Imaging Recommendations for Diagnosis, Staging, and Management of Uterine Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 110-118
DOI: DOI: 10.1055/s-0042-1759519
Abstract
Uterine cancers are classified into cancers of the corpus uteri (uterine carcinomas and carcinosarcoma) and corpus uteri (sarcomas) by the AJCC staging system (eighth edition). Endometrial carcinoma is the most common amongst these with prolonged estrogen exposure being a well-known risk factor. The FIGO staging system for endometrial carcinoma is primarily surgical and includes total hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy. Imaging is useful in the preoperative evaluation of tumor stage, especially assessment of myometrial invasion and cervical stromal extension. Dynamic contrast enhanced MRI with DWI has a high staging accuracy and is the preferred imaging modality for primary evaluation with contrast-enhanced CT abdomen being indicated for recurrent disease. PET/CT is considered superior in evaluation of lymph nodes and extra pelvic metastases.
Keywords
dynamic contrast-enhanced MRI - endometrial neoplasms - gynecology and obstetrics - leiomyosarcoma - magnetic resonance imaging - medical oncology - positron emission tomography computed tomography - radiologyPublication History
Article published online:
24 January 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Uterine cancers are classified into cancers of the corpus uteri (uterine carcinomas and carcinosarcoma) and corpus uteri (sarcomas) by the AJCC staging system (eighth edition). Endometrial carcinoma is the most common amongst these with prolonged estrogen exposure being a well-known risk factor. The FIGO staging system for endometrial carcinoma is primarily surgical and includes total hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy. Imaging is useful in the preoperative evaluation of tumor stage, especially assessment of myometrial invasion and cervical stromal extension. Dynamic contrast enhanced MRI with DWI has a high staging accuracy and is the preferred imaging modality for primary evaluation with contrast-enhanced CT abdomen being indicated for recurrent disease. PET/CT is considered superior in evaluation of lymph nodes and extra pelvic metastases.
Keywords
dynamic contrast-enhanced MRI - endometrial neoplasms - gynecology and obstetrics - leiomyosarcoma - magnetic resonance imaging - medical oncology - positron emission tomography computed tomography - radiologyIntroduction
Tumors of the uterine corpus include epithelial tumors, mesenchymal tumors, mixed epithelial and mesenchymal, miscellaneous tumors (neuroendocrine or germ cell), lymphoid, myeloid and secondary tumors.[1] [2] The American Joint Committee on Cancer (AJCC) staging system has classified uterine cancers into two groups: corpus uteri (uterine carcinomas and carcinosarcoma) and corpus uteri (sarcomas).[3] Out of these, endometrial cancer is the most common and is classified histologically into Type I and Type II. Definitive diagnosis is usually made through endometrial biopsy or dilatation and curettage; however, pre-operative radiological imaging is required to stage the disease and to tailor patient's management. The treatment comprises surgical staging and adjuvant radiotherapy and/or chemotherapy depending on the final surgico-pathological stage.
Risk Factors and Etiopathogenesis
Long-term estrogen excess (exogenous or endogenous) is postulated to have a causative effect on Type I cancers.[4] Early menarche, late menopause, nulliparity, anovulatory states (polycystic ovary syndrome) and estrogen only hormonal therapy are causes of prolonged estrogen exposure. The other risk factors include obesity, diabetes mellitus, Lynch syndrome, Cowden syndrome, tamoxifen therapy, and previous pelvic irradiation.[5] Most patients present at an early stage and are associated with a good prognosis, which depends on several factors, including the clinical stage, depth of myometrial invasion, histological grade, cell type, lymphovascular invasion, nodal status, and patient age. In contrast, Type II cancers have a worse prognosis and risk factors include Black race, older age, and lower body mass index.[4] [5]
Epidemiology, Clinical Presentation in India and Global
There has been an increase in the incidence and prevalence of cancers in female population worldwide. Though breast and carcinoma cervix are the most common causes of morbidity and mortality, carcinoma of the uterine corpus continues to pose a significant concern. It is the sixth most common cancer with detection of 417,000 new cases and 97,000 deaths, as per GLOBOCAN 2020.[6] Uterine malignancies are predominantly seen in developed countries as compared with the developing countries; however, the incidence shows a rising trend in both due to an increase in the prevalence of associated risk factors such as excess body weight and diabetes. The National Cancer Registry of India showed heterogeneous distribution of cancers in India with breast and carcinoma cervix being the most common since 2012.[7] It had projected the risk of uterine corpus in 26,514 patients in 2020 with cumulative risk of 1 in 190, indicating its potential risk. GLOBOCAN 2020 showed 16,413 new cases of carcinoma uterine corpus, with 6,385 deaths, estimating its 5-year prevalence of 6.56 per 100,000 in Indian population.[6]
Imaging Referral Guidelines
Endometrial Carcinoma
Endometrial carcinoma is staged surgically according to the joint 2017 International Federation of Gynecology and Obstetrics (FIGO)/Tumour, Node, Metastasis (TNM) classification system.[3] The staging procedure includes total hysterectomy, bilateral salpingo-oophorectomy, and lymphadenectomy, unless the patients desire fertility sparing surgery (and are candidates for the same). Imaging serves as an adjunct in the treatment stratification of endometrial carcinoma.[8] [9] According to the NCCN guidelines,[10] the initial imaging workup varies according to the treatment offered.
