Imaging Recommendations for Diagnosis, Staging, and Management of Sinonasal Tumors
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 047-053
DOI: DOI: 10.1055/s-0042-1759520
Abstract
Sinonasal tumors are a relatively rare and heterogeneous group of tumors. Owing to their nonspecific presentation and rarity, they can be potentially overlooked resulting in delayed diagnosis and management, and increased patient morbidity. Imaging is crucial for the detection, staging, surgical planning, follow-up as well as surveillance of sinonasal masses, wherein computed tomography (CT) and magnetic resonance imaging (MRI) play complementary roles. CT is better at depicting bony changes, while MRI is useful for delineating the extent of soft tissue lesion, detect perineural, intracranial, or intraorbital spread as well as differentiate trapped sinus secretions from tumor tissue. Other modalities like fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) and arteriography can be selectively employed. FDG-PET is useful for metastatic workup and detection of residual/ recurrent disease. Arteriography and endovascular image-guided interventions are useful to delineate supply of vascular tumors and perform preoperative embolization. A systematic evidence-based approach to a possible case of sinonasal tumor can go a long way in streamlining the detection and management of these tumors, while optimizing the use of available healthcare resources.
Keywords
guidelines - malignancy - tumors - neoplasms - paranasal sinus - radiology - sinonasal imagingAuthors' Contributions
ASB, GMNI, SM contributed in the concept, design, literature search, manuscript preparation, editing, and review. AG, RK, AS, AT, and AI contributed to the manuscript editing and review.
The authors hereby declare to have read and given their approval for this manuscript.
Supplementary MaterialPublication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Sinonasal tumors are a relatively rare and heterogeneous group of tumors. Owing to their nonspecific presentation and rarity, they can be potentially overlooked resulting in delayed diagnosis and management, and increased patient morbidity. Imaging is crucial for the detection, staging, surgical planning, follow-up as well as surveillance of sinonasal masses, wherein computed tomography (CT) and magnetic resonance imaging (MRI) play complementary roles. CT is better at depicting bony changes, while MRI is useful for delineating the extent of soft tissue lesion, detect perineural, intracranial, or intraorbital spread as well as differentiate trapped sinus secretions from tumor tissue. Other modalities like fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) and arteriography can be selectively employed. FDG-PET is useful for metastatic workup and detection of residual/ recurrent disease. Arteriography and endovascular image-guided interventions are useful to delineate supply of vascular tumors and perform preoperative embolization. A systematic evidence-based approach to a possible case of sinonasal tumor can go a long way in streamlining the detection and management of these tumors, while optimizing the use of available healthcare resources.
Keywords
guidelines - malignancy - tumors - neoplasms - paranasal sinus - radiology - sinonasal imagingIntroduction
Sinonasal tumors are rare with sinonasal malignancies accounting for about 3%-of the head and neck cancers.[1] Many are diagnosed in advanced stages owing to innocuous symptoms until late into disease. The clinical manifestation often is similar to inflammatory sinus conditions including nasal discharge, nasal blockade, headache or epistaxis, thereby making a clinical diagnosis difficult. At times, a mass may be visualized on clinical examination or nasal endoscopy. Even when a mass is visualized, cross sectional imaging is essential to elucidate the origin and extent of the mass.
Computed tomography (CT) and magnetic resonance imaging (MRI) play a complementary role with bony and cartilaginous lesions better depicted by CT, whereas soft tissue extension of tumor, differentiation between tumor and trapped secretions, perineural spread, intraorbital, dural, cavernous sinus, and intracranial involvement are better seen on MRI.
Although pointing out a histological diagnosis is often impractical on imaging, it does help characterize the lesion into benign versus malignant and guide further management.
Risk Factors and Etiopathogenesis
Predilections and risk factors of the sinonasal tumors vary by the histological subtype.
