Imaging Recommendations for Diagnosis, Staging, and Management of Pancreatic Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 077-083
DOI: DOI: 10.1055/s-0042-1759521
Abstract
Pancreatic cancer is the fourth most prevalent cause of cancer-related death worldwide, with a fatality rate equal to its incidence rate. Pancreatic cancer is a rare malignancy with a global incidence and death ranking of 14th and 7th, respectively. Pancreatic cancer cases are divided into three categories without metastatic disease: resectable, borderline resectable, or locally advanced disease. The category is determined by the tumor's location in the pancreas and whether it is abutting or encasing the adjacent arteries and/or vein/s.
The stage of disease and the location of the primary tumor determine the clinical presentation: the pancreatic head, neck, or uncinate process, the body or tail, or multifocal disease. Imaging plays a crucial role in the diagnosis and follow-up of pancreatic cancers. Various imaging modalities available for pancreatic imaging are ultrasonography (USG), contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI), and 18-fluoro-deoxy glucose positron emission tomography (FDG PET).
Even though surgical resection is possible in both resectable and borderline resectable non-metastatic cases, neoadjuvant chemotherapy with or without radiotherapy has become the standard practice for borderline resectable cases as it gives a high yield of R0 resection.
Keywords
ablation - biopsy - IRE - magnetic resonance imaging - multi-detector computed tomography - oncology - pancreatic neoplasms - PET-CTPublication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Pancreatic cancer is the fourth most prevalent cause of cancer-related death worldwide, with a fatality rate equal to its incidence rate. Pancreatic cancer is a rare malignancy with a global incidence and death ranking of 14th and 7th, respectively. Pancreatic cancer cases are divided into three categories without metastatic disease: resectable, borderline resectable, or locally advanced disease. The category is determined by the tumor's location in the pancreas and whether it is abutting or encasing the adjacent arteries and/or vein/s.
The stage of disease and the location of the primary tumor determine the clinical presentation: the pancreatic head, neck, or uncinate process, the body or tail, or multifocal disease. Imaging plays a crucial role in the diagnosis and follow-up of pancreatic cancers. Various imaging modalities available for pancreatic imaging are ultrasonography (USG), contrast-enhanced computed tomography (CECT), magnetic resonance imaging (MRI), and 18-fluoro-deoxy glucose positron emission tomography (FDG PET).
Even though surgical resection is possible in both resectable and borderline resectable non-metastatic cases, neoadjuvant chemotherapy with or without radiotherapy has become the standard practice for borderline resectable cases as it gives a high yield of R0 resection.
Keywords
ablation - biopsy - IRE - magnetic resonance imaging - multi-detector computed tomography - oncology - pancreatic neoplasms - PET-CT
Introduction
Pancreatic cancer is the fourth most prevalent cause of cancer-related deathworldwide,[1] with a fatality rate equal to its incidence rate.[2] [3] While other cancers such as colorectal cancer, breast cancer, and prostate cancer have made significant advances in early detection and treatment, the prognosis for pancreatic cancer remains bleak. As per the latest American Cancer Society Cancer Facts & Figures Report, the 5-year survival rate is 11%-across all stages and a mortality rate that has not decreased over the last few decades.[4] [5] As a result, pancreatic cancer appears to be one of the most challenging cancers to combat.[6] In this article, we review the imaging findings relevant to diagnosis and surgical staging of pancreatic carcinoma. Apart of diagnosis, emphasis has been given to image guided management of pancreatic carcinoma.
Risk Factors and Etiopathogenesis