For Non-Fertility Sparing treatment, pelvic contrast-enhanced magnetic resonance imaging (CEMRI) is recommended (to establish origin of tumor as endometrial versus endocervical and local disease extent evaluation).[11] In early stages ([Fig. 1]), evaluation with transvaginal sonography can be done followed by MRI as an optional modality.[12] Chest X-ray is the baseline evaluation, to be followed by non-contrast computed tomography (NCCT) chest in case of any abnormality. CECT chest and abdomen (including pelvis) is recommended for metastatic evaluation in high-grade carcinomas (poorly differentiated endometrioid, clear cell, serous, undifferentiated carcinoma, and carcinosarcoma) and PET/CT (neck/chest/abdomen/pelvis/groin) can be done in select cases. In case of postoperative incidental finding of endometrial cancer or incompletely staged cancer with uterine risk factors (tumor > 2 cm, high-grade carcinomas, invasion > 50%-myometrium, cervical stromal involvement and LVSI), CECT chest and abdomen is suggested to evaluate for metastatic disease. Additional imaging can be considered based on the clinical concern for metastases (delay in presentation or treatment, abnormal physical exam finding, abdominal or pulmonary symptoms, bulky uterine tumor and vaginal or extrauterine disease).[10] [13]
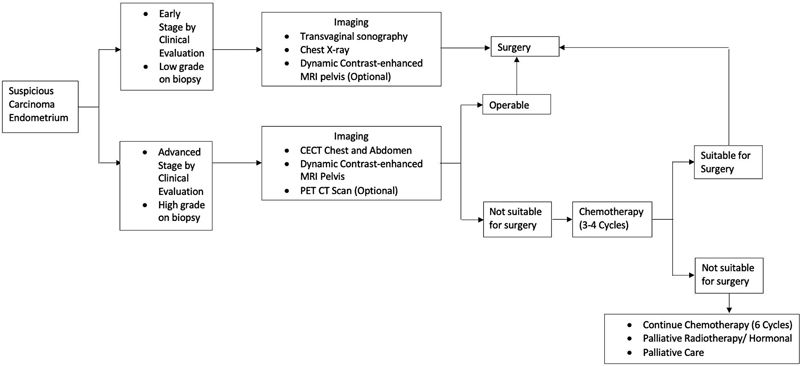
| Figure 1:Imaging referral and treatment algorithm for endometrial carcinoma.Adapted from references [10] [12].
For Fertility-Sparing treatment, CEMRI pelvis is preferred to exclude any myoinvasion and assess the local disease extent.[14] If MRI is contraindicated, transvaginal ultrasound pelvis can be considered. Chest X-ray is the baseline evaluation to be followed by non-contrast computed tomography (NCCT) chest in case of any abnormality. If metastasis is suspected in select patients, PET/CT (neck/chest/abdomen/pelvis/groin) is recommended. Additional imaging can be considered based on the clinical concern for metastases.[10]
Uterine Sarcoma
Uterine sarcomas may be diagnosed after total/supracervical hysterectomy (SCH) or after biopsy/myomectomy and the imaging workup varies accordingly. For the initial workup of patients with incidental finding of uterine sarcoma or incompletely resected uterus/adnexa, CEMRI abdomen and pelvis is recommended with non-contrast CT chest for metastatic disease. In cases of SCH, suspicious tumor fragmentation, myomectomy, or intraperitoneal morcellation local tumor extension and residual disease is to be evaluated with pelvic MRI. PET/CT (neck/chest/abdomen/pelvis/groin) is recommended to clarify ambiguous findings. Additional imaging can be considered based on the clinical concern for metastases (as in case of endometrial carcinoma).[10] [15]
Clinical/ Diagnostic Workup Excluding Imaging
The clinical presentation is usually abnormal uterine bleeding in premenopausal women and postmenopausal bleeding in the elderly age group. A detailed history including use of hormones, tamoxifen use, diabetes mellitus, and family history is essential followed by a complete systemic and gynecological examination. Endometrial and endocervical sampling is required to make a definitive diagnosis and endocervical curettage is done before endometrial aspiration. Endometrial biopsy is done with endometrial aspiration using devices such as Pipelle or a fine Karman's cannula.[4] In women with inadequate or negative sampling and strong suspicion of malignancy, hysteroscopy and directed biopsy is advised.
The preoperative laboratory evaluation includes a complete blood count, liver and renal function tests, blood sugar, serum electrolytes serum electrolytes, viral marker and viral marker and urinalysis. In selected patients with extrauterine spread of disease (especially nodal involvement in high-risk tumors), serum levels of CA 125 maybe elevated and can be used to monitor response to therapy.[16] The serum human epididymis protein (HE4) levels are elevated in aggressive types of disease and are useful for detecting early disease recurrence.[17]
Genetic evaluation is suggested for younger patients (< 50>
Imaging Guidelines
Screening
Routine screening is not recommended for endometrial carcinoma because majority of the patients with endometrial cancer present with abnormal uterine bleeding and at a stage with disease confined to the uterus. In addition, there is no non-invasive test available with sufficiently high specificity and sensitivity for screening. However, in patients with Lynch syndrome, endometrial biopsy is recommended every 1 to 2 years beginning at the age of 30 to 35 years as a screening procedure.[18]
Diagnosis and Staging
Staging of endometrial carcinoma is primarily surgical and typically performed with laparoscopy.[8] The diagnosis is established by histopathological evaluation and MRI maybe done in equivocal cases to distinguish between cancers of endometrial and cervical origin. Preoperative disease assessment requires depth of myometrial invasion (MRI) and histologic type and grade (endometrial biopsy).
