Imaging Recommendations for Diagnosis, Staging, and Management of Renal Tumors
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 084-092
DOI: DOI: 10.1055/s-0042-1759718
Abstract
Renal cell carcinomas accounts for 2% of all the cancers globally. Most of the renal tumors are detected incidentally. Ultrasound remains the main screening modality to evaluate the renal masses. A multi -phase contrast enhanced computer tomography is must for characterizing the renal lesions. Imaging plays an important role in staging, treatment planning and follow up of renal cancers. In this review , we discuss the imaging guidelines for the management of renal tumors.
Publication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Renal cell carcinomas accounts for 2%-of all the cancers globally. Most of the renal tumors are detected incidentally. Ultrasound remains the main screening modality to evaluate the renal masses. A multi -phase contrast enhanced computer tomography is must for characterizing the renal lesions. Imaging plays an important role in staging, treatment planning and follow up of renal cancers. In this review , we discuss the imaging guidelines for the management of renal tumors.
Keywords
CT scan - MRI - PET-CT scan - renal cell carcinoma - ultrasoundIntroduction
Continuous advancements in various imaging modalities have revolutionized the imaging algorithm of renal masses. A majority of renal masses are detected incidentally when the patient is scanned for unrelated complaints. Radiologists need to be able to characterize renal mass on imaging. The foremost step is to differentiate between cystic and solid masses as up to 90%-of solid tumors are malignant, whereas purely cystic lesions are usually benign or indolent.
Imaging is also important for staging, treatment planning, and follow-up of malignant renal masses. Ultrasound (US) is the screening modality for the evaluation of renal masses but cannot distinctly differentiate between benign and malignant lesions accurately and is also operator dependent. Contrast-enhanced US (CEUS) is a valuable addition and is especially useful in characterizing complex cystic lesions and the identification of pseudotumors. Multiphasic contrast-enhanced computed tomography (CT) is the current gold standard for the evaluation of renal masses and multiparametric magnetic resonance imaging (MRI) is used mainly as a problem-solving tool.
Risk Factors and Etiopathogenesis
Renal cell carcinoma (RCC) accounts for 2%-of all cancers globally and is responsible for 2%-of cancer deaths. It is the seventh most common cancer in men and the tenth most common in women[1] .
Risk factors and etiopathogenesis include:[2]
Obesity: Obesity is a risk factor for kidney cancer in both men and women. The mechanisms by which obesity influences renal carcinogenesis are unclear, with chronic inflammation in adipose tissue and immune dysregulation potentially promoting carcinogenesis.
Smoking: Ever-smokers have a higher risk of renal cancer than never-smokers with a dose-dependent increase in risk related to the number of cigarettes smoked per day.
Hypertension: Hypertension is an independent risk factor for RCC.
Acquired cystic disease: Patients on long-term hemodialysis due to end-stage renal disease develop renal cysts and have an increased risk of renal cancer
Occupational exposure: Exposure to metal dyes increases the risk of developing RCCs.
Genetic susceptibility: Many genetic syndromes are associated with the development of RCC.
Epidemiology and Clinical Presentation
Abdominal mass, pain abdomen, and hematuria are the three cardinal clinical signs to suspect RCC. However, this constellation of symptoms is rarely seen at presentation these days and rather suggests advanced disease. About half of the RCCs are detected incidentally; such masses are small in size and pretend to have a good prognosis. Other common manifestations can be fever, leukocytosis, and weight loss. A variety of paraneoplastic syndromes may occur like polycythemia due to secretion of erythropoietin, hypercalcemia due to oversecretion of parathormone-related hormone peptide, hypertension due to renin, or Cushing syndrome due to adrenocorticotropic hormone.[3]
Clinical/ Diagnostic Workup
The initial workup of patients with suspected RCC includes history, physical examination, and blood investigations including a complete blood count with differential white blood count, serum calcium, liver functions, and renal functions. The workup allows the patient with metastatic RCC to be classified into favorable, intermediate, and poor risk categories, as per the International Metastatic RCC Database Consortium (IMDC) classification[4] . The factors include
Less than 1 year from the time of diagnosis to systemic therapy
Karnofsky performance status less than 70
Hemoglobin less than lower limit of normal
Corrected calcium more than upper limit of normal
Neutrophils more than upper limit of normal
Platelets more than upper limit of normal
The presence of none, 1 to 2, and 3 or more of the above factors categorizes the patient into favorable, intermediate, and poor risk categories, respectively. The choice of appropriate systemic therapy is based on the IMDC risk categories. For example, intermediate and poor-risk patients are treated with immune checkpoint inhibitor combinations (nivolumab and ipilimumab) or a combination of an immune checkpoint inhibitor with vascular endothelial growth factor tyrosine kinase inhibitor (VEGF TKI) pembrolizumab and lenvatinib/axitinib, nivolumab and cabozantinib, and avelumab and axitinib), while good risk patients are treated with VEGF TKIs (sunitinib or pazopanib) or a combination of immune checkpoint inhibitor and VEGF TKIs.[5]
Imaging Guidelines
RCC may be detected by an abdominal US either incidentally or in symptomatic patients. US serves as the most convenient and reliable screening tool for the detection of renal mass. It can accurately detect simple and minimally complicated cysts (Bosniak categories 1 and II). No further imaging is required in such cases. The accuracy of US falls in complex renal cysts from Bosniak 2F onwards. Differentiation of benign versus malignant masses cannot be confidently made by B mode US.