Notable associations are that of the juvenile nasal angiofibroma (JNA) occurring exclusively in males, bimodal distribution of olfactory neuroblastoma with a peak at age 45-55 years and smaller peak at 10 to 25 years, human papillomavirus (HPV) association of inverted papilloma, Epstein-Barr virus association of sinonasal lymphoepithelial carcinoma.[2]
Important risk factors for malignant sinonasal tumors are inhaled wood dust (particularly hardwood), leather dust, nickel and chrome pigments. The aforementioned reportedly cause 600-fold increased risk for adenocarcinoma and 20-fold increased risk for squamous cell carcinoma (SCC).[3] HPV infection and smoking are the other lesser risk factors.[4] Carcinogens like formaldehyde, diisopropyl sulfate, dichloroethyl sulfide, and thorotrast have also been implicated.
Sinonasal malignancies are not notable for lymphadenopathy or distant metastases. They, however, tend to demonstrate contiguous multicompartmental local invasion with destroyed intervening bones.[5]
Epidemiology, Clinical Presentation in India and Globally
Sinonasal tumors are rare with incidence of less than 1 in 100,000 per year.[6] The sinonasal malignancies comprise 3%-of the head and neck cancers and 1%-of all malignancies. Peak incidence is in the fifth to seventh decade with a male preponderance.[1] SCC is the most common malignancy accounting for 50 to 80%-of epithelial sinonasal malignancies.[2] The nasal cavity, maxillary and ethmoid sinuses are common sites, whereas frontal and sphenoid sinuses are rarely involved. Benign tumors are commoner in the second to third decade, with papilloma being the most common benign epithelial neoplasm.
Studies investigating the epidemiology of sinonasal tumors in India are limited. Few retrospective studies done have found a similar distribution of these tumors as seen globally. A study by Satarkar and Srikanth in North India retrospectively analyzed 206 cases of sinonasal tumors and tumor-like conditions during a period of 5 years, and found similar results. In their study, JNA was the most common benign tumor and SCC the most common malignant tumour.[7]
Clinical manifestations of sinonasal tumors are often ambiguous and mimic rhinosinusitis, thereby delaying presentation and diagnosis. Advanced disease with orbital or skull base involvement may present with visual impairment, proptosis, diplopia, epiphora, anosmia, or cranial neuropathies.
Imaging Referral Guidelines
Guidelines proposed by various societies around the world for referral and imaging in sinonasal tumors primarily advocate CT and MRI of the head and neck in complementary roles.
Imaging in the form of combination of CT and MRI is recommended by the Royal College of Radiologists (RCR) in all biopsy proven cases of sinonasal cancer to stage disease ([Fig. 1]).[8] CT with contrast or MRI with contrast of head and neck is indicated in suspected cases of paranasal sinus (PNS) tumors by the National Comprehensive Cancer Network (NCCN).[9] Maxillofacial CT with or without intravenous (IV) contrast and MRI of orbits face neck with and without IV contrast are usually appropriate as initial imaging for suspected sinonasal mass, as per the American College of Radiology (ACR) appropriateness criteria.[10] If an MRI is planned, then a complementary noncontrast maxillofacial CT is usually sufficient as only bony changes need be assessed on CT. Imaging is to be done ideally before biopsy, if possible, as a biopsy procedure may lead to edema of the tumor and surrounding mucosa and so spuriously overstage disease extent. If advanced disease is detected on CT making a poor surgical candidate, further sinonasal imaging is not recommended by the RCR. It is frequent for tumors to cause obstruction of sinus drainage thus leading to inspissated secretions clogging the sinuses with blocked drainage pathways. Noncontrast CT may not clearly differentiate clogged secretions from tumor, and it is therefore necessary to image with either contrast enhanced CT or with MR for this differentiation.
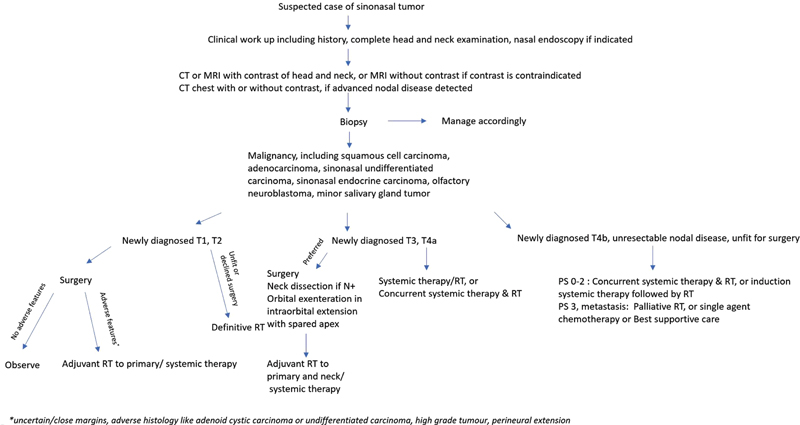
| Figure 1:Simplified flowchart for the management of suspected sinonasal mass (adapted from the NCCN v1.2022 guidelines[9]). CT, computed tomography; MRI, magnetic resonance imaging; RT, radiotherapy.