Transabdominal and transvaginal ultrasound are used as baseline screening modalities in patients presenting with abnormal uterine bleeding or postmenopausal bleeding. Transvaginal US has accuracies ranging from 73%-to 84%-in assessing myometrial invasion ([Fig. 2]) with insufficient data about prediction of cervical extension or lymphadenopathy.[19] [20]
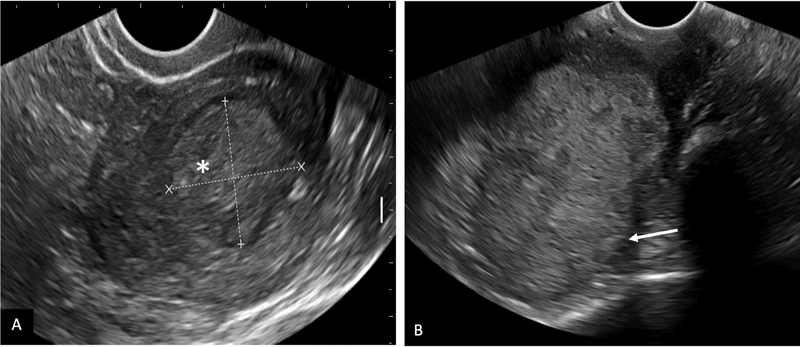
| Figure 2:Transvaginal ultrasound in carcinoma endometrium. A: A large relatively well-defined iso to hyperechoic mass lesion (*) in the endometrial cavity with < 50%-myometrial invasion. B: An ill-defined hyperechoic mass in the endometrial cavity with > 50%-invasion into myometrium (arrow).
Computed tomography (CT) has a limited role in evaluating myometrial invasion ([Fig. 3]) and cervical extension in endometrial cancer. In comparative studies of CT with US or MRI for myometrial invasion, the accuracy of CT is reported to be 58%-to 61%-versus 68%-to 69%-for US and 88%-to 89%-for MRI.[21] [22] In select cases, CT chest and abdomen is indicated as a part of metastatic workup.
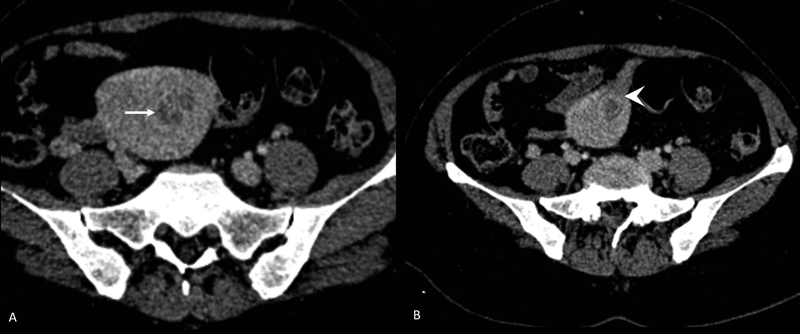
| Figure 3:CECT abdomen in endometrial carcinoma. Patient had pacemaker and MRI was contraindicated. An ill-defined heterogeneously enhancing lesion in the endometrial cavity (arrow in A) with >50%-invasion into myometrium (arrowhead in B).
Dynamic contrast-enhanced MRI ([Fig. 4]) is the preferred imaging modality to evaluate myometrial invasion with high accuracy (59% to 100%), sensitivity (71%- to 100%), and specificity (72%-to 100%).[23] The staging accuracy ranges from 83%-to 92%.[24] [25]

| Figure 4:Dynamic contrast-enhanced MRI pelvis in carcinoma endometrium (stage IB). T1 axial oblique (A) and T2 sagittal (B) show an ill-defined polypoidal mass lesion (*) in the endometrial cavity. DCE MRI (C) shows mild contrast enhancement of tumor and disruption of subendometrial zone of enhancement (arrow in C) with myometrial invasion of >50%. DWI (D) shows diffusion restriction (*) with low ADC value in the ADC map (E). Post contrast T1 axial oblique (F) shows myometrial invasion of >50%-with intact serosal margin (arrow in F).
PET CT is considered to be better in the evaluation of lymph node metastases and metabolically active nodes of any size are considered to be metastatic.[26] It is also superior in the assessment of extrapelvic disease and bone metastases.[3] [27]
MRI Sequences and Imaging Protocols
Scanner: There is improved signal-to-noise ratio (SNR), spatial resolution, anatomic detail and faster scanning techniques with the use of 3 Tesla (T) scanners. The use of phase-array surface abdominopelvic coil is recommended for both 1.5 T and 3.0 T scanners.
Patient preparation: Fasting is advised for 4 hours, but water intake is encouraged before the scan. A moderately full bladder is required during the scan and the patient should be asked to void ∼30 to 45 minutes before the examination. Antispasmodic drugs such as butylscopolamine (40 mg) IM/IV or glucagon IV/IM (0.5–1.0 mg) are recommended to reduce bowel motion. Vaginal opacification with ∼10 mL of lignocaine 2%-jelly gives optimal contrast resolution.[28]
MRI Technique and Sequences: In the pelvis, T2W FSE sequences are the mainstay of evaluation. Sequences are oriented in relation to the pelvis or dedicated to the uterine axis.[29] T2-weighted images include a small FOV (512 × 256 matrix, 24 cm FOV) sagittal T2WI of the pelvis and a small FOV T2W sequences of pelvis in the axial oblique plane perpendicular to the uterine corpus. T1W sequence of the pelvis in the axial plane is followed by diffusion-weighted imaging (DWI) in axial oblique (in sync with the axial oblique T2WI). Large FOV (256 × 256 matrix, 32 cm FOV) T1- or T2-weighted image image of upper abdomen is obtained to evaluate for lymph nodes and hydronephrosis. DWI in the axial plane (large FOV) is also acquired in sync with the large FOV T2 sequence.[8] The dynamic contrast-enhanced sequences are acquired for the assessment of preservation of endometrial halo and differential enhancement of the endometrial soft tissue and the myometrium. Dynamic acquisition can be done in the sagittal plane using a three-dimensional gradient echo T1WI, fat-saturated sequence following the administration of 0.1 mmol/kg of gadolinium at 2 mL/s. Images are acquired before contrast injection and then at 25 seconds, 1 minute and 2 minutes after injection followed by a delayed sequence in the axial oblique plane 4 minutes after injection.[30]
Staging
Endometrial Carcinoma
Carcinomas are usually isointense on T1WI and hyperintense (relative to the myometrium) on T2WI. The lesion shows diffusion restriction with low mean ADC values.[31] Post-contrast administration, tumor enhances slowly and less avidly than the myometrium.