The last decade has seen an upsurge in renal applications of CEUS and it has shown fair potential in the characterization of renal tumors, especially in patients with chronic kidney disease or allergy to CT or MRI contrast. CEUS has shown great potential specifically in the differentiation of pseudotumors from renal tumors. The same enhancement characteristics along with the normal vascular pattern of the mass as the background normal kidney favor pseudotumors.[6] [7] In addition, characterization of indeterminate masses, classification of the cystic renal mass into one of the Bosniak categories,[8] [9] postablative treatment assessment,[10] [11] differentiating bland versus malignant thrombus,[12] [13] and renal transplant evaluation are other potential applications of CEUS.[14] Subtyping of the RCC by CEUS requires more studies for validation. Further characterization of the renal mass requires a dedicated tailored imaging protocol.
As per the guidelines issued by the American Association of Urology, high-quality, multiphasic, cross-sectional imaging is mandatory in any patient detected to have renal mass. This is essential for the optimum characterization and staging of the mass. Multiphasic CT forms the mainstay for the diagnosis of renal tumors. Morphology of the lesion, presence, and dynamic nature of enhancement are the important criteria in these modalities for differentiating benign from malignant masses.[14] [15] In all suspected cases, a renal protocol is followed. Patient is given neutral oral contrast. A noncontrast scan is done followed by a postcontrast nephrographic phase at 40 to 70 seconds, a corticomedullary phase at 100 to 120 seconds, and an excretory phase at 7 to 10 minutes. Renal carcinoma is best identified in the nephrographic phase. The various subtypes of RCC can be better appreciated in the corticomedullary phase. The involvement of the pelvicalyceal system can be seen in the excretory phase. The split bolus technique is a newer modification that is currently followed in our institute as well. Conventional CT includes four phases in total that amounts to a high radiation dose. The split bolus technique has allowed a reduction in the number of phases with reduced total radiation dose and comparable imaging quality.
A baseline multiphasic CT is required in all diagnosed cases of RCC. The first step is to determine whether the mass is cystic or solid. If the mass is cystic, depending upon the complexity of the lesion, it should be classified in one of the Bosniak categories. Bosniak classification, version 2019, is used on a renal mass protocol CT or MRI for predicting the risk of malignancy in cystic renal masses and guides treatment in each category. Any cystic lesion can be classified into one of the five categories namely I, II, IIF, III, and IV. Risk of malignancy increases from category IIF onwards. Bosniak III cysts can be managed with either active surveillance or primary surgery.[14] [16] [17] When the attenuation of the renal lesion is between −10 and +20 Hounsfield unit (HU), it is likely to be a simple cyst. If the attenuation is greater than 70 HU, it is likely to be a proteinaceous or hemorrhagic cyst. No further investigation is required for Bosniak category 1, II cysts. Bosniak category II F cysts require active surveillance and Bosniak category III and IV cysts should be considered for surgery.[18] If it is solid, it should be characterized as malignant (RCC, metastasis, lymphoma) or benign (angiomyolipoma [AML] or oncocytoma). About 90%-of the solid masses are malignant. When the attenuation is between 20 and 70 HU on plain CT, contrast enhancement of greater than 15 to 20HU with less than 5%-fat is highly suspicious for RCC.[16]
Such lesions mandate urological consultation for possible surgical management. Once the mass is characterized and labeled to be malignant, further staging is done to decide on the appropriate management. Essential points to consider for the staging of the mass are the size of the mass, the morphology of the mass including complexity, enhancement, and presence of fat, exophytic or endophytic nature of the mass, crossing the polar lines, involvement of PCS, invasion of perinephric fat, amount of perinephric fat, involvement of renal vein/IVC, invasion of adrenal/ surrounding organs, lymphadenopathy, distant metastasis and status of the contralateral kidney. TNM staging (8th edition) has been elaborated in [Table 1].