Noncontrast MRI orbits face neck may be appropriate conditionally, if contrast is contraindicated. Noncontrast CT head or combined pre- and postcontrast maxillofacial CT imaging is not appropriate. Radiography of PNS, fluorodeoxyglucose-positron emission tomography (FDG-PET) whole body, single-photon emission computed tomography of PNS, CT cone-beam PNS and computed tomography angiography/magnetic resonance angiography (CTA/MRA) of head are considered usually not appropriate for initial imaging. CT and MRA may be useful for preoperative planning of a vascular mass. Similarly, craniofacial arteriography is not appropriate as initial imaging, and may be employed in cases of vascular tumors for preoperative embolization, preoperative planning and to control severe epistaxis.
While the RCR recommends CT chest in all clinical stages to rule out lung metastases, the NCCN recommends chest CT with or without contrast in cases of advanced nodal disease to screen for lung metastases.
FDG-PET is not generally indicated for staging. It serves to screen for lymph nodal and distant metastases in advanced disease (stage III or IV)[9] and as a problem-solving modality in cases of suspected recurrence and for suspected cancerous lymph nodes not accessible for fine-needle aspiration (FNA) or with equivocal FNA cytology results.[8]
Contrast CT or FDG-PET/CT of the abdomen and chest as well as contrast MR of the brain is indicated to rule out distant metastases if biopsy reveals a sinonasal mucosal melanoma.[9]
Clinical/Diagnostic Workup (Excluding Imaging)
As per NCCN guidelines, the workup comprises history including documentation and quantification of tobacco use (pack years smoked) and physical examination including complete head and neck examination with nasal endoscopy as clinically indicated. Dental consultation, nutritional, speech and swallowing evaluation, screening for depression, smoking cessation counselling, and fertility/reproductive counselling are also to be considered as clinically indicated.
Although most tumors require biopsy to establish a histopathological diagnosis, exceptions do exist, like JNA wherein the diagnosis is clinicoradiological and biopsy is usually avoided. Transnasal route for endoscopic/punch biopsy is preferred, when performed. Needle biopsy is acceptable. In sampling of maxillary tumors, canine fossa puncture and Caldwell-Luc approach are to be avoided for biopsy.
Imaging Guidelines
Screening
Sinonasal cancers are rare, and general population screening is ineffective, with a potential for false positive diagnoses. Currently, there is no evidence to support screening of head and neck cancers in general as well as high-risk populations.[11] [12] [13] [14]
Diagnosis
Imaging is done with contrast unless contraindicated.[9] The diagnostic CT protocol entails spiral CT following intravenous contrast administration from skull base to thoracic inlet with hands by the sides of the patient. The slice thickness should be no greater than 3 mm. It is viewed in the axial and coronal reformatted planes in soft-tissue as well as bone windows for local extent of tumor and lymph nodal disease.