Assessment of myometrial invasion is crucial in the staging of endometrial carcinoma. Deep myometrial invasion is excluded in the presence of an intact junctional zone (JZ) along with smooth early subendometrial enhancement (25–60 seconds). Disruption of the JZ with the tumor within the outer myometrium is suggestive of myometrial invasion (> 50%). The presence of leiomyomas or adenomyosis can result in an overestimation of the depth of myometrial invasion. Deep myometrial invasion is best assessed during the equilibrium phase (2–3 minutes after contrast injection). An imaging delay of ∼90 seconds is considered optimal timing for best tumor-myometrium contrast.[32] Delayed-phase images (4–5 minutes after contrast) are useful for detecting cervical stromal invasion.[30]
For extrauterine extension, T2WI should be interpreted in conjunction with the DWI. The presence of intermediate to high signal intensity tumor causing disruption of the normal low signal intensity cervical stroma is suggestive of cervical stromal invasion on T2WI.
Serosal involvement is suggested by an irregular uterine contour/disrupted low signal intensity of the uterine serosa on T2WI, and a loss of the normal edge of enhancing myometrium on DCE sequences.[8]
Tumor abutting or indenting the bladder/rectum over a significant area; tumor interrupting the low signal intensity of the bladder/rectal muscular layer or tumor invading the bladder/rectal muscular wall on T2WI is suggestive of bladder/ rectal involvement. The presence of bullous edema alone is not sufficient to label it as stage IVA disease. Adnexal deposits can be well picked up on DWI and T2WI.[30]
Lymph node involvement can be well picked up on T1WI and DWI. Morphological features such as short-axis diameter of more than 10 mm, rounded shape, loss of fatty hilum are features that help to identify suspicious lymph nodes.[33] However, there is a degree of overlap in the sizes and ADC values of benign and malignant pelvic lymph nodes.
For treatment response, the role of CEMRI and ADC values is still evolving. The commonest site for recurrence is the vagina[34] followed by pelvic and paraaortic lymph nodes. The presence of a T2 hyperintense mass with disruption of the normal low signal intensity linear configuration of the vault is suggestive of vault recurrence.
Uterine Sarcomas
The primary uterine sarcomas are leiomyosarcoma (LMS), endometrial stromal sarcoma (ESS), and adenosarcoma. Usually, the diagnosis of sarcomas is made after hysterectomy or myomectomy. The staging of LMS and ESS is different and that of adenosarcoma is the same as endometrial carcinoma. Size is an important criterion in staging though myometrial invasion in LMS and ESS is definitional.[3]
T2WI and contrast enhanced T1WI ([Fig. 5]) are useful in assessing the size, spread into adnexa, abdominal tissues, bladder or rectum (key T descriptors). LMS are usually solid masses with irregular margins, hemorrhagic T1 hyperintense areas, and intermediate to high signal on T2WI with heterogenous post contrast enhancement and diffusion restriction. ADC values in LMS range from 0.791 ± 0.145 × 10−3 to 1.17 ± 0.15 × 10−3 mm2/s.[35]
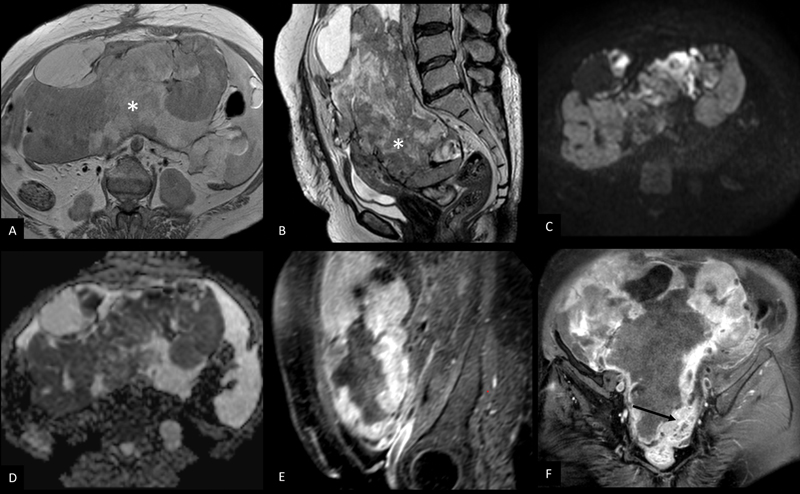
| Figure 5:Contrast enhanced MRI of leiomyosarcoma of uterus (stage IV A). T1 axial (A) and T2 sagittal (B) images show a large, ill-defined, heterogeneous lesion (*) replacing the entire uterus and involving bilateral adnexa, reaching up to the bilateral pelvic side walls. DWI (C) and corresponding ADC map (D) show areas of diffusion restriction. Post contrast sagittal (E) and coronal (F) images show heterogeneous enhancement of the mass and infiltration of the rectum (arrow in F).