|
T—Primary tumor |
|---|
|
· T1—tumor < 7 cm or less in greatest dimension, limited to the kidney |
|
o T1a: tumor confined to kidney, <4> |
|
o T1b: tumor confined to kidney, >4 cm but <7> |
|
· T2: limited to kidney >7 cm |
|
o T2a: tumor confined to kidney, >7 cm but not >10 cm |
|
o T2b: tumor confined to kidney, >10 cm |
|
· T3: tumor extension into major veins or perinephric tissues, but not into ipsilateral adrenal gland or beyond Gerota fascia |
|
o T3a: T3a tumor extends into the renal vein or its segmental branches, or tumor invades the pelvicalyceal system or tumor invades perirenal and/or renal sinus fat (peripelvic fat), but not beyond Gerota fascia |
|
o T3b: Tumor extends into the vena cava below diaphragm |
|
o T3c: T3c tumor extends into vena cava above the diaphragm or invades the wall of the vena cava |
|
· T4: Tumor invades beyond Gerota fascia (including contiguous extension into the ipsilateral adrenal gland) |
|
N—Regional lymph nodes |
|
· NX regional lymph nodes cannot be assessed |
|
· N0: no nodal involvement |
|
· N1: metastatic involvement of regional lymph node(s) |
|
M |
|
· M0: no distant metastases |
|
· M1: distant metastases |
|
Stage groupings |
|
Stage I T1 N0 M0 |
|
Stage II T2 N0 M0 |
|
Stage III T3 N0 M0 T1, T2, T3 N1 M0 |
|
Stage IV T4 Any N M0 Any T Any N M1 |
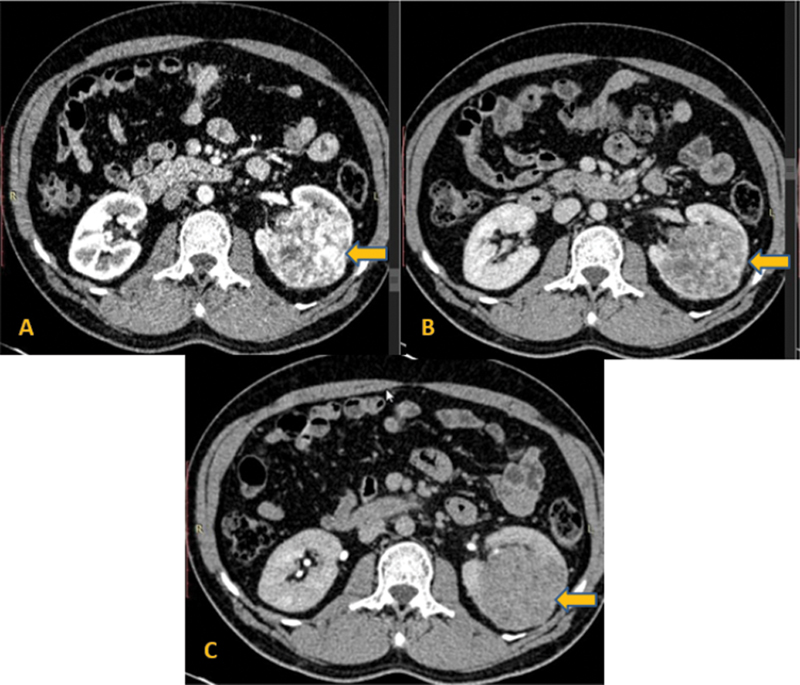
| Figure 1:Multiphase computed tomography axial images of the abdomen show an exo-endophytic mass arrow in the left kidney, showing marked enhancement in the corticomedullary phase (A) with relative washout in the nephrographic (B) and delayed phases (C). Diagnosis of clear cell renal cell carcinoma was made, confirmed on postsurgical histopathology.
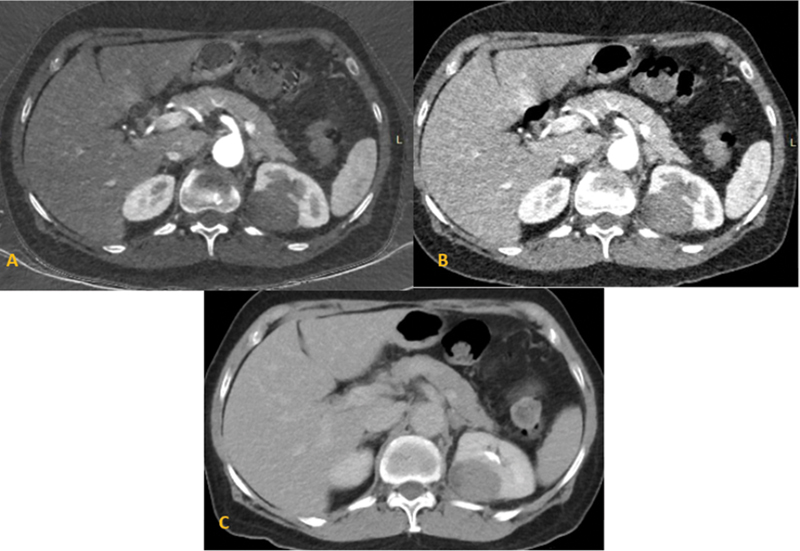
| Figure 12:Multiphase computed tomography axial images of the abdomen show an exo-endophytic mass in the right kidney, showing hypoenhancement in the corticomedullary phase (A) nephrographic (B), and delayed phases (C). Diagnosis of papillary renal cell carcinoma was made, confirmed on histopathology.
Key imaging mimics of RCC are oncocytoma and fat-poor AML.[16] Due to their hypervascular nature, they are often confused with RCC due to which the patient unnecessarily undergoes surgical treatment.[29] Fat-rich AML is easy to diagnose with fat as the major component. They are hyperechoic on US and the fat component in the mass shows low attenuation (<10 href="https://www.thieme-connect.de/products/ejournals/html/10.1055/s-0042-1759718#JR221150451-30" xss=removed>30] [31] Second common benign neoplasm of the kidney is the oncocytoma that closely resembles chromophobe variety of RCC. Oncocytoma usually occurs in elderly patients, and are well-defined, homogenous masses, showing stellate scar with spoke wheel enhancement and segmental enhancement inversion on postcontrast images in different phases.[32]
In the pediatric age group, the most common tumor of the kidney (∼80%-cases) is Wilms tumor having a good prognosis.[33] It presents as a large heterogenous mass with hemorrhage and necrosis and infrequent areas of calcification. One should always look for multifocal/ bilateral disease (in hereditary syndromes), an extension of the mas into vascular structures, invasion of surrounding structures, lymphadenopathy, ascites, and distant metastasis.[21]
Positron Emission Tomography
Positron emission tomography (PET) is not recommended for the staging of RCC. 18F-fluoro-2-deoxy-2-d-glucose, the substrate utilized for PET imaging, is excreted through the kidneys. The renal mass may fallaciously get obscured. The primary role of PET is in the re-evaluation of RCC post-treatment and also to detect recurrent or metastatic disease.[34] Quantitative PET helps evaluate the grade of the tumor, thereby helping in prognostication.[35]
Synoptic reporting formats for radiograph, US, CT, MRI, PET-CT scan are provided in [Table 2]. Also, a concise imaging algorithm for renal mass has been detailed in [Fig. 3].