The risk factor can be inherited and non-inherited. Inherited include hereditary pancreatitis, cystic fibrosis, Peutz–Jeghers syndrome, hereditary nonpolyposis colorectal cancer with MLH1 mutation, familial atypical multiple mole melanoma syndromes, hereditary breast and ovarian cancer 23.1%-in BRCA1 carriers and 6%-in BRCA2 carriers,[7] and familial pancreatic cancer. Noninherited risk factors include smoking, diabetes mellitus, chronic pancreatitis, obesity, physical activity, and cystic lesions.[7] During the development and spread of pancreatic cancer, multiple groups of genes undergo genomic alterations, i.e., either activation or inactivation. Oncogene activation and tumor suppressor gene inactivation are involved in the beginning and progression of pancreatic malignancies. Furthermore, dysregulation of molecules in various cell signaling pathways, such as EGFR, Akt, NF-B, and others, and their molecular interaction, play essential roles in pancreatic cancer molecular pathogenesis.[8]
Epidemiology
Pancreatic cancer is a rare malignancy with a global incidence and death ranking of 14th and 7th, respectively. In India, pancreas ranks 24th with 10,860 new cases (1.03%) and 18th in mortality (9). The incidence is higher in the older population (more than 50%-in those aged 65–75 years).[3] The incidence is the highest among Northeastern Indian regions. In India, pancreatic carcinoma is ranked 21st in males and 17th in females. Mizoram has the highest AAR (age-adjusted incidence rates), followed by Mumbai, Thiruvananthapuram, and Delhi in males and Mumbai, Delhi, Bengaluru, and Thiruvananthapuram in females.[9] In Indian registries, there is an inconsistent pattern due to the absence of reporting of all cases in registries.[9]
Staging
The TNM staging system is used by the American Joint Committee on Cancer to assess immediate and long-term clinical prognosis and to create survival data for patients based on their illness stage. The T stage is determined by the tumor's size and its relationship (abuts/encases the vessels) with the vessels when there is an extra-pancreatic disease. The lack or presence of metastasis to regional lymph nodes or other distant sites determines the regional lymph node (N) and distant metastasis (M) stages.[10] The N categories only comprise regional lymph nodes found along lymphatic drainage channels that would be included in the surgical field and would be removed along with the underlying tumor. M1 stage lymph nodes are those that have spread outside of the usual drainage channels or are not routinely included in surgical resection.[11] The NCCN consensus report guidelines describe a tumor staging system and therapy recommendations based on the amount of the tumor. The NCCN uses the American Hepato-Pancreatico-Biliary Association (AHPBA) consensus report to determine resectability status. Pancreatic cancer cases are divided into three categories without metastatic disease: resectable, borderline resectable, or locally advanced disease.[12] The category is determined by the tumor's location in the pancreas and whether it is abutting or encasing the adjacent arteries and/or vein/s. The recommendations define “abutment” as less than or equal to 180° tumor contact of the vessel circumference and “encasement” as more significant than 180° tumor contact of the vessel circumference.[12] [13] The term borderline resectable had extensive debate in the literature, hence many consensus such as AHPBA, MD Anderson and others had defined it and are listed in [Table 1].[14] [15] [16] A few authors tried subclassifying borderline resectable (BR) further into BR-resectable and BR-locally advanced. NCCN had defined all BR-cases were vascular reconstruction is possible as borderline resectable and the rest as unresectable-locally advanced, provided no distant metastases.[17] [18]
|
Vessel involved |
AHPBA/SSAT/SSO/NCCN |
MD Anderson |
Alliance (TVI) |
|---|---|---|---|
|
Superior mesenteric vein/portal vein |
Abutment/impingement/encasement/short segment occlusion |
Occlusion |
TVI ≥ 180° of vessel wall circumference and or reconstructable occlusion |
|
Superior mesenteric artery |
Abutment |
Abutment |
TVI < 180° of vessel wall circumference |
|
Hepatic artery |
Abutment/short segment encasement |
Abutment/short segment encasement |
Reconstructable short segment interface of any degree between tumor and vessel wall |
|
Celiac artery |
Uninvolved |
Abutment |
TVI < 180° of vessel wall circumference |
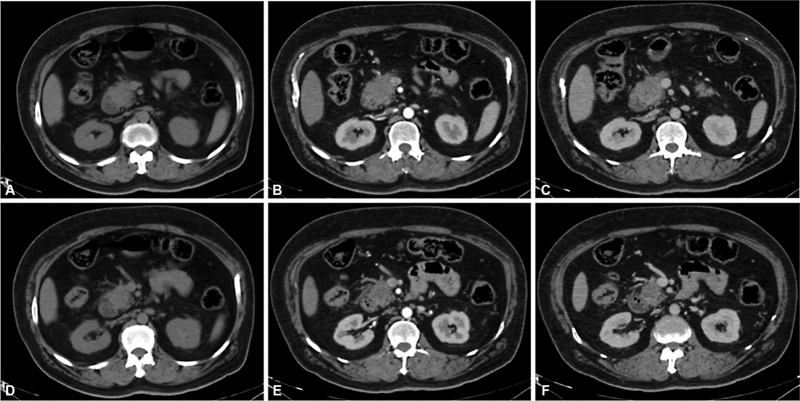
| Figure 1:Axial CT images A, D-plain, B, E-pancreatic parenchymal, and C, F-portovenous phase depicts a hypodense hypoenhancing mass involving the head of the pancreas. Radiologically, this represents a resectable pancreatic carcinoma.
