in lung biopsy.

| Figure 6:(A-B) Instrumentation used in TRUS guided biopsy and 12 containers for 12 core biopsy of the prostate. (C-D) Axial CT shows an ill-defined cervical mass sampled through TRUS guided approach.
References
- Gupta S, Madoff DC. Image-guided percutaneous needle biopsy in cancer diagnosis and staging. Tech Vasc Interv Radiol 2007; 10 (02) 88-101
- Cardella JF, Bakal CW, Bertino RE. et al; Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for image-guided percutaneous biopsy in adults. J Vasc Interv Radiol 2003; 14 (9 Pt 2): S227-S230
- Schiavon LHO, Tyng CJ, Travesso DJ, Rocha RD, Schiavon ACSA, Bitencourt AGV. Computed tomography-guided percutaneous biopsy of abdominal lesions: indications, techniques, results, and complications. Radiol Bras 2018; 51 (03) 141-146
- Veltri A, Bargellini I, Giorgi L, Almeida PA, Akhan O. CIRSE guidelines on percutaneous needle biopsy (PNB). Cardiovascular and Interventional Radiology 2017; 40 (10) 1501-1513
- Gutierrez A, Abtin F, Suh RD. Percutaneous transthoracic lung biopsy. In percutaneous image-guided biopsy. New York, NY: Springer; 2014. :141–166
- Gupta S, Seaberg K, Wallace MJ. et al. Imaging-guided percutaneous biopsy of mediastinal lesions: different approaches and anatomic considerations. Radiographics 2005; 25 (03) 763-786 , discussion 786–788
- Shankar S, van Sonnenberg E, Silverman SG, Tuncali K. Interventional radiology procedures in the liver. Biopsy, drainage, and ablation. Clin Liver Dis 2002; 6 (01) 91-118
- Heyer CM, Al-Hadari A, Mueller KM, Stachon A, Nicolas V. Effectiveness of CT-guided percutaneous biopsies of the spine: an analysis of 202 examinations. Acad Radiol 2008; 15 (07) 901-911
- Wu EH, Chen YL, Wu YM, Huang YT, Wong HF, Ng SH. CT-guided core needle biopsy of deep suprahyoid head and neck lesions. Korean J Radiol 2013; 14 (02) 299-306
- Tam AL, Lim HJ, Wistuba II. et al. Image-guided biopsy in the era of personalized cancer care: proceedings from the society of interventional radiology research consensus panel. J Vasc Interv Radiol 2016; 27 (01) 8-19
- Lorentzen T, Nolsøe CP, Ewertsen C. et al; EFSUMB. EFSUMB Guidelines on Interventional Ultrasound (INVUS), part I–general aspects (long version). Ultraschall Med 2015; 36 (05) E1-E14
- Sato S, Mishiro T, Miyake T. et al. Prophylactic administration of antibiotics unnecessary following ultrasound-guided biopsy and ablation therapy for liver tumors: open-labeled randomized prospective study. Hepatol Res 2009; 39 (01) 40-46
- Patel IJ, Rahim S, Davidson JC. et al. Society of Interventional Radiology Consensus Guidelines for the Periprocedural Management of Thrombotic and Bleeding Risk in Patients Undergoing Percutaneous Image-Guided Interventions-part II: recommendations: endorsed by the Canadian Association for Interventional Radiology and the Cardiovascular and Interventional Radiological Society of Europe. J Vasc Interv Radiol 2019; 30 (08) 1168-1184.e1 DOI: 10.1016/j.jvir.2019.04.017.
- Gupta S, Wallace MJ, Cardella JF, Kundu S, Miller DL, Rose SC. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous needle biopsy. J Vasc Interv Radiol 2010; 21 (07) 969-975
- Wu CC, Maher MM, Shepard JA. CT-guided percutaneous needle biopsy of the chest: preprocedural evaluation and technique. Am J Roentgenol 2011; 196 (05) W511-4
- Tyng CJ, Bitencourt AG, Martins EB, Pinto PN, Chojniak R. Technical note: CT-guided paravertebral adrenal biopsy using hydrodissection–a safe and technically easy approach. Br J Radiol 2012; 85 (1015): e339-e342
- Grasso RF, Faiella E, Luppi G. et al. Percutaneous lung biopsy: comparison between an augmented reality CT navigation system and standard CT-guided technique. Int J CARS 2013; 8 (05) 837-848
- Rosati A, Gueli Alletti S, Capozzi VA. et al. Role of ultrasound in the detection of recurrent ovarian cancer: a review of the literature. Gland Surg 2020; 9 (04) 1092-1101
- Sodickson A, Weiss M. Effects of patient size on radiation dose reduction and image quality in low-kVp CT pulmonary angiography performed with reduced IV contrast dose. Emerg Radiol 2012; 19 (05) 437-445
- Du J, Huang Z, Luo Q. et al. Rapid diagnosis of pleural tuberculosis by Xpert MTB/RIF assay using pleural biopsy and pleural fluid specimens. J Res Med Sci 2015; 20 (01) 26-31
- Atwell TD, Smith RL, Hesley GK. et al. Incidence of bleeding after 15,181 percutaneous biopsies and the role of aspirin. Am J Roentgenol 2010; 194 (03) 784-789
- Loeb S, Vellekoop A, Ahmed HU. et al. Systematic review of complications of prostate biopsy. Eur Urol 2013; 64 (06) 876-892
- Lang EK, Ghavami R, Schreiner VC, Archibald S, Ramirez J. Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 2000; 216 (01) 93-96
- Burke DR. Society of Interventional Radiology Standards of Practice Committee. Quality improvement guidelines for percutaneous transhepatic cholangiography and biliary drainage. J Vasc Interv Radiol 2003; 14: 243-246
- Adiga S, Athreya S. Safety, efficacy, and feasibility of an ultra-low dose radiation protocol for CT-guided percutaneous needle biopsy of pulmonary lesions: initial experience. Clin Radiol 2014; 69 (07) 709-714


 PDF
PDF  Views
Views  Share
Share