MRI is better for assessing skull base invasion, soft tissue intracranial, or intraorbital extension, differentiating retained secretions from tumor and perineural spread ([Figs. 2] and [3]). CT is complementary to MRI to assess bony destruction/remodeling ([Figs. 4] and [5]). The basic MR sequences acquired are pre- and post-gadolinium T1-weighted (T1W), post-gadolinium T1-fat suppressed, T2W, and short tau inversion recovery images in orthogonal planes (axial and coronal) with slice thickness no greater than 4 mm. Additionally, T2W sagittal images and T2W coronal images with increased matrix (512 × 512) may also be acquired. MRA may be done to delineate arterial involvement, and volumetric post-gadolinium images acquired for radiotherapy (RT) planning.[8]
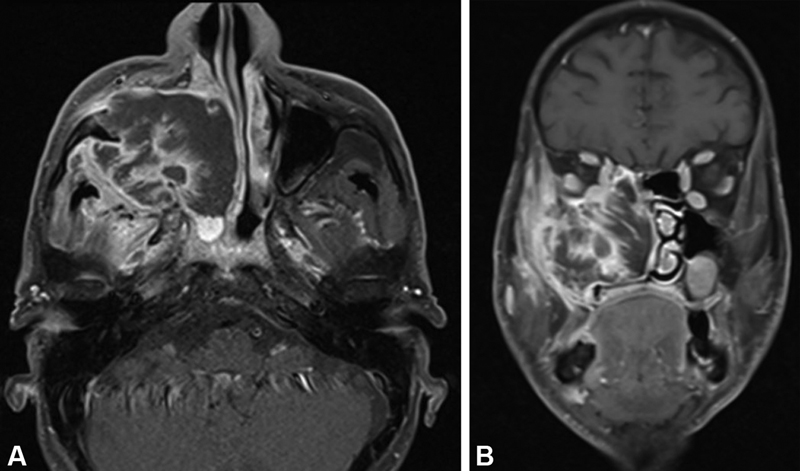
| Figure 2:Well-differentiated squamous cell carcinoma of maxillary sinus. (A) Axial post-gadolinium T1-weighted image with fat suppression shows heterogeneously enhancing irregular mass in the right maxillary sinus with destruction of its anterior and posterior walls and invasion of the subcutaneous tissue and pterygoid fossa, respectively. (B) Coronal post-gadolinium T1-weighted image with fat suppression shows the mass invading into the orbital fat and ethmoid sinus.
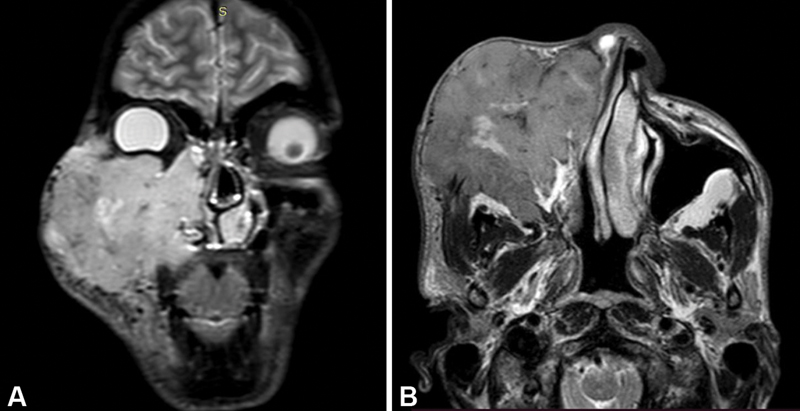
| Figure 3:Nonkeratinizing squamous cell carcinoma of right maxillary sinus. (A) Coronal short tau inversion recovery image shows hyperintense irregular mass lesion of the right maxillary sinus invading the subcutaneous tissue and skin of cheek, hard palate, nasal cavity, right ethmoid sinus, and right orbit. (B) Axial T2-weighted image shows extension of mass into the right infratemporal fossa as well.

| Figure 4:Sinonasal adenocarcinoma. (A) Axial contrast computed tomographic image in soft tissue window shows irregular lobulated mass in the right nasal cavity protruding as far as the anterior choana, and the nasopharynx. Retained secretions in the maxillary sinus noted. (B) Axial bone window image shows destruction of the right turbinate.