Magnetic resonance imaging: The main indication for MRI is when an isoattenuating lesion is when no obvious lesion is appreciated on CECT in a case of suspected pancreatic cancer. Pancreatic adenocarcinoma is a hypovascular tumor rich in fibrous stroma. It appears hypointense on T1 and T2 and shows diffusion restriction, hypointense in the venous phase, and isointense in the delayed phase due to wash-in of contrast. Magnetic resonance cholangiopancreatography (MRCP) is the modality of choice to evaluate the ductal system. It is superior to CT and ERCP as it effectively demonstrates ducts both proximal and distal to the stricture.[30] The MRI protocol for pancreatic cancer imaging is provided in [Supplementary Table S4], available online only. The major drawback with MRI is cost, time, and availability compared with CT. Compared to computed tomography, MRI is a non-ionizing cross-sectional imaging technique with a safer intravenous contrast profile (CT). This is crucial, especially for patients who need to have repeated imaging follow-up and are at a higher risk of radiation harm (such as younger patients). Less than 1 cm non-contour-deforming focal ductal adenocarcinomas that typically present as non-contour-deforming pancreatic lesions on CT can be well characterized using MRI.[31] With a sensitivity and specificity of 93%-and 75%, respectively, the MR method using fat-suppressed T1-weighted 3D-GRE sequence is able to distinguish ductal adenocarcinoma from chronic pancreatitis.[32]
Fluoro-deoxyglucose positron emission tomography: Integrated FDG PET-CT has incremental value in detecting subtle lesions in CT-negative or equivocal cases. A study by Heinrich et al showed the sensitivity and specificity of PET-CT versus CT alone were 89%-versus 93%-and 69%-versus 21%, respectively.[33] NCCN criteria suggest that PET-CT cannot be substituted for the conventional dual-phase high-resolution CECT. But PET-CT has added advantage in the detection of distant metastases and staging of pancreatic cancer.[34] Neurotensin receptors are overexpressed in pancreatic cancer cells and can be specifically targeted using radiolabeled neurotensin analogs. In a study of six patients with metastatic pancreatic adenocarcinoma using a neurotensin receptor antagonist coupled to 177Lu (177Lu-3BP-227) demonstrated feasibility, improvement of symptoms, and quality of life in all patients.[35]
In the current era, tissue diagnosis, including immunohistochemistry and molecular markers, is essential before any chemotherapy. EUS-FNA is still the gold standard for sampling pancreatic masses because of its high diagnostic accuracy, especially when combined with rapid on-site evaluation (ROSE) and low-risk profile. However, FNA has some inherent flaws, which include a limited volume of tissue with poor cellularity and the difficulty of ensuring a core tissue with intact histological architecture, making immunohistochemistry and molecular profiling difficult.[36] Pancreatic tissue sampling can be performed under USG and CT guidance. The preferred modality for immunohistochemistry is the biopsy, as it requires more tissue samples than conventional FNAC. USG-guided sampling is preferred in large masses, mass involving the head of the pancreas when there is no intervening bowel shadow. CT-guided sampling is the preferred modality in many cases as the pancreas is a retroperitoneal structure and in smaller lesions or lesions involving the body and tail of the pancreas where an adequate acoustic window is not possible. In cases where good access is not available, either a transgastric approach or hydrodissection with saline can be performed to create a window. Presently, all biopsies are performed with an 18-gauge semiautomatic biopsy needle, and for FNAC 22-gauge needle is used. Post biopsy dual-phase contrast CT has to be performed routinely for all patients to rule out any possible complications.[37] [38] The approach for CT-guided pancreatic biopsy is depicted in [Supplementary Fig. S1], available online only. FDG PET-CT has an advantage in guiding the biopsy to the most avid part of the tumor, thereby increasing the diagnostic yield.
Staging
Dual-phase CECT is the modality of choice for the staging of pancreatic cancers and is done according to mostly followed TNM classification by AJCC or the resectability criteria proposed by the NCCN guidelines. Staging involves defining the tumor's location, extent, vascular involvement, nodal spread, and distant metastatic evaluation. The arterial and venous encasement is shown in [Supplementary Fig. S2], available online only. Distant metastasis most commonly involves the liver, lungs, and peritoneum.[39] Hence, CT chest is usually acquired as a part of the venous phase of the dual-phase CECT. It is crucial to look for nodal spread and the number and location of the nodes, peritoneal disease, as these factors can affect the surgical resection. Alternative to CECT, MRI and FDG PET-CT can also be used for staging pancreatic cancer. FDG PET-CT effectively detects subtle nodal, peritoneal, and lung metastases, while MRI is better for local disease extent and liver, peritoneal and nodal metastatic evaluation.[40]
Management
Even though surgical resection is possible in both resectable and borderline resectable non-metastatic cases, neoadjuvant chemotherapy with or without radiotherapy has become the standard practice for borderline resectable cases as it gives a high yield of R0 resection. Dual-phase CECT is the imaging modality of choice for response assessment in both neoadjuvant settings and in the immediate postoperative period. Post chemotherapy response assessment scan shown in [Supplementary Fig. S3], available online only shows a decrease in the tumor size. In immediate post-surgery settings, dual-phase CECT is necessary to rule out complications such as pancreatitis, gastroduodenal artery (GDA) stump pseudoaneurysm or bleeding, abdominal collections, and anastomotic leaks.[41] [42]
Imaging in the neoadjuvant and adjuvant setting is challenging as the radiological response lags behind the histological response due to persistent soft tissue around the vessels as the tumor is mainly composed of fibrous stroma even if there is no viable tumor on histology. A recent study by Lee et al concluded that a reduction in metabolic tumor parameters of FDG-PET/CT after neoadjuvant chemotherapy indicates an improved overall survival and recurrence-free survival.[43] Other challenges are local edema and inflammatory reaction induced by radiation therapy. These factors necessitate careful reading of images to avoid overcalling the resectability status. The role of imaging in a palliative setting is to assess the response to therapy and detect the presence of new lesions or metastases.[44] [45]
Follow-up
NCCN recommends CECT as the modality of choice for post-treatment surveillance with a 3 to 6 monthly CECT for up to 2 years and yearly later. The average 5-year survival post curative therapy in pancreatic cancer is 20%.[45] Studies have demonstrated that routine imaging follow-up has survival benefits compared to performing imaging in symptomatic patients.