PET CT is considered superior in evaluation of lymph nodes and extra pelvic metastases. [Table 1] summarizes the TNM and FIGO staging of uterine cancers including carcinoma and sarcoma.
|
FIGO |
Carcinoma Endometrium & Carcinosarcoma |
Sarcoma (Leiomyosarcoma & Endometrial stromal sarcoma) |
|
|---|---|---|---|
|
T |
|||
|
TX |
Primary lesion cannot be assessed |
Primary lesion cannot be assessed |
|
|
T0 |
No evidence of primary lesion. |
No evidence of primary lesion |
|
|
T1 T1a T1b |
I IA IB |
Lesion confined to the body of uterus including endocervical glandular involvement Lesion limited to the endometrium or░<░50%-myometrial invasion Lesion invading ≥ 50%-myometrium |
Growth limited to the uterus Size of the lesion ≤ 5 cm in greatest dimension Lesion░>░5 cm |
|
T2 T2a T2b |
II IIA IIB |
Lesion invading the cervical stroma (not endocervix) but not extending beyond the uterus. - - |
Lesion seen beyond the uterus, within the pelvis Lesion involves adnexa Lesion involves other pelvic tissues |
|
T3 T3a T3b |
III IIIA IIIB |
Lesion involving serosa, adnexa, vagina or parametrium Direct extension or metastasis to serosa and/or adnexa Direct extension or metastasis to vagina or parametrium |
Lesion infiltrates abdominal tissues One site More than one site |
|
T4 |
IVA |
Infiltration of bladder mucosa and/or bowel mucosa (bullous edema is not sufficient to classify a tumor as T4) |
Lesion invades bladder or rectum |
|
N |
|||
|
NX |
Regional lymph nodes cannot be assessed |
Regional lymph nodes cannot be assessed |
|
|
N0 |
No regional lymph node metastasis |
No regional lymph node metastasis |
|
|
N0(i + ) |
Isolated cancer cells in regional lymph node(s), not > 0.2 mm |
Isolated cancer cells in regional lymph node(s) not > 0.2 mm |
|
|
N1 N1mi N1a |
IIIC1 IIIC1 IIIC1 |
Regional lymph node metastasis in pelvic lymph nodes >0.2 mm to ≤ 2.0 mm in diameter (pelvic lymph nodes) > 2.0 mm in diameter (pelvic lymph nodes) |
Regional lymph node metastasis (FIGO IIIC) |
|
N2 N2mi N2a |
IIIC2 IIIC2 IIIC2 |
Regional lymph node metastasis to para-aortic lymph nodes with or without positive pelvic lymph nodes >0.2 mm ≤ 2.0 mm in diameter (para-aortic lymph nodes) > 2.0 mm in diameter (para-aortic lymph nodes) |
− |
|
M |
|||
|
M0 |
No distant metastasis |
No distant metastasis |
|
|
M1 |
IVB |
Distant metastasis including metastasis to inguinal lymph nodes, intraperitoneal disease, liver, lung, or bone. (excludes metastasis to pelvic or para-aortic lymph nodes, uterine serosa, vagina, or adnexa). |
Distant metastasis (excluding adnexa, pelvic, and abdominal tissues) |
|
Risk group |
Common treatment recommendation |
|
|
Low risk |
No adjuvant treatment |
|
|
Intermediate risk |
Vaginal brachytherapy Consider observation if age < 60 years |
|
|
High-intermediate risk |
Vaginal brachytherapy Consider EBRT, if LVSI un-equivocally positive, especially if no lymph node dissection or sentinel nodes have been performed. |
|
|
High-risk |
I. EBRT, Consider VBT, if no LVSI II. Vaginal brachytherapy if grade 1–2 disease (e.g., stage II disease) III. Pelvic radiotherapy if Stage I, grade 3 LVSI un equivocally positive, Stage II IV. Stage III-combined radiotherapy and chemotherapy |
Non-endometrioid I. Stage IA-vaginal brachytherapy after full surgical staging, II. LVSI negative Stage IB-III: combined external beam RT and chemo |
References
- Female Genital Tumours. WHO Classification of Tumors, 5th ed. Vol. 4. Lyon, France. International Agency for Research on Cancer; 2020
- Abu-Rustum NR, Yashar CM, Bradley K. et al. NCCN Guidelines® Insights: Uterine Neoplasms, Version 3.2021. J Natl Compr Canc Netw 2021; 19 (08) 888-895
- Amin MB, Greene FL, Edge SB. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-99
- ICMR Indian Council of Medical Research Consensus document for management of uterine cancer 2019. Accessed on February 20, 2022, at: https://main.icmr.nic.in/sites/default/files/guidelines/Uterine_Cancer.pdf
- McMeekin DS, Yashar C, Campos SM, Zaino RJ. Corpus: epithelial tumors. In: Barakat RR, Berchuk A, Markman M, Randall M, eds. Principles and Practice of Gynecologic Oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins(LWW); 2013: 661-714
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Nougaret S, Horta M, Sala E. et al. Endometrial cancer MRI staging: updated guidelines of the European Society of Urogenital Radiology. Eur Radiol 2019; 29 (02) 792-805
- Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology 2013; 266 (03) 717-740
- National Comprehensive Cancer Network. (2021). NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. Version 1.2022. Accessed on February 20, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
- Vargas HA, Akin O, Zheng J. et al. The value of MR imaging when the site of uterine cancer origin is uncertain. Radiology 2011; 258 (03) 785-792
- NCG Guidelines for endometrial cancer. Accessed on February 20, 2022, at: https://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/Gynaec/fwdncgcaendometriumguidelines/NCG_Endometrial_Cancer_Management_Guidelines.pdf
- Colombo N, Creutzberg C, Amant F. et al; ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (01) 16-41
- Haldorsen IS, Salvesen HB. What is the best preoperative imaging for endometrial cancer?. Curr Oncol Rep 2016; 18 (04) 25
- Hensley ML, Barrette BA, Baumann K. et al. Gynecologic Cancer InterGroup (GCIG) consensus review: uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer 2014; 24 (9, Suppl 3) S61-S66