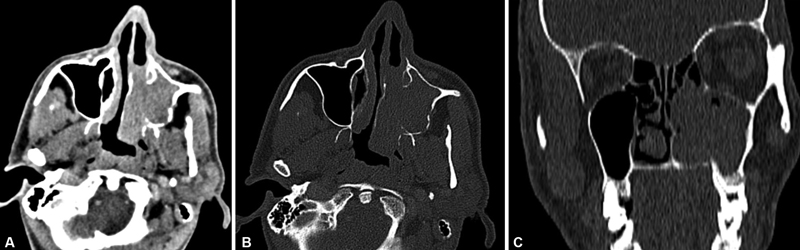
| Figure 5:Sinonasal undifferentiated carcinoma. (A) Axial contrast computed tomographic image in soft tissue window shows enhancing mass in the left maxillary sinus invading the left nasal cavity. (B) Bone window image better depicts the erosion of the posterior wall of left maxillary sinus, and the left medial pterygoid plate. The turbinates are destroyed. (C) Coronal reformatted bone window image shows erosion of the hard palate.
Ultrasonography (US) may be used for the assessment of clinically occult neck nodes and post-treatment surveillance of neck nodes. In nodal assessment by US, it is noteworthy that size criterion (short axis diameter > 1.0 cm) has poor sensitivity and additional features in form of shape, contour, echogenicity, grouping, internal architecture, necrosis and pattern of Doppler vascularity must be taken into account to achieve greater accuracy (reportedly more than 90%).[15] US-guided FNA cytology is useful to detect metastatic nodes with high specificity.
In resource-poor settings, a holistic workup might not always be practical. The bare minimum investigations as recommended by the National Cancer Grid in such scenarios include diagnostic nasal endoscopy with biopsy and IHC, CT PNS, and chest X-ray.[16] It recommends MRI of face and neck, CT Thorax, ophthalmic and endocrine evaluation for optimal assessment. PET CT is deemed optional in initial assessment.
The synoptic reporting formats for CT PNS have been provided in [Supplementary Material].
Staging
More than 70 histopathological entities of sinonasal neoplasms have been classified by the World Health Organization, based on tissue of origin and differentiation, and grouped under benign and malignant tumours.[2]
The American Joint Committee on Cancer—Tumour Node Metastasis (AJCC TNM) staging system for malignancies of the nasal cavity and PNS is referred to for staging of epithelial (non-melanoma) sinonasal tumours.[17] TNM staging of head and neck mucosal melanomas is separately described to stage them. The current edition (8th at the time of publication) distinguishes and separately describes the staging of maxillary sinus and ethmoid sinus tumors ([Table 1]). No system for staging of malignancies of the frontal and sphenoid sinus is defined. Staging systems other than the TNM exist too for certain tumor histologies, like the Kadish staging for olfactory neuroblastoma.
|
T staging |
Maxillary sinus |
Nasal cavity and ethmoid sinus |
|
|---|---|---|---|
|
Tx |
Primary tumor cannot be assessed |
||
|
Tis |
Carcinoma in situ |
||
|
T1 |
Limited to mucosa with no bony erosion/destruction |
Limited to one subsite with or without bony invasion |
|
|
T2 |
Bony destruction including extension to hard palate and/or middle meatus, but not including posterior wall of maxillary sinus and pterygoid plates |
Tumor involves two subsites within the same region or extends to an adjacent region within the nasoethmoidal complex ± bony invasion |
|
|
T3 |
Involvement of one of the following—bone of posterior wall of maxillary sinus, subcutaneous tissues, floor or medial wall of orbit, pterygoid fossa, ethmoid sinuses |
Invasion of medial wall or floor of orbit, maxillary sinus, palate or cribriform plate |
|
|
T4 |
T4a—termed moderately advanced local disease. Invasion of anterior orbital contents, skin of cheek, pterygoid plates, infra-temporal fossa, cribriform plate, sphenoid or frontal sinuses T4b—termed very advanced local disease. Invasion of orbital apex, dura, brain, middle cranial fossa, cranial nerves other than V2, nasopharynx or clivus |