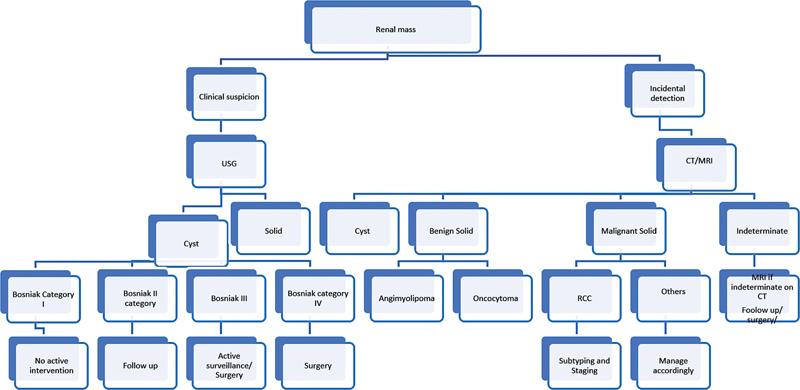
| Figure 3:Imaging algorithm. CT, computed tomography; MRI, magnetic resonance imaging; RCC, renal cell carcinoma; USG, ultrasonography.
|
Ultrasound |
|---|
|
· Presence of mass lesion |
|
· Size |
|
· Growth rate (if previous imaging done) |
|
· Solid or cystic |
|
· Simple or complex cyst |
|
· Echogenicity of the lesion |
|
· Necrosis |
|
· Axial location |
|
· Craniocaudal location |
|
· Margins of lesion |
|
· Capsule present or absent |
|
· Vascularity on color and spectral doppler—present or absent, if present wave form and velocity |
|
· Extent of the lesion |
|
· Distance to collecting system |
|
· Perinephric extension |
|
· Involvement of surrounding organs |
|
· Renal arterial and venous anatomy |
|
· Renal vein/IVC thrombus |
|
· Whether bland or tumor thrombus |
|
· Tumor thrombus if present extent |
|
· Lymphadenopathy |
|
· Obvious metastases (liver, other abdominal organs) |
|
· Status of opposite kidney |
|
MPCT |
|
· Presence of mass lesion |
|
· Size |
|
· Growth rate (if previous imaging done) |
|
· Solid or cystic |
|
· Bosniak classification if cystic |
|
· Macroscopic fat |
|
· Necrosis |
|
· Presence and degree of enhancement |
|
· Axial location |
|
· Craniocaudal location |
|
· Margins of lesion |
|
· Capsule present or absent |
|
· Extent of the lesion |
|
· Distance to collecting system |
|
· Perinephric extension |
|
· Involvement of surrounding organs |
|
· Renal arterial and venous anatomy |
|
· Renal vein/IVC thrombus |
|
· Whether bland or tumor thrombus |
|
· Tumor thrombus if present extent |
|
· Lymphadenopathy |
|
· Distant metastases (lung, liver, bone) |
|
MRI |
|
· Presence of mass lesion |
|
· Size |
|
· Growth rate (if previous imaging done) |
|
· Solid or cystic |
|
· Bosniak classification if cystic |
|
· Signal on T1W,T2W sequences, diffusion restriction |
|
· Macroscopic fat |
|
· Necrosis |
|
· Microscopic fat |
|
· Presence and degree of enhancement |
|
· Possible histology |
|
· Axial location |
|
· Craniocaudal location |
|
· Margins of lesion |
|
· Capsule present or absent |
|
· Extent of the lesion |
|
· Distance to collecting system |
|
· Perinephric extension |
|
· Involvement of surrounding organs |
|
· Renal arterial and venous anatomy |
|
· Renal vein /IVC thrombus |
|
· Whether bland or tumor thrombus |
|
· Tumor thrombus if present extent |
|
· Caval wall invasion |
|
· Lymphadenopathy |
|
· Distant metastases (lung, liver, bone) |
|
PET-CT with IV contrast: |
|
Presence of mass lesion |
|
Size |
|
FDG avid |
|
SUVmax |
|
Solid or cystic |
|
Necrosis |
|
Presence and degree of enhancement |
|
Axial location |
|
Craniocaudal location |
|
Margins of lesion |
|
Capsule present or absent |
|
Extent of the lesion |
|
Distance to collecting system |
|
Perinephric extension |
|
Involvement of surrounding organs |
|
Renal vein/IVC thrombus |
|
Tumor thrombus |
|
Tumor thrombus if present extent |
|
Lymphadenopathy |
|
Distant metastases (lung, liver, bone) |

| Figure 4:Algorithm for renal cell carcinoma (RCC) management. CKD, chronic kidney disease; IVC, inferior vena cava;
Summary of Recommendations
A contrast-enhanced, triple-phase helical CT scan is the preferred imaging study for evaluating renal masses.