- Jiang T, Huang L, Zhang S. Preoperative serum CA125: a useful marker for surgical management of endometrial cancer. BMC Cancer 2015; 15: 396
- Bignotti E, Ragnoli M, Zanotti L. et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer 2011; 104 (09) 1418-1425
- Committee on Practice Bulletins-Gynecology, Society of Gynecologic Oncology. ACOG practice bulletin no. 147: Lynch syndrome. Obstet Gynecol 2014; 124 (05) 1042-1054
- Arko D, Takac I. High frequency transvaginal ultrasonography in preoperative assessment of myometrial invasion in endometrial cancer. J Ultrasound Med 2000; 19 (09) 639-643
- Savelli L, Ceccarini M, Ludovisi M. et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet Gynecol 2008; 31 (05) 560-566
- Kim SH, Kim HD, Song YS, Kang SB, Lee HP. Detection of deep myometrial invasion in endometrial carcinoma: comparison of transvaginal ultrasound, CT, and MRI. J Comput Assist Tomogr 1995; 19 (05) 766-772
- Lakhman Y, Katz SS, Goldman DA. et al. Diagnostic performance of computed tomography for preoperative staging of patients with non-endometrioid carcinomas of the uterine corpus. Ann Surg Oncol 2016; 23 (04) 1271-1278
- Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol 2012; 67 (01) 2-12
- Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics 2012; 32 (06) 1805-1827
- Manfredi R, Mirk P, Maresca G. et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 2004; 231 (02) 372-378
- Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol 2009; 19 (06) 1529-1536
- Otero-García MM, Mesa-Álvarez A, Nikolic O. et al. Role of MRI in staging and follow-up of endometrial and cervical cancer: pitfalls and mimickers. Insights Imaging 2019; 10 (01) 19
- Lucas R, Cunha TM. ESUR quick guide to female pelvis imaging 1.0. 2019: European Society of Urogenital Radiology. Accessed March 01, 2022, at: http://www.esur.org/fileadmin/content/2019/ESUR2019-ESURQuickGuidetoFemale_Pelvis_Imaging.pdf
- Anand R. ICRI guidelines for imaging protocols in women's imaging 2020. https://icri.iria.org.in/wpcontent/uploads/2022/03/ICRI Guidelines for Imaging Protocols in Women's Imaging.pdf. Accessed on 22.11.2022
- Nougaret S, Lakhman Y, Vargas HA. et al. From staging to prognostication: achievements and challenges of MR imaging in the assessment of endometrial cancer. Magn Reson Imaging Clin N Am 2017; 25 (03) 611-633
- Vora Z, Manchanda S, Sharma R. et al. Normalized apparent diffusion coefficient: a novel paradigm for characterization of endometrial and subendometrial lesions. Br J Radiol 2021; 94 (1117): 20201069
- Park SB, Moon MH, Sung CK, Oh S, Lee YH. Dynamic contrast-enhanced MR imaging of endometrial cancer: optimizing the imaging delay for tumour-myometrium contrast. Eur Radiol 2014; 24 (11) 2795-2799
- Lalwani N, Dubinsky T, Javitt MC. et al; American College of Radiology. ACR Appropriateness criteria® pretreatment evaluation and follow-up of endometrial cancer. Ultrasound Q 2014; 30 (01) 21-28
- Mahajan A, Sable NP, Popat PB. et al. Magnetic resonance imaging of gynecological malignancies: role in personalized management. Semin Ultrasound CT MR 2017; 38 (03) 231-268
- Barral M, Placé V, Dautry R. et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol (NY) 2017; 42 (06) 1762-1772
- Reinhold C, Ueno Y, Akin EA. et al; Expert Panel on GYN and OB Imaging. ACR appropriateness criteria® pretreatment evaluation and follow-up of endometrial cancer. J Am Coll Radiol 2020; 17 (11S): S472-S486
- Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol 2007; 62 (01) 28-34 , discussion 35–36
- Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol 2017; 146 (01) 3-10
- Elit L, Reade CJ. Recommendations for follow-up care for gynecologic cancer survivors. Obstet Gynecol 2015; 126 (06) 1207-1214
- Concin N, Creutzberg CL, Vergote I. et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch 2021; 478 (02) 153-190
- Asher R, Obermair A, Janda M, Gebski V. Disease-free and survival outcomes for total laparoscopic hysterectomy compared with total abdominal hysterectomy in early-stage endometrial carcinoma: a meta-analysis. Int J Gynecol Cancer 2018; 28 (03) 529-538
- Li X, Cheng Y, Dong Y. et al. Development and validation of predictive model for lymph node metastasis in endometrial cancer: a SEER analysis. Ann Transl Med 2021; 9 (07) 538
- Kim SR, van der Zanden C, Ikiz H, Kuzelijevic B, Havelock J, Kwon JS. Fertility-sparing management using progestin for young women with endometrial cancer from a population-based study. J Obstet Gynaecol Can 2018; 40 (03) 328-333
- Wortman BG, Creutzberg CL, Putter H. et al; PORTEC Study Group. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer 2018; 119 (09) 1067-1074
- Nout RA, Smit VT, Putter H. et al; PORTEC Study Group. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010; 375 (9717): 816-823
- Jamieson A, Bosse
T, McAlpine JN. The emerging role of
molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol
2021;13:17588359211035959
Address for correspondence
Smita Manchanda, MD, DNBDepartment of Radiodiagnosis and Interventional Radiology, All India Institute of Medical SciencesNew Delhi 110029IndiaEmail: smitamanchanda@gmail.comPublication History
Article published online:
24 January 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Imaging referral and treatment algorithm for endometrial carcinoma.Adapted from references [10] [12].