T4a—termed moderately advanced local disease. Invasion of anterior orbital contents, skin of cheek, minimal extension to anterior cranial fossa, pterygoid plates, sphenoid or frontal sinuses. T4b—termed very advanced local disease. Invasion of orbital apex, dura, brain, middle cranial fossa, cranial nerves other than V2, nasopharynx or clivus |
|
|
N staging (clinical) |
|||
|
Nx |
Regional lymph nodes cannot be assessed |
||
|
N0 |
No regional lymph node metastasis |
||
|
N1 |
Single ipsilateral lymph node metastasis < 3cm in greatest dimension and no extranodal extension (ENE) |
||
|
N2 |
N2a—Single ipsilateral lymph node metastasis (3-6 cm) with ENE (−) N2b—Multiple ipsilateral lymph node metastasis, all < 6 cm and ENE (−) N2c—Bilateral or contralateral lymph node metastasis < 6 cm and ENE (−) |
||
|
N3 |
N3a—Metastasis in a lymph node with greatest dimension > 6 cm and ENE (−) N3b—metastasis in any node(s) with clinically overt ENE |
||
|
M staging |
|||
|
M0 |
No distant metastasis |
||
|
M1 |
Distant metastasis |
||
|
Prognostic stage groups |
|||
|
Stage 0 |
Tis |
N0 |
M0 |
|
Stage I |
T1 |
N0 |
M0 |
|
Stage II |
T2 |
N0 |
M0 |
|
Stage III |
T1, 2 |
N1 |
M0 |
|
T3 |
N0, 1 |
M0 |
|
|
Stage IVA |
T1, 2, 3 |
N2 |
M0 |
|
T4a |
N0, 1, 2 |
M0 |
|
|
Stage IVB |
Any T |
N3 |
M0 |
|
T4b |
Any N |
M0 |
|
|
Stage IVC |
Any T |
Any N |
M1 |
Conflict of Interest
None declared.
Authors' Contributions
ASB, GMNI, SM contributed in the concept, design, literature search, manuscript preparation, editing, and review. AG, RK, AS, AT, and AI contributed to the manuscript editing and review.
The authors hereby declare to have read and given their approval for this manuscript.
Supplementary MaterialReferences
- Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging 2012; 12: 136-152
- McCollister KB, Hopper BD, Michel MA. Sinonasal neoplasms: Update on classification, imaging features, and management. Appl Radiol 2015; 44 (12) 7-15
- Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014; 11 (08) 460-472
- Haerle SK, Gullane PJ, Witterick IJ, Zweifel C, Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am 2013; 24 (01) 39-49
- Sen S, Chandra A, Mukhopadhyay S, Ghosh P. Imaging approach to sinonasal neoplasms. Neuroimaging Clin N Am 2015; 25 (04) 577-593
- Lund VJ, Clarke PM, Swift AC, McGarry GW, Kerawala C, Carnell D. Nose and paranasal sinus tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016; 130 (S2): S111-S118
- Satarkar RN, Srikanth S. Tumors and tumor-like conditions of the nasal cavity, paranasal sinuses, and nasopharynx: a study of 206 cases. Indian J Cancer 2016; 53 (04) 478-482
- Olliff J, Richards P, Connor S. et al. Head and Neck Cancers. In: Nicholson T. ed. Recommendations for Cross-Sectional Imaging in Cancer Management. 2nd ed.. London: The Royal College of Radiologists; 2014
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 1.2022). Accessed November 11, 2022, at: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- American College of Radiology. ACR Appropriateness Criteria Sinonasal Disease (2021 revision). Accessed November 11, 2022, at: http://acsearch.acr.org/docs/69502/narrative
- Genden EM, Rinaldo A, Bradley PJ. et al. Referral guidelines for suspected cancer of the head and neck. Auris Nasus Larynx 2006; 33 (01) 1-5
- Hawkins RJ, Wang EE, Leake JL. Preventive health care, 1999 update: prevention of oral cancer mortality. The Canadian Task Force on Preventive Health Care. J Can Dent Assoc 1999; 65 (11) 617
- Prout MN, Sidari JN, Witzburg RA, Grillone GA, Vaughan CW. Head and neck cancer screening among 4611 tobacco users older than forty years. Otolaryngol Head Neck Surg 1997; 116 (02) 201-208