Principles of Management
The current management strategies are based on the resectability criteria. The non-metastatic pancreatic carcinomas are subdivided into resectable, borderline resectable, and non-resectable. The management of choice for resectable cancers is upfront surgical resection. However, only 20%-of the newly diagnosed cases fulfill the resectability criteria.[46] [47] For tumors involving the head, uncinate process, and neck of the pancreas, Whipple's pancreatoduodenectomy and pylorus-preserving pancreatoduodenectomy are performed. In contrast, distal pancreatectomy is commonly performed for pancreatic body and tail tumors.[48] [49]
For borderline resectable cases, the standard practice is to downstage the tumor with neoadjuvant chemotherapy with or without radiation, increasing the likelihood of future R0 resection. The widely used first-line chemotherapy regimen is FOLFIRINOX. The average 5-year survival percentage for pancreatic carcinoma for stages I–IV is 14%, 7%, 3%, and 1%.[50] [51] Moreover, most patients will develop disease recurrence after curative-intent surgery, resulting in a 5-year survival rate of only 12 to 27%-and median overall survival (OS) of 16.8 months. Newer advances in radiotherapy such as stereotactic body radiotherapy (SBRT) and intensity-modulated radiotherapy (IMRT) are widely used in borderline resectable cases to improve R0 resection rates.[43] Recently, irreversible electroporation (IRE), a nonthermal ablation technique, has been used in borderline resectable cases to improve survival.[52] [53] Treatment options include chemotherapy, radiotherapy, and palliative bypass surgeries in unresectable and metastatic cases. Chemotherapy with or without radiation therapy is recommended for patients with unresectable disease, followed by attempted resection if the tumor is downstaged. FOLFIRINOX and gemcitabine-based regimes are the first lines of chemotherapeutic agents, with gemcitabine having lower efficacy but with a more tolerable side effects profile compared to FOLFIRINOX. Patients who have a response or stable disease after 4 months of chemotherapy may undergo maintenance therapy. For supportive care, ERCP or PTBD can be done for biliary obstruction or celiac plexus neurolysis for pain palliation is helpful.[54] [55]
Follow-Up Imaging and Management of Recurrent Disease
As per the NCCN guidelines, clinical evaluation and history for symptoms every 3 to 6 months for 2 years, then every 6 to 12 months as clinically indicated. CA 19-9 and follow-up contrast CT every 3 to 6 months is also recommended. Careful evaluation for postoperative bed soft tissue, peritoneal disease, and lung and liver metastases is essential.[56]
In a considerable percentage of patients, a multimodality approach to pancreatic cancer recurrence appears to provide effective palliation. In a small number of patients, radical excision of tumor recurrence may be possible. When compared to patients who receive chemoradiotherapy or supportive treatment, this subgroup of patients has a better chance of surviving longer. Furthermore, combining traditional therapies (e.g., chemoradiotherapy, surgery) with novel therapeutic modalities (e.g., RFA, IRE, stereotactic radiotherapy) may provide a new perspective on an otherwise fatal disease. To optimize the management of recurring tumors, accurate follow-up is required.[57] [58]
Summary
Imaging is crucial for pancreatic cancer surveillance, diagnosis, resectability assessment, and response assessment. To prevent unnecessary surgery, it is crucial for the radiologist to be aware of PDAC mimics. Structured reporting for complete and accurate assessment of the primary tumor, its relationship to/involvement of neighboring structures is an effective method for reporting pancreatic cancer and that it enhances assessment and surgeons' confidence. Future pancreatic cancer care will likely see a significant increase in the utilization of novel imaging tools and therapies, such as dual-energy CT, functional MR imaging techniques, and image guided techniques such as PTBD/SEMS, celiac plexus neurolysis, and IRE.
Conflicting Interest
None declared.