Chest CT should be done for the staging of renal cancers except in cT1a renal tumors.
A multiparametric MRI can be performed as a problem-solving tool in characterizing indeterminate renal masses.
The contrast-enhanced US can be helpful in specific cases.
Characterization of small renal mass and response assessment following targeted therapy for advanced RCC are key challenges for current imaging modalities.
No conflict of interest has been declared by the author(s).
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Kabel AM, Zamzami F, Al-Talhi M, Al-Dwila K, Habib R. Renal cell carcinoma: insights into risk factors, diagnosis and management. J Cancer Research Treatment 2017; 5: 15-19
- Padala SA, Barsouk A, Thandra KC. et al. Epidemiology of renal cell carcinoma. World J Oncol 2020; 11 (03) 79-87
- Heng DYC, Xie W, Regan MM. et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27 (34) 5794-5799
- Ko JJ, Xie W, Kroeger N. et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015; 16 (03) 293-300
- Bertolotto M, Bucci S, Valentino M, Currò F, Sachs C, Cova MA. Contrast-enhanced ultrasound for characterizing renal masses. Eur J Radiol 2018; 105: 41-48
- Sidhu PS, Cantisani V, Dietrich CF. et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Long Version). Ultraschall Med 2018; 39 (02) e2-e44
- Clevert D-A, Minaifar N, Weckbach S. et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc 2008; 39 (1-4): 171-178
- Cantisani V, Bertolotto M, Clevert D-A. et al. EFSUMB 2020 proposal for a contrast-enhanced ultrasound-adapted Bosniak cyst categorization - position statement. Ultraschall Med 2021; 42 (02) 154-166
- Wang ZJ, Nikolaidis P, Khatri G. et al; Expert Panel on Urologic Imaging. ACR Appropriateness Criteria® Indeterminate Renal Mass. J Am Coll Radiol 2020; 17 (11S): S415-S428
- Meloni MF, Bertolotto M, Alberzoni C. et al. Follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: contrast-enhanced sonography versus contrast-enhanced CT or MRI. AJR Am J Roentgenol 2008; 191 (04) 1233-1238
- Vikram R, Beland MD, Blaufox MD. et al. ACR appropriateness criteria renal cell carcinoma staging. J Am Coll Radiol 2016; 13 (05) 518-525
- Li Q, Wang Z, Ma X, Tang J, Luo Y. Diagnostic accuracy of contrast-enhanced ultrasound for detecting bland thrombus from inferior vena cava tumor thrombus in patients with renal cell carcinoma. Int Braz J Urol 2020; 46 (01) 92-100
- Ljungberg B, Albiges L, Abu-Ghanem Y. et al. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2022 update. Eur Urol 2022; 82 (04) 399-410
- Ward RD, Tanaka H, Campbell SC, Remer EM. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics 2018; 38 (07) 2021-2033
- Pedrosa I, Cadeddu JA. How we do it: managing the indeterminate renal mass with the MRI clear cell likelihood score. Radiology 2022; 302 (02) 256-269
- Silverman SG, Pedrosa I, Ellis JH. et al. Bosniak classification of cystic renal masses, Version 2019: an update proposal and needs assessment. Radiology 2019; 292 (02) 475-488
- Birnbaum BA, Hindman N, Lee J, Babb JS. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology 2007; 244 (03) 767-775
- Lim DJ, Carter MF. Computerized tomography in the preoperative staging for pulmonary metastases in patients with renal cell carcinoma. J Urol 1993; 150 (04) 1112-1114
- Mano R, Vertosick E, Sankin AI. et al. Subcentimeter pulmonary nodules are not associated with disease progression in patients with renal cell carcinoma. J Urol 2015; 193 (03) 776-782
- Kotecha RR, Flippot R, Nortman T. et al. Prognosis of incidental brain metastases in patients with advanced renal cell carcinoma. J Natl Compr Canc Netw 2021; 19 (04) 432-438
- Dilauro M, Quon M, McInnes MDF. et al. Comparison of contrast-enhanced multiphase renal protocol CT versus MRI for diagnosis of papillary renal cell carcinoma. AJR Am J Roentgenol 2016; 206 (02) 319-325
- Schieda N, Lim RS, McInnes MDF. et al. Characterization of small (<4cm>. Diagn Interv Imaging 2018; 99 (7-8): 443-455
- Herts BR, Silverman SG, Hindman NM. et al. Management of the incidental renal mass on CT: a white paper of the ACR incidental findings committee. J Am Coll Radiol 2018; 15 (02) 264-273
- Krishna S, Schieda N, Pedrosa I. et al. Update on MRI of cystic renal masses including Bosniak Version 2019. J Magn Reson Imaging 2021; 54 (02) 341-356
- Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003; 27 (05) 612-624
- Roy C, Sauer B, Lindner V, Lang H, Saussine C, Jacqmin D. MR imaging of papillary renal neoplasms: potential application for characterization of small renal masses. Eur Radiol 2007; 17 (01) 193-200
- Cornelis F, Grenier N. Multiparametric magnetic resonance imaging of solid renal tumors: a practical algorithm. Semin Ultrasound CT MR 2017; 38 (01) 47-58