- Calabrese L, Tradati N, Nickolas TL. et al. Cancer screening in otorhinolaryngology. Oral Oncol 1998; 34 (01) 1-4
- Richards PS, Peacock TE. The role of ultrasound in the detection of cervical lymph node metastases in clinically N0 squamous cell carcinoma of the head and neck. Cancer Imaging 2007; 7: 167-178
- Guidelines Version NCG. 3 Management of Head and Neck Cancers. Accessed November 11, 2022, at: http://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/Headandneck/Management_of_HN_cancer_Ver.3-AT-15Nov19.pdf
- Amin MB, Edge SB, Greene FL. et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017
- Higgins TS, Thorp B, Rawlings BA, Han JK. Outcome results of endoscopic vs craniofacial resection of sinonasal malignancies: a systematic review and pooled-data analysis. Int Forum Allergy Rhinol 2011; 1 (04) 255-261
- Lund VJ, Stammberger H, Nicolai P. et al; European Rhinologic Society Advisory Board on Endoscopic Techniques in the Management of Nose, Paranasal Sinus and Skull Base Tumours. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl 2010; 22: 1-143
- Robbins KT, Ferlito A, Silver CE. et al. Contemporary management of sinonasal cancer. Head Neck 2011; 33 (09) 1352-1365
- Carrau RL, Segas J, Nuss DW. et al. Squamous cell carcinoma of the sinonasal tract invading the orbit. Laryngoscope 1999; 109 (2 Pt 1): 230-235
- Sangal NR, Lee Y-J, Brady JS. et al. The role of elective neck dissection in the treatment of maxillary sinus squamous cell carcinoma. Laryngoscope 2018; 128 (08) 1835-1841
- Guntinas-Lichius O, Kreppel MP, Stuetzer H, Semrau R, Eckel HE, Mueller RP. Single modality and multimodality treatment of nasal and paranasal sinuses cancer: a single institution experience of 229 patients. Eur J Surg Oncol 2007; 33 (02) 222-228
Address for correspondence
Publication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Simplified flowchart for the management of suspected sinonasal mass (adapted from the NCCN v1.2022 guidelines[9]). CT, computed tomography; MRI, magnetic resonance imaging; RT, radiotherapy.

| Figure 2:Well-differentiated squamous cell carcinoma of maxillary sinus. (A) Axial post-gadolinium T1-weighted image with fat suppression shows heterogeneously enhancing irregular mass in the right maxillary sinus with destruction of its anterior and posterior walls and invasion of the subcutaneous tissue and pterygoid fossa, respectively. (B) Coronal post-gadolinium T1-weighted image with fat suppression shows the mass invading into the orbital fat and ethmoid sinus.

| Figure 3:Nonkeratinizing squamous cell carcinoma of right maxillary sinus. (A) Coronal short tau inversion recovery image shows hyperintense irregular mass lesion of the right maxillary sinus invading the subcutaneous tissue and skin of cheek, hard palate, nasal cavity, right ethmoid sinus, and right orbit. (B) Axial T2-weighted image shows extension of mass into the right infratemporal fossa as well.

| Figure 4:Sinonasal adenocarcinoma. (A) Axial contrast computed tomographic image in soft tissue window shows irregular lobulated mass in the right nasal cavity protruding as far as the anterior choana, and the nasopharynx. Retained secretions in the maxillary sinus noted. (B) Axial bone window image shows destruction of the right turbinate.

| Figure 5:Sinonasal undifferentiated carcinoma. (A) Axial contrast computed tomographic image in soft tissue window shows enhancing mass in the left maxillary sinus invading the left nasal cavity. (B) Bone window image better depicts the erosion of the posterior wall of left maxillary sinus, and the left medial pterygoid plate. The turbinates are destroyed. (C) Coronal reformatted bone window image shows erosion of the hard palate.