| Figure 2:Transvaginal ultrasound in carcinoma endometrium. A: A large relatively well-defined iso to hyperechoic mass lesion (*) in the endometrial cavity with < 50% myometrial invasion. B: An ill-defined hyperechoic mass in the endometrial cavity with > 50% invasion into myometrium (arrow).

| Figure 3:CECT abdomen in endometrial carcinoma. Patient had pacemaker and MRI was contraindicated. An ill-defined heterogeneously enhancing lesion in the endometrial cavity (arrow in A) with >50%-invasion into myometrium (arrowhead in B).

| Figure 4:Dynamic contrast-enhanced MRI pelvis in carcinoma endometrium (stage IB). T1 axial oblique (A) and T2 sagittal (B) show an ill-defined polypoidal mass lesion (*) in the endometrial cavity. DCE MRI (C) shows mild contrast enhancement of tumor and disruption of subendometrial zone of enhancement (arrow in C) with myometrial invasion of >50%. DWI (D) shows diffusion restriction (*) with low ADC value in the ADC map (E). Post contrast T1 axial oblique (F) shows myometrial invasion of >50% with intact serosal margin (arrow in F).

| Figure 5:Contrast enhanced MRI of leiomyosarcoma of uterus (stage IV A). T1 axial (A) and T2 sagittal (B) images show a large, ill-defined, heterogeneous lesion (*) replacing the entire uterus and involving bilateral adnexa, reaching up to the bilateral pelvic side walls. DWI (C) and corresponding ADC map (D) show areas of diffusion restriction. Post contrast sagittal (E) and coronal (F) images show heterogeneous enhancement of the mass and infiltration of the rectum (arrow in F).
References
- Female Genital Tumours. WHO Classification of Tumors, 5th ed. Vol. 4. Lyon, France. International Agency for Research on Cancer; 2020
- Abu-Rustum NR, Yashar CM, Bradley K. et al. NCCN Guidelines® Insights: Uterine Neoplasms, Version 3.2021. J Natl Compr Canc Netw 2021; 19 (08) 888-895
- Amin MB, Greene FL, Edge SB. et al. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-99
- ICMR Indian Council of Medical Research Consensus document for management of uterine cancer 2019. Accessed on February 20, 2022, at: https://main.icmr.nic.in/sites/default/files/guidelines/Uterine_Cancer.pdf
- McMeekin DS, Yashar C, Campos SM, Zaino RJ. Corpus: epithelial tumors. In: Barakat RR, Berchuk A, Markman M, Randall M, eds. Principles and Practice of Gynecologic Oncology. 6th ed. Philadelphia: Lippincott Williams & Wilkins(LWW); 2013: 661-714
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Mathur P, Sathishkumar K, Chaturvedi M. et al; ICMR-NCDIR-NCRP Investigator Group. Cancer Statistics, 2020: Report From National Cancer Registry Programme, India. JCO Glob Oncol 2020; 6: 1063-1075
- Nougaret S, Horta M, Sala E. et al. Endometrial cancer MRI staging: updated guidelines of the European Society of Urogenital Radiology. Eur Radiol 2019; 29 (02) 792-805
- Sala E, Rockall AG, Freeman SJ, Mitchell DG, Reinhold C. The added role of MR imaging in treatment stratification of patients with gynecologic malignancies: what the radiologist needs to know. Radiology 2013; 266 (03) 717-740
- National Comprehensive Cancer Network. (2021). NCCN Clinical Practice Guidelines in Oncology: Uterine Neoplasms. Version 1.2022. Accessed on February 20, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf
- Vargas HA, Akin O, Zheng J. et al. The value of MR imaging when the site of uterine cancer origin is uncertain. Radiology 2011; 258 (03) 785-792
- NCG Guidelines for endometrial cancer. Accessed on February 20, 2022, at: https://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/Gynaec/fwdncgcaendometriumguidelines/NCG_Endometrial_Cancer_Management_Guidelines.pdf
- Colombo N, Creutzberg C, Amant F. et al; ESMO-ESGO-ESTRO Endometrial Consensus Conference Working Group. ESMO-ESGO-ESTRO consensus conference on endometrial cancer: diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (01) 16-41
- Haldorsen IS, Salvesen HB. What is the best preoperative imaging for endometrial cancer?. Curr Oncol Rep 2016; 18 (04) 25
- Hensley ML, Barrette BA, Baumann K. et al. Gynecologic Cancer InterGroup (GCIG) consensus review: uterine and ovarian leiomyosarcomas. Int J Gynecol Cancer 2014; 24 (9, Suppl 3) S61-S66
- Jiang T, Huang L, Zhang S. Preoperative serum CA125: a useful marker for surgical management of endometrial cancer. BMC Cancer 2015; 15: 396
- Bignotti E, Ragnoli M, Zanotti L. et al. Diagnostic and prognostic impact of serum HE4 detection in endometrial carcinoma patients. Br J Cancer 2011; 104 (09) 1418-1425
- Committee on Practice Bulletins-Gynecology, Society of Gynecologic Oncology. ACOG practice bulletin no. 147: Lynch syndrome. Obstet Gynecol 2014; 124 (05) 1042-1054
- Arko D, Takac I. High frequency transvaginal ultrasonography in preoperative assessment of myometrial invasion in endometrial cancer. J Ultrasound Med 2000; 19 (09) 639-643
- Savelli L, Ceccarini M, Ludovisi M. et al. Preoperative local staging of endometrial cancer: transvaginal sonography vs. magnetic resonance imaging. Ultrasound Obstet Gynecol 2008; 31 (05) 560-566