- Tsili AC, Andriotis E, Gkeli MG. et al; Oncologic Imaging Subcommittee Working Group of the Hellenic Radiological Society. The role of imaging in the management of renal masses. Eur J Radiol 2021; 141: 109777 https://www.ejradiology.com/article/S0720-048X(21)00258-8/fulltext [Internet]
- Yang C-W, Shen S-H, Chang Y-H. et al. Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma?. AJR Am J Roentgenol 2013; 201 (05) 1017-1028
- Galia M, Albano D, Bruno A. et al. Imaging features of solid renal masses. Br J Radiol 2017; 90 (1077): 20170077
- Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 2016; 8 (05) 484-500
- Servaes SE, Hoffer FA, Smith EA, Khanna G. Imaging of Wilms tumor: an update. Pediatr Radiol 2019; 49 (11) 1441-1452
- Wang H-Y, Ding H-J, Chen J-H. et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 2012; 12: 464-474
- Liu Y. The place of FDG PET/CT in renal cell carcinoma: value and limitations. Front Oncol 2016; 6: 201
- Petrelli F, Coinu A, Vavassori I. et al. Cytoreductive nephrectomy in metastatic renal cell carcinoma treated with targeted therapies: a systematic review with a meta-analysis. Clin Genitourin Cancer 2016; 14 (06) 465-472
- Pindoria N, Raison N, Blecher G, Catterwell R, Dasgupta P. Cytoreductive nephrectomy in the era of targeted therapies: a review. BJU Int 2017; 120 (03) 320-328
- Amin MB, Greene FL, Edge SB. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-9
- Ljungberg B, Albiges L, Abu-Ghanem Y. et al. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2019 update. Eur Urol 2019; 75 (05) 799-810
- Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am 2008; 35 (04) 613-625 , vii vii
- Sun M, Vetterlein M, Harshman LC, Chang SL, Choueiri TK, Trinh Q-D. Risk assessment in small renal masses: a review article. Urol Clin North Am 2017; 44 (02) 189-202
- Martínez-Salamanca JI, Huang WC, Millán I. et al; International Renal Cell Carcinoma-Venous Thrombus Consortium. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011; 59 (01) 120-127
- Margulis V, Sánchez-Ortiz RF, Tamboli P, Cohen DD, Swanson DA, Wood CG. Renal cell carcinoma clinically involving adjacent organs: experience with aggressive surgical management. Cancer 2007; 109 (10) 2025-2030
- Tran J, Ornstein MC. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol Pract 2022; 18 (03) 187-196
- Campbell SC, Novick AC, Belldegrun A. et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182 (04) 1271-1279
- Lee Z, Jegede OA, Haas NB. et al. Local recurrence following resection of intermediate-high risk nonmetastatic renal cell carcinoma: an anatomical classification and analysis of the ASSURE (ECOG-ACRIN E2805) adjuvant trial. J Urol 2020; 203 (04) 684-689
- Thomas AZ, Adibi M, Borregales LD. et al. Surgical management of local retroperitoneal recurrence of renal cell carcinoma after radical nephrectomy. J Urol 2015; 194 (02) 316-322
- Donat SM, Diaz M, Bishoff JT. et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 2013; 190 (02) 407-416
- Antonelli A, Furlan M, Tardanico R. et al. Features of ipsilateral renal recurrences after partial nephrectomy: a proposal of a pathogenetic classification. Clin Genitourin Cancer 2017; 15 (05) 540-547
- Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol 2008; 180 (01) 89-93 , discussion 93
- Marchioni M, Sountoulides P, Furlan M. et al. Management of local recurrence after radical nephrectomy: surgical removal with or without systemic treatment is still the gold standard. Results from a multicenter international cohort. Int Urol Nephrol 2021; 53 (11) 2273-2280
- Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol 2000; 164 (02) 322-325
- Ierardi AM,
Carnevale A, Rossi UG. et al. Percutaneous microwave
ablation therapy of renal cancer local relapse after radical nephrectomy: a feasibility and efficacy study.
Med Oncol 2020; 37 (04) 27
Address for correspondence
Chandan J Das, MD, DNB, FRCP EdinDepartment of Radiodiagnosis and Interventional Radiology AIIMSAurobindo Marg, New Delhi, 110029IndiaPublication History
Article published online:
06 March 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Multiphase computed tomography axial images of the abdomen show an exo-endophytic mass arrow in the left kidney, showing marked enhancement in the corticomedullary phase (A) with relative washout in the nephrographic (B) and delayed phases (C). Diagnosis of clear cell renal cell carcinoma was made, confirmed on postsurgical histopathology.

| Figure 12:Multiphase computed tomography axial images of the abdomen show an exo-endophytic mass in the right kidney, showing hypoenhancement in the corticomedullary phase (A) nephrographic (B), and delayed phases (C). Diagnosis of papillary renal cell carcinoma was made, confirmed on histopathology.

| Figure 3:Imaging algorithm. CT, computed tomography; MRI, magnetic resonance imaging; RCC, renal cell carcinoma; USG, ultrasonography.