References
- Eggesbø HB. Imaging of sinonasal tumours. Cancer Imaging 2012; 12: 136-152
- McCollister KB, Hopper BD, Michel MA. Sinonasal neoplasms: Update on classification, imaging features, and management. Appl Radiol 2015; 44 (12) 7-15
- Llorente JL, López F, Suárez C, Hermsen MA. Sinonasal carcinoma: clinical, pathological, genetic and therapeutic advances. Nat Rev Clin Oncol 2014; 11 (08) 460-472
- Haerle SK, Gullane PJ, Witterick IJ, Zweifel C, Gentili F. Sinonasal carcinomas: epidemiology, pathology, and management. Neurosurg Clin N Am 2013; 24 (01) 39-49
- Sen S, Chandra A, Mukhopadhyay S, Ghosh P. Imaging approach to sinonasal neoplasms. Neuroimaging Clin N Am 2015; 25 (04) 577-593
- Lund VJ, Clarke PM, Swift AC, McGarry GW, Kerawala C, Carnell D. Nose and paranasal sinus tumours: United Kingdom National Multidisciplinary Guidelines. J Laryngol Otol 2016; 130 (S2): S111-S118
- Satarkar RN, Srikanth S. Tumors and tumor-like conditions of the nasal cavity, paranasal sinuses, and nasopharynx: a study of 206 cases. Indian J Cancer 2016; 53 (04) 478-482
- Olliff J, Richards P, Connor S. et al. Head and Neck Cancers. In: Nicholson T. ed. Recommendations for Cross-Sectional Imaging in Cancer Management. 2nd ed.. London: The Royal College of Radiologists; 2014
- National Comprehensive Cancer Network. Head and Neck Cancers (Version 1.2022). Accessed November 11, 2022, at: http://www.nccn.org/professionals/physician_gls/pdf/head-and-neck.pdf
- American College of Radiology. ACR Appropriateness Criteria Sinonasal Disease (2021 revision). Accessed November 11, 2022, at: http://acsearch.acr.org/docs/69502/narrative
- Genden EM, Rinaldo A, Bradley PJ. et al. Referral guidelines for suspected cancer of the head and neck. Auris Nasus Larynx 2006; 33 (01) 1-5
- Hawkins RJ, Wang EE, Leake JL. Preventive health care, 1999 update: prevention of oral cancer mortality. The Canadian Task Force on Preventive Health Care. J Can Dent Assoc 1999; 65 (11) 617
- Prout MN, Sidari JN, Witzburg RA, Grillone GA, Vaughan CW. Head and neck cancer screening among 4611 tobacco users older than forty years. Otolaryngol Head Neck Surg 1997; 116 (02) 201-208
- Calabrese L, Tradati N, Nickolas TL. et al. Cancer screening in otorhinolaryngology. Oral Oncol 1998; 34 (01) 1-4
- Richards PS, Peacock TE. The role of ultrasound in the detection of cervical lymph node metastases in clinically N0 squamous cell carcinoma of the head and neck. Cancer Imaging 2007; 7: 167-178
- Guidelines Version NCG. 3 Management of Head and Neck Cancers. Accessed November 11, 2022, at: http://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/Headandneck/Management_of_HN_cancer_Ver.3-AT-15Nov19.pdf
- Amin MB, Edge SB, Greene FL. et al, eds. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer, 2017
- Higgins TS, Thorp B, Rawlings BA, Han JK. Outcome results of endoscopic vs craniofacial resection of sinonasal malignancies: a systematic review and pooled-data analysis. Int Forum Allergy Rhinol 2011; 1 (04) 255-261
- Lund VJ, Stammberger H, Nicolai P. et al; European Rhinologic Society Advisory Board on Endoscopic Techniques in the Management of Nose, Paranasal Sinus and Skull Base Tumours. European position paper on endoscopic management of tumours of the nose, paranasal sinuses and skull base. Rhinol Suppl 2010; 22: 1-143
- Robbins KT, Ferlito A, Silver CE. et al. Contemporary management of sinonasal cancer. Head Neck 2011; 33 (09) 1352-1365
- Carrau RL, Segas J, Nuss DW. et al. Squamous cell carcinoma of the sinonasal tract invading the orbit. Laryngoscope 1999; 109 (2 Pt 1): 230-235
- Sangal NR, Lee Y-J, Brady JS. et al. The role of elective neck dissection in the treatment of maxillary sinus squamous cell carcinoma. Laryngoscope 2018; 128 (08) 1835-1841
- Guntinas-Lichius O, Kreppel MP, Stuetzer H, Semrau R, Eckel HE, Mueller RP. Single modality and multimodality treatment of nasal and paranasal sinuses cancer: a single institution experience of 229 patients. Eur J Surg Oncol 2007; 33 (02) 222-228


 PDF
PDF  Views
Views  Share
Share