- Kim SH, Kim HD, Song YS, Kang SB, Lee HP. Detection of deep myometrial invasion in endometrial carcinoma: comparison of transvaginal ultrasound, CT, and MRI. J Comput Assist Tomogr 1995; 19 (05) 766-772
- Lakhman Y, Katz SS, Goldman DA. et al. Diagnostic performance of computed tomography for preoperative staging of patients with non-endometrioid carcinomas of the uterine corpus. Ann Surg Oncol 2016; 23 (04) 1271-1278
- Haldorsen IS, Salvesen HB. Staging of endometrial carcinomas with MRI using traditional and novel MRI techniques. Clin Radiol 2012; 67 (01) 2-12
- Freeman SJ, Aly AM, Kataoka MY, Addley HC, Reinhold C, Sala E. The revised FIGO staging system for uterine malignancies: implications for MR imaging. Radiographics 2012; 32 (06) 1805-1827
- Manfredi R, Mirk P, Maresca G. et al. Local-regional staging of endometrial carcinoma: role of MR imaging in surgical planning. Radiology 2004; 231 (02) 372-378
- Kitajima K, Murakami K, Yamasaki E, Kaji Y, Sugimura K. Accuracy of integrated FDG-PET/contrast-enhanced CT in detecting pelvic and paraaortic lymph node metastasis in patients with uterine cancer. Eur Radiol 2009; 19 (06) 1529-1536
- Otero-García MM, Mesa-Álvarez A, Nikolic O. et al. Role of MRI in staging and follow-up of endometrial and cervical cancer: pitfalls and mimickers. Insights Imaging 2019; 10 (01) 19
- Lucas R, Cunha TM. ESUR quick guide to female pelvis imaging 1.0. 2019: European Society of Urogenital Radiology. Accessed March 01, 2022, at: http://www.esur.org/fileadmin/content/2019/ESUR2019-ESURQuickGuidetoFemale_Pelvis_Imaging.pdf
- Anand R. ICRI guidelines for imaging protocols in women's imaging 2020. https://icri.iria.org.in/wpcontent/uploads/2022/03/ICRI Guidelines for Imaging Protocols in Women's Imaging.pdf. Accessed on 22.11.2022
- Nougaret S, Lakhman Y, Vargas HA. et al. From staging to prognostication: achievements and challenges of MR imaging in the assessment of endometrial cancer. Magn Reson Imaging Clin N Am 2017; 25 (03) 611-633
- Vora Z, Manchanda S, Sharma R. et al. Normalized apparent diffusion coefficient: a novel paradigm for characterization of endometrial and subendometrial lesions. Br J Radiol 2021; 94 (1117): 20201069
- Park SB, Moon MH, Sung CK, Oh S, Lee YH. Dynamic contrast-enhanced MR imaging of endometrial cancer: optimizing the imaging delay for tumour-myometrium contrast. Eur Radiol 2014; 24 (11) 2795-2799
- Lalwani N, Dubinsky T, Javitt MC. et al; American College of Radiology. ACR Appropriateness criteria® pretreatment evaluation and follow-up of endometrial cancer. Ultrasound Q 2014; 30 (01) 21-28
- Mahajan A, Sable NP, Popat PB. et al. Magnetic resonance imaging of gynecological malignancies: role in personalized management. Semin Ultrasound CT MR 2017; 38 (03) 231-268
- Barral M, Placé V, Dautry R. et al. Magnetic resonance imaging features of uterine sarcoma and mimickers. Abdom Radiol (NY) 2017; 42 (06) 1762-1772
- Reinhold C, Ueno Y, Akin EA. et al; Expert Panel on GYN and OB Imaging. ACR appropriateness criteria® pretreatment evaluation and follow-up of endometrial cancer. J Am Coll Radiol 2020; 17 (11S): S472-S486
- Sohaib SA, Houghton SL, Meroni R, Rockall AG, Blake P, Reznek RH. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol 2007; 62 (01) 28-34 , discussion 35–36
- Salani R, Khanna N, Frimer M, Bristow RE, Chen LM. An update on post-treatment surveillance and diagnosis of recurrence in women with gynecologic malignancies: Society of Gynecologic Oncology (SGO) recommendations. Gynecol Oncol 2017; 146 (01) 3-10
- Elit L, Reade CJ. Recommendations for follow-up care for gynecologic cancer survivors. Obstet Gynecol 2015; 126 (06) 1207-1214
- Concin N, Creutzberg CL, Vergote I. et al. ESGO/ESTRO/ESP Guidelines for the management of patients with endometrial carcinoma. Virchows Arch 2021; 478 (02) 153-190
- Asher R, Obermair A, Janda M, Gebski V. Disease-free and survival outcomes for total laparoscopic hysterectomy compared with total abdominal hysterectomy in early-stage endometrial carcinoma: a meta-analysis. Int J Gynecol Cancer 2018; 28 (03) 529-538
- Li X, Cheng Y, Dong Y. et al. Development and validation of predictive model for lymph node metastasis in endometrial cancer: a SEER analysis. Ann Transl Med 2021; 9 (07) 538
- Kim SR, van der Zanden C, Ikiz H, Kuzelijevic B, Havelock J, Kwon JS. Fertility-sparing management using progestin for young women with endometrial cancer from a population-based study. J Obstet Gynaecol Can 2018; 40 (03) 328-333
- Wortman BG, Creutzberg CL, Putter H. et al; PORTEC Study Group. Ten-year results of the PORTEC-2 trial for high-intermediate risk endometrial carcinoma: improving patient selection for adjuvant therapy. Br J Cancer 2018; 119 (09) 1067-1074
- Nout RA, Smit VT, Putter H. et al; PORTEC Study Group. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010; 375 (9717): 816-823
- Jamieson A, Bosse T, McAlpine JN. The emerging role of molecular pathology in directing the systemic treatment of endometrial cancer. Ther Adv Med Oncol 2021;13:17588359211035959


 PDF
PDF  Views
Views  Share
Share