| Figure 4:Algorithm for renal cell carcinoma (RCC) management. CKD, chronic kidney disease; IVC, inferior vena cava;
References
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- Kabel AM, Zamzami F, Al-Talhi M, Al-Dwila K, Habib R. Renal cell carcinoma: insights into risk factors, diagnosis and management. J Cancer Research Treatment 2017; 5: 15-19
- Padala SA, Barsouk A, Thandra KC. et al. Epidemiology of renal cell carcinoma. World J Oncol 2020; 11 (03) 79-87
- Heng DYC, Xie W, Regan MM. et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol 2009; 27 (34) 5794-5799
- Ko JJ, Xie W, Kroeger N. et al. The International Metastatic Renal Cell Carcinoma Database Consortium model as a prognostic tool in patients with metastatic renal cell carcinoma previously treated with first-line targeted therapy: a population-based study. Lancet Oncol 2015; 16 (03) 293-300
- Bertolotto M, Bucci S, Valentino M, Currò F, Sachs C, Cova MA. Contrast-enhanced ultrasound for characterizing renal masses. Eur J Radiol 2018; 105: 41-48
- Sidhu PS, Cantisani V, Dietrich CF. et al. The EFSUMB guidelines and recommendations for the clinical practice of contrast-enhanced ultrasound (CEUS) in non-hepatic applications: update 2017 (Long Version). Ultraschall Med 2018; 39 (02) e2-e44
- Clevert D-A, Minaifar N, Weckbach S. et al. Multislice computed tomography versus contrast-enhanced ultrasound in evaluation of complex cystic renal masses using the Bosniak classification system. Clin Hemorheol Microcirc 2008; 39 (1-4): 171-178
- Cantisani V, Bertolotto M, Clevert D-A. et al. EFSUMB 2020 proposal for a contrast-enhanced ultrasound-adapted Bosniak cyst categorization - position statement. Ultraschall Med 2021; 42 (02) 154-166
- Wang ZJ, Nikolaidis P, Khatri G. et al; Expert Panel on Urologic Imaging. ACR Appropriateness Criteria® Indeterminate Renal Mass. J Am Coll Radiol 2020; 17 (11S): S415-S428
- Meloni MF, Bertolotto M, Alberzoni C. et al. Follow-up after percutaneous radiofrequency ablation of renal cell carcinoma: contrast-enhanced sonography versus contrast-enhanced CT or MRI. AJR Am J Roentgenol 2008; 191 (04) 1233-1238
- Vikram R, Beland MD, Blaufox MD. et al. ACR appropriateness criteria renal cell carcinoma staging. J Am Coll Radiol 2016; 13 (05) 518-525
- Li Q, Wang Z, Ma X, Tang J, Luo Y. Diagnostic accuracy of contrast-enhanced ultrasound for detecting bland thrombus from inferior vena cava tumor thrombus in patients with renal cell carcinoma. Int Braz J Urol 2020; 46 (01) 92-100
- Ljungberg B, Albiges L, Abu-Ghanem Y. et al. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2022 update. Eur Urol 2022; 82 (04) 399-410
- Ward RD, Tanaka H, Campbell SC, Remer EM. 2017 AUA renal mass and localized renal cancer guidelines: imaging implications. Radiographics 2018; 38 (07) 2021-2033
- Pedrosa I, Cadeddu JA. How we do it: managing the indeterminate renal mass with the MRI clear cell likelihood score. Radiology 2022; 302 (02) 256-269
- Silverman SG, Pedrosa I, Ellis JH. et al. Bosniak classification of cystic renal masses, Version 2019: an update proposal and needs assessment. Radiology 2019; 292 (02) 475-488
- Birnbaum BA, Hindman N, Lee J, Babb JS. Renal cyst pseudoenhancement: influence of multidetector CT reconstruction algorithm and scanner type in phantom model. Radiology 2007; 244 (03) 767-775
- Lim DJ, Carter MF. Computerized tomography in the preoperative staging for pulmonary metastases in patients with renal cell carcinoma. J Urol 1993; 150 (04) 1112-1114
- Mano R, Vertosick E, Sankin AI. et al. Subcentimeter pulmonary nodules are not associated with disease progression in patients with renal cell carcinoma. J Urol 2015; 193 (03) 776-782
- Kotecha RR, Flippot R, Nortman T. et al. Prognosis of incidental brain metastases in patients with advanced renal cell carcinoma. J Natl Compr Canc Netw 2021; 19 (04) 432-438
- Dilauro M, Quon M, McInnes MDF. et al. Comparison of contrast-enhanced multiphase renal protocol CT versus MRI for diagnosis of papillary renal cell carcinoma. AJR Am J Roentgenol 2016; 206 (02) 319-325
- Schieda N, Lim RS, McInnes MDF. et al. Characterization of small (<4cm>. Diagn Interv Imaging 2018; 99 (7-8): 443-455
- Herts BR, Silverman SG, Hindman NM. et al. Management of the incidental renal mass on CT: a white paper of the ACR incidental findings committee. J Am Coll Radiol 2018; 15 (02) 264-273
- Krishna S, Schieda N, Pedrosa I. et al. Update on MRI of cystic renal masses including Bosniak Version 2019. J Magn Reson Imaging 2021; 54 (02) 341-356
- Cheville JC, Lohse CM, Zincke H, Weaver AL, Blute ML. Comparisons of outcome and prognostic features among histologic subtypes of renal cell carcinoma. Am J Surg Pathol 2003; 27 (05) 612-624