Supplementary Material
References
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008; 10 (01) 58-62
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362 (17) 1605-1617
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68 (01) 7-30
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363 (9414): 1049-1057
- American Cancer Society. Accessed November 10, 2022, at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures
- Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014; 20 (24) 7864-7877
- Decker GA, Batheja MJ, Collins JM. et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y) 2010; 6 (04) 246-254
- Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: pathogenesis, prevention and treatment. Toxicol Appl Pharmacol 2007; 224 (03) 326-336
- Gaidhani RH, Balasubramaniam G. An epidemiological review of pancreatic cancer with special reference to India. Indian J Med Sci 2021; (May): 1-1
- Tempero MA, Malafa MP, Chiorean EG. et al. NCCN guidelines insights: pancreatic adenocarcinoma, version 1.2019: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2019; 17 (03) 202-210
- Daly MB, Pilarski R, Yurgelun MB. et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw 2020; 18 (04) 380-391
- Dimitrokallis N, Karachaliou GS, Moris D. New NCCN guidelines for locally advanced pancreatic cancer: new horizons in extending resectability. J BUON 2020; 25 (04) 2125-2126
- van Roessel S, Kasumova GG, Verheij J. et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg 2018; 153 (12) e183617
- Soweid AM. The borderline resectable and locally advanced pancreatic ductal adenocarcinoma: definition. Endosc Ultrasound 2017; 6 (Suppl. 03) S76-S78
- He J, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: where do we stand?. World J Gastroenterol 2014; 20 (09) 2255-2266
- Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol 2014; 20 (31) 10740-10751
- Kurdia KC, Kapoor VK. Pancreatic cancer: “whether to cross the border”?. Indian J Surg Oncol 2021; 12 (02) 235-237
- Hong SB, Lee SS, Kim JH. et al. Pancreatic cancer CT: prediction of resectability according to NCCN criteria. Radiology 2018; 289 (03) 710-718
- Cabasag CJ, Arnold M, Piñeros M. et al. Population-based cancer staging for oesophageal, gastric, and pancreatic cancer 2012-2014: International Cancer Benchmarking Partnership SurvMark-2. Int J Cancer 2021; 149 (06) 1239-1246
- Yabar CS, Winter JM. Pancreatic cancer: a review. Gastroenterol Clin North Am 2016; 45 (03) 429-445
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020; 395 (10242): 2008-2020
- McAllister F, Montiel MF, Uberoi GS, Uberoi AS, Maitra A, Bhutani MS. Current status and future directions for screening patients at high risk for pancreatic cancer. Gastroenterol Hepatol (N Y) 2017; 13 (05) 268-275
- Henrikson NB, Aiello Bowles EJ, Blasi PR. et al. Screening for pancreatic cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019; 322 (05) 445-454
- Burk KS, Lo GC, Gee MS, Sahani DV. Imaging and screening of pancreatic cancer. Radiol Clin North Am 2017; 55 (06) 1223-1234
- Canto MI, Hruban RH, Fishman EK. et al; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142 (04) 796-804 , quiz e14–e15
- Rickes S, Unkrodt K, Neye H, Ocran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol 2002; 37 (11) 1313-1320
- Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol 2011; 2 (03) 168-174
- Al-Hawary MM, Francis IR, Chari ST. et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014; 270 (01) 248-260
- Almeida RR, Lo GC, Patino M, Bizzo B, Canellas R, Sahani DV. Advances in pancreatic CT imaging. AJR Am J Roentgenol 2018; 211 (01) 52-66
- Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol 2015; 6 (04) 343-357
- Kim JK, Altun E, Elias Jr J, Pamuklar E, Rivero H, Semelka RC. Focal pancreatic mass: distinction of pancreatic cancer from chronic pancreatitis using gadolinium-enhanced 3D-gradient-echo MRI. J Magn Reson Imaging 2007; 26 (02) 313-322
- Ansari NA, Ramalho M, Semelka RC, Buonocore V, Gigli S, Maccioni F. Role of magnetic resonance imaging in the detection and characterization of solid pancreatic nodules: an update. World J Radiol 2015; 7 (11) 361-374
- Heinrich S, Goerres GW, Schäfer M. et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005; 242 (02) 235-243
- Tempero MA, Arnoletti JP, Behrman S. et al; NCCN Pancreatic Adenocarcinoma. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010; 8 (09) 972-1017
- Yin X, Wang M, Wang H. et al. Evaluation of neurotensin receptor 1 as a potential imaging target in pancreatic ductal adenocarcinoma. Amino Acids 2017; 49 (08) 1325-1335
- Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come?. World J Gastrointest Endosc 2019; 11 (08) 454-471
- Paulsen SD, Nghiem HV, Negussie E, Higgins EJ, Caoili EM, Francis IR. Evaluation of imaging-guided core biopsy of pancreatic masses. AJR Am J Roentgenol 2006; 187 (03) 769-772
- Stella SF, Van Borsel M, Markose G, Nair SB. Image-guided percutaneous biopsy for pancreatic lesions: 10-year experience in a tertiary cancer center. Can Assoc Radiol J 2019; 70 (02) 199-203
- Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology 2018; 287 (02) 374-390
- Elbanna KY, Jang HJ, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging 2020; 11 (01) 58
- Lee W, Oh M, Kim JS. et al. Metabolic activity by FDG-PET/CT after neoadjuvant chemotherapy in borderline resectable and locally advanced pancreatic cancer and association with survival. Br J Surg 2021; 109 (01) 61-70
- Shanbhogue KP, Pourvaziri A, Jeyaraj SK, Kambadakone A. Endoscopic and surgical treatment options for chronic pancreatitis: an imaging perspective. Abdom Radiol (NY) 2020; 45 (05) 1397-1409
- Xia BT, Fu B, Wang J. et al. Does radiologic response correlate to pathologic response in patients undergoing neoadjuvant therapy for borderline resectable pancreatic malignancy?. J Surg Oncol 2017; 115 (04) 376-383
- Yang HK, Park MS, Choi M. et al. Systematic review and meta-analysis of diagnostic performance of CT imaging for assessing resectability of pancreatic ductal adenocarcinoma after neoadjuvant therapy: importance of CT criteria. Abdom Radiol (NY) 2021; 46 (11) 5201-5217
- Elmi A, Murphy J, Hedgire S. et al. Post-Whipple imaging in patients with pancreatic ductal adenocarcinoma: association with overall survival: a multivariate analysis. Abdom Radiol (NY) 2017; 42 (08) 2101-2107
- Zhang Q, Zeng L, Chen Y. et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract 2016; 2016: 8962321
- Fang Y, Yao Q, Chen Z. et al. Genetic and molecular alterations in pancreatic cancer: implications for personalized medicine. Med Sci Monit 2013; 19: 916-926
- Brunner TB, Scott-Brown M. The role of radiotherapy in multimodal treatment of pancreatic carcinoma. Radiat Oncol 2010; 5 (01) 64
- Son SH, Song JH, Choi BO. et al. The technical feasibility of an image-guided intensity-modulated radiotherapy (IG-IMRT) to perform a hypofractionated schedule in terms of toxicity and local control for patients with locally advanced or recurrent pancreatic cancer. Radiat Oncol 2012; 7 (01) 203
- Martin II RC. Use of irreversible electroporation in unresectable pancreatic cancer. Hepatobiliary Surg Nutr 2015; 4 (03) 211-215
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10 (01) 10-27
- Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw 2019; 17 (5.5): 603-605
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371 (11) 1039-1049
- Truty MJ, Thomas RM, Katz MH. et al. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg 2012; 215 (01) 41-51 , discussion 51–52
- Skau Rasmussen L, Vittrup B, Ladekarl M. et al. The effect of postoperative gemcitabine on overall survival in patients with resected pancreatic cancer: a nationwide population-based Danish register study. Acta Oncol 2019; 58 (06) 864-871
- Tempero MA, Malafa MP, Al-Hawary M. et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15 (08) 1028-1061
- Sperti C, Moletta L, Merigliano S. Multimodality treatment of recurrent pancreatic cancer: Mith or reality?. World J Gastrointest Oncol 2015; 7 (12) 375-382
- Paiella S,
Butturini G, Frigerio I. et al. Safety and feasibility
of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a
prospective study. Dig Surg 2015; 32 (02) 90-97
Address for correspondence
Suyash S. Kulkarni, DMRD, DNBRoom No. 71, Tata Memorial Hospital, Parel, Mumbai, MaharashtraIndiaEmail: suyashkulkarnitmh@gmail.comPublication History
Article published online:
06 March 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Axial CT images A, D-plain, B, E-pancreatic parenchymal, and C, F-portovenous phase depicts a hypodense hypoenhancing mass involving the head of the pancreas. Radiologically, this represents a resectable pancreatic carcinoma.
References
- Hariharan D, Saied A, Kocher HM. Analysis of mortality rates for pancreatic cancer across the world. HPB (Oxford) 2008; 10 (01) 58-62
- Hidalgo M. Pancreatic cancer. N Engl J Med 2010; 362 (17) 1605-1617
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018; 68 (01) 7-30
- Li D, Xie K, Wolff R, Abbruzzese JL. Pancreatic cancer. Lancet 2004; 363 (9414): 1049-1057
- American Cancer Society. Accessed November 10, 2022, at: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/annual-cancer-facts-and-figures/2022/2022-cancer-facts-and-figures