- Roy C, Sauer B, Lindner V, Lang H, Saussine C, Jacqmin D. MR imaging of papillary renal neoplasms: potential application for characterization of small renal masses. Eur Radiol 2007; 17 (01) 193-200
- Cornelis F, Grenier N. Multiparametric magnetic resonance imaging of solid renal tumors: a practical algorithm. Semin Ultrasound CT MR 2017; 38 (01) 47-58
- Tsili AC, Andriotis E, Gkeli MG. et al; Oncologic Imaging Subcommittee Working Group of the Hellenic Radiological Society. The role of imaging in the management of renal masses. Eur J Radiol 2021; 141: 109777 https://www.ejradiology.com/article/S0720-048X(21)00258-8/fulltext [Internet]
- Yang C-W, Shen S-H, Chang Y-H. et al. Are there useful CT features to differentiate renal cell carcinoma from lipid-poor renal angiomyolipoma?. AJR Am J Roentgenol 2013; 201 (05) 1017-1028
- Galia M, Albano D, Bruno A. et al. Imaging features of solid renal masses. Br J Radiol 2017; 90 (1077): 20170077
- Low G, Huang G, Fu W, Moloo Z, Girgis S. Review of renal cell carcinoma and its common subtypes in radiology. World J Radiol 2016; 8 (05) 484-500
- Servaes SE, Hoffer FA, Smith EA, Khanna G. Imaging of Wilms tumor: an update. Pediatr Radiol 2019; 49 (11) 1441-1452
- Wang H-Y, Ding H-J, Chen J-H. et al. Meta-analysis of the diagnostic performance of [18F]FDG-PET and PET/CT in renal cell carcinoma. Cancer Imaging 2012; 12: 464-474
- Liu Y. The place of FDG PET/CT in renal cell carcinoma: value and limitations. Front Oncol 2016; 6: 201
- Petrelli F, Coinu A, Vavassori I. et al. Cytoreductive nephrectomy in metastatic renal cell carcinoma treated with targeted therapies: a systematic review with a meta-analysis. Clin Genitourin Cancer 2016; 14 (06) 465-472
- Pindoria N, Raison N, Blecher G, Catterwell R, Dasgupta P. Cytoreductive nephrectomy in the era of targeted therapies: a review. BJU Int 2017; 120 (03) 320-328
- Amin MB, Greene FL, Edge SB. et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 2017; 67: 93-9
- Ljungberg B, Albiges L, Abu-Ghanem Y. et al. European Association of Urology Guidelines on Renal Cell Carcinoma: the 2019 update. Eur Urol 2019; 75 (05) 799-810
- Lane BR, Kattan MW. Prognostic models and algorithms in renal cell carcinoma. Urol Clin North Am 2008; 35 (04) 613-625 , vii vii
- Sun M, Vetterlein M, Harshman LC, Chang SL, Choueiri TK, Trinh Q-D. Risk assessment in small renal masses: a review article. Urol Clin North Am 2017; 44 (02) 189-202
- Martínez-Salamanca JI, Huang WC, Millán I. et al; International Renal Cell Carcinoma-Venous Thrombus Consortium. Prognostic impact of the 2009 UICC/AJCC TNM staging system for renal cell carcinoma with venous extension. Eur Urol 2011; 59 (01) 120-127
- Margulis V, Sánchez-Ortiz RF, Tamboli P, Cohen DD, Swanson DA, Wood CG. Renal cell carcinoma clinically involving adjacent organs: experience with aggressive surgical management. Cancer 2007; 109 (10) 2025-2030
- Tran J, Ornstein MC. Clinical review on the management of metastatic renal cell carcinoma. JCO Oncol Pract 2022; 18 (03) 187-196
- Campbell SC, Novick AC, Belldegrun A. et al; Practice Guidelines Committee of the American Urological Association. Guideline for management of the clinical T1 renal mass. J Urol 2009; 182 (04) 1271-1279
- Lee Z, Jegede OA, Haas NB. et al. Local recurrence following resection of intermediate-high risk nonmetastatic renal cell carcinoma: an anatomical classification and analysis of the ASSURE (ECOG-ACRIN E2805) adjuvant trial. J Urol 2020; 203 (04) 684-689
- Thomas AZ, Adibi M, Borregales LD. et al. Surgical management of local retroperitoneal recurrence of renal cell carcinoma after radical nephrectomy. J Urol 2015; 194 (02) 316-322
- Donat SM, Diaz M, Bishoff JT. et al. Follow-up for clinically localized renal neoplasms: AUA guideline. J Urol 2013; 190 (02) 407-416
- Antonelli A, Furlan M, Tardanico R. et al. Features of ipsilateral renal recurrences after partial nephrectomy: a proposal of a pathogenetic classification. Clin Genitourin Cancer 2017; 15 (05) 540-547
- Johnson A, Sudarshan S, Liu J, Linehan WM, Pinto PA, Bratslavsky G. Feasibility and outcomes of repeat partial nephrectomy. J Urol 2008; 180 (01) 89-93 , discussion 93
- Marchioni M, Sountoulides P, Furlan M. et al. Management of local recurrence after radical nephrectomy: surgical removal with or without systemic treatment is still the gold standard. Results from a multicenter international cohort. Int Urol Nephrol 2021; 53 (11) 2273-2280
- Itano NB, Blute ML, Spotts B, Zincke H. Outcome of isolated renal cell carcinoma fossa recurrence after nephrectomy. J Urol 2000; 164 (02) 322-325
- Ierardi AM, Carnevale A, Rossi UG. et al. Percutaneous microwave ablation therapy of renal cancer local relapse after radical nephrectomy: a feasibility and efficacy study. Med Oncol 2020; 37 (04) 27


 PDF
PDF  Views
Views  Share
Share