- Lee ES, Lee JM. Imaging diagnosis of pancreatic cancer: a state-of-the-art review. World J Gastroenterol 2014; 20 (24) 7864-7877
- Decker GA, Batheja MJ, Collins JM. et al. Risk factors for pancreatic adenocarcinoma and prospects for screening. Gastroenterol Hepatol (N Y) 2010; 6 (04) 246-254
- Sarkar FH, Banerjee S, Li Y. Pancreatic cancer: pathogenesis, prevention and treatment. Toxicol Appl Pharmacol 2007; 224 (03) 326-336
- Gaidhani RH, Balasubramaniam G. An epidemiological review of pancreatic cancer with special reference to India. Indian J Med Sci 2021; (May): 1-1
- Tempero MA, Malafa MP, Chiorean EG. et al. NCCN guidelines insights: pancreatic adenocarcinoma, version 1.2019: featured updates to the NCCN guidelines. J Natl Compr Canc Netw 2019; 17 (03) 202-210
- Daly MB, Pilarski R, Yurgelun MB. et al. NCCN guidelines insights: genetic/familial high-risk assessment: breast, ovarian, and pancreatic, version 1.2020. J Natl Compr Canc Netw 2020; 18 (04) 380-391
- Dimitrokallis N, Karachaliou GS, Moris D. New NCCN guidelines for locally advanced pancreatic cancer: new horizons in extending resectability. J BUON 2020; 25 (04) 2125-2126
- van Roessel S, Kasumova GG, Verheij J. et al. International validation of the eighth edition of the American Joint Committee on Cancer (AJCC) TNM staging system in patients with resected pancreatic cancer. JAMA Surg 2018; 153 (12) e183617
- Soweid AM. The borderline resectable and locally advanced pancreatic ductal adenocarcinoma: definition. Endosc Ultrasound 2017; 6 (Suppl. 03) S76-S78
- He J, Page AJ, Weiss M, Wolfgang CL, Herman JM, Pawlik TM. Management of borderline and locally advanced pancreatic cancer: where do we stand?. World J Gastroenterol 2014; 20 (09) 2255-2266
- Lopez NE, Prendergast C, Lowy AM. Borderline resectable pancreatic cancer: definitions and management. World J Gastroenterol 2014; 20 (31) 10740-10751
- Kurdia KC, Kapoor VK. Pancreatic cancer: “whether to cross the border”?. Indian J Surg Oncol 2021; 12 (02) 235-237
- Hong SB, Lee SS, Kim JH. et al. Pancreatic cancer CT: prediction of resectability according to NCCN criteria. Radiology 2018; 289 (03) 710-718
- Cabasag CJ, Arnold M, Piñeros M. et al. Population-based cancer staging for oesophageal, gastric, and pancreatic cancer 2012-2014: International Cancer Benchmarking Partnership SurvMark-2. Int J Cancer 2021; 149 (06) 1239-1246
- Yabar CS, Winter JM. Pancreatic cancer: a review. Gastroenterol Clin North Am 2016; 45 (03) 429-445
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet 2020; 395 (10242): 2008-2020
- McAllister F, Montiel MF, Uberoi GS, Uberoi AS, Maitra A, Bhutani MS. Current status and future directions for screening patients at high risk for pancreatic cancer. Gastroenterol Hepatol (N Y) 2017; 13 (05) 268-275
- Henrikson NB, Aiello Bowles EJ, Blasi PR. et al. Screening for pancreatic cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA 2019; 322 (05) 445-454
- Burk KS, Lo GC, Gee MS, Sahani DV. Imaging and screening of pancreatic cancer. Radiol Clin North Am 2017; 55 (06) 1223-1234
- Canto MI, Hruban RH, Fishman EK. et al; American Cancer of the Pancreas Screening (CAPS) Consortium. Frequent detection of pancreatic lesions in asymptomatic high-risk individuals. Gastroenterology 2012; 142 (04) 796-804 , quiz e14–e15
- Rickes S, Unkrodt K, Neye H, Ocran KW, Wermke W. Differentiation of pancreatic tumours by conventional ultrasound, unenhanced and echo-enhanced power Doppler sonography. Scand J Gastroenterol 2002; 37 (11) 1313-1320
- Tummala P, Junaidi O, Agarwal B. Imaging of pancreatic cancer: an overview. J Gastrointest Oncol 2011; 2 (03) 168-174
- Al-Hawary MM, Francis IR, Chari ST. et al. Pancreatic ductal adenocarcinoma radiology reporting template: consensus statement of the Society of Abdominal Radiology and the American Pancreatic Association. Radiology 2014; 270 (01) 248-260
- Almeida RR, Lo GC, Patino M, Bizzo B, Canellas R, Sahani DV. Advances in pancreatic CT imaging. AJR Am J Roentgenol 2018; 211 (01) 52-66
- Pietryga JA, Morgan DE. Imaging preoperatively for pancreatic adenocarcinoma. J Gastrointest Oncol 2015; 6 (04) 343-357
- Kim JK, Altun E, Elias Jr J, Pamuklar E, Rivero H, Semelka RC. Focal pancreatic mass: distinction of pancreatic cancer from chronic pancreatitis using gadolinium-enhanced 3D-gradient-echo MRI. J Magn Reson Imaging 2007; 26 (02) 313-322
- Ansari NA, Ramalho M, Semelka RC, Buonocore V, Gigli S, Maccioni F. Role of magnetic resonance imaging in the detection and characterization of solid pancreatic nodules: an update. World J Radiol 2015; 7 (11) 361-374
- Heinrich S, Goerres GW, Schäfer M. et al. Positron emission tomography/computed tomography influences on the management of resectable pancreatic cancer and its cost-effectiveness. Ann Surg 2005; 242 (02) 235-243
- Tempero MA, Arnoletti JP, Behrman S. et al; NCCN Pancreatic Adenocarcinoma. Pancreatic adenocarcinoma. J Natl Compr Canc Netw 2010; 8 (09) 972-1017
- Yin X, Wang M, Wang H. et al. Evaluation of neurotensin receptor 1 as a potential imaging target in pancreatic ductal adenocarcinoma. Amino Acids 2017; 49 (08) 1325-1335
- Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come?. World J Gastrointest Endosc 2019; 11 (08) 454-471
- Paulsen SD, Nghiem HV, Negussie E, Higgins EJ, Caoili EM, Francis IR. Evaluation of imaging-guided core biopsy of pancreatic masses. AJR Am J Roentgenol 2006; 187 (03) 769-772
- Stella SF, Van Borsel M, Markose G, Nair SB. Image-guided percutaneous biopsy for pancreatic lesions: 10-year experience in a tertiary cancer center. Can Assoc Radiol J 2019; 70 (02) 199-203
- Zins M, Matos C, Cassinotto C. Pancreatic adenocarcinoma staging in the era of preoperative chemotherapy and radiation therapy. Radiology 2018; 287 (02) 374-390
- Elbanna KY, Jang HJ, Kim TK. Imaging diagnosis and staging of pancreatic ductal adenocarcinoma: a comprehensive review. Insights Imaging 2020; 11 (01) 58
- Lee W, Oh M, Kim JS. et al. Metabolic activity by FDG-PET/CT after neoadjuvant chemotherapy in borderline resectable and locally advanced pancreatic cancer and association with survival. Br J Surg 2021; 109 (01) 61-70
- Shanbhogue KP, Pourvaziri A, Jeyaraj SK, Kambadakone A. Endoscopic and surgical treatment options for chronic pancreatitis: an imaging perspective. Abdom Radiol (NY) 2020; 45 (05) 1397-1409
- Xia BT, Fu B, Wang J. et al. Does radiologic response correlate to pathologic response in patients undergoing neoadjuvant therapy for borderline resectable pancreatic malignancy?. J Surg Oncol 2017; 115 (04) 376-383
- Yang HK, Park MS, Choi M. et al. Systematic review and meta-analysis of diagnostic performance of CT imaging for assessing resectability of pancreatic ductal adenocarcinoma after neoadjuvant therapy: importance of CT criteria. Abdom Radiol (NY) 2021; 46 (11) 5201-5217
- Elmi A, Murphy J, Hedgire S. et al. Post-Whipple imaging in patients with pancreatic ductal adenocarcinoma: association with overall survival: a multivariate analysis. Abdom Radiol (NY) 2017; 42 (08) 2101-2107
- Zhang Q, Zeng L, Chen Y. et al. Pancreatic cancer epidemiology, detection, and management. Gastroenterol Res Pract 2016; 2016: 8962321
- Fang Y, Yao Q, Chen Z. et al. Genetic and molecular alterations in pancreatic cancer: implications for personalized medicine. Med Sci Monit 2013; 19: 916-926
- Brunner TB, Scott-Brown M. The role of radiotherapy in multimodal treatment of pancreatic carcinoma. Radiat Oncol 2010; 5 (01) 64
- Son SH, Song JH, Choi BO. et al. The technical feasibility of an image-guided intensity-modulated radiotherapy (IG-IMRT) to perform a hypofractionated schedule in terms of toxicity and local control for patients with locally advanced or recurrent pancreatic cancer. Radiat Oncol 2012; 7 (01) 203
- Martin II RC. Use of irreversible electroporation in unresectable pancreatic cancer. Hepatobiliary Surg Nutr 2015; 4 (03) 211-215
- Rawla P, Sunkara T, Gaduputi V. Epidemiology of pancreatic cancer: global trends, etiology and risk factors. World J Oncol 2019; 10 (01) 10-27
- Tempero MA. NCCN guidelines updates: pancreatic cancer. J Natl Compr Canc Netw 2019; 17 (5.5): 603-605
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014; 371 (11) 1039-1049
- Truty MJ, Thomas RM, Katz MH. et al. Multimodality therapy offers a chance for cure in patients with pancreatic adenocarcinoma deemed unresectable at first operative exploration. J Am Coll Surg 2012; 215 (01) 41-51 , discussion 51–52
- Skau Rasmussen L, Vittrup B, Ladekarl M. et al. The effect of postoperative gemcitabine on overall survival in patients with resected pancreatic cancer: a nationwide population-based Danish register study. Acta Oncol 2019; 58 (06) 864-871
- Tempero MA, Malafa MP, Al-Hawary M. et al. Pancreatic adenocarcinoma, version 2.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw 2017; 15 (08) 1028-1061
- Sperti C, Moletta L, Merigliano S. Multimodality treatment of recurrent pancreatic cancer: Mith or reality?. World J Gastrointest Oncol 2015; 7 (12) 375-382
- Paiella S, Butturini G, Frigerio I. et al. Safety and feasibility of Irreversible Electroporation (IRE) in patients with locally advanced pancreatic cancer: results of a prospective study. Dig Surg 2015; 32 (02) 90-97


 PDF
PDF  Views
Views  Share
Share

