Imaging Recommendations for Diagnosis, Staging, and Management of Nasopharynx Carcinoma
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 175-180
DOI: DOI: 10.1055/s-0042-1760309
Abstract
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from lining of the nasopharyngeal mucosa usually at the fossa of Rosenmuller (pharyngeal recess). An early detection on endoscopy can be rewarding, however, often difficult as the tumor at the pharyngeal recess is hidden from the endoscopic view. Magnetic resonance imaging and positron emission tomography–computed tomography form the backbone of detection and spread of the carcinoma into local and distant regions. These modalities help further characterize the precise locoregional infiltration and lymph nodal involvement which aids in the planning of the surgery/chemoradiotherapy. They also help in the follow-up evaluation and further management strategies. Many research and treatment groups namely American Joint Committee on Cancer, National Comprehensive Cancer Network, American Society of Clinical Oncology, American College of Radiology, Radiological Society of North America, European Society of Radiology (iGuide), Indian Radiological & Imaging Association/Indian College of Radiology and Imaging, National Cancer Grid, etc. have devised guidelines for the optimal assessment and treatment of NPC. The present document aims at providing a comprehensive review of the clinicoradiological recommendations for the diagnosis and management of NPC based on these guidelines as well as personalized experience of the contributors.
Keywords
nasopharyngeal carcinoma - diagnosis - staging - magnetic resonance imaging - computed tomographyPublication History
Article published online:
01 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from lining of the nasopharyngeal mucosa usually at the fossa of Rosenmuller (pharyngeal recess). An early detection on endoscopy can be rewarding, however, often difficult as the tumor at the pharyngeal recess is hidden from the endoscopic view. Magnetic resonance imaging and positron emission tomography–computed tomography form the backbone of detection and spread of the carcinoma into local and distant regions. These modalities help further characterize the precise locoregional infiltration and lymph nodal involvement which aids in the planning of the surgery/chemoradiotherapy. They also help in the follow-up evaluation and further management strategies. Many research and treatment groups namely American Joint Committee on Cancer, National Comprehensive Cancer Network, American Society of Clinical Oncology, American College of Radiology, Radiological Society of North America, European Society of Radiology (iGuide), Indian Radiological & Imaging Association/Indian College of Radiology and Imaging, National Cancer Grid, etc. have devised guidelines for the optimal assessment and treatment of NPC. The present document aims at providing a comprehensive review of the clinicoradiological recommendations for the diagnosis and management of NPC based on these guidelines as well as personalized experience of the contributors.
Keywords
nasopharyngeal carcinoma - diagnosis - staging - magnetic resonance imaging - computed tomographyIntroduction and Etiopathogenesis
Nasopharyngeal carcinoma (NPC) is an epithelial carcinoma originating from the mucosal lining of the nasopharynx. The tumor occurs more commonly in males than females (two- to threefold) and occurs with a bimodal incidence in second and sixth decades of life.[1] Epidemiological trends have shown a gradual decline in incidence of the disease with reduction in mortality reflecting lifestyle and environmental changes. NPC usually originates at the fossa of Rosenmuller (pharyngeal recess) which is located posterosuperior to the Eustachian tube opening, from where it spreads to adjacent areas.[2] NPC is multifactorial disease with strong genetic basis among first-degree relatives.[3] Other major risk factors include Epstein–Barr virus (EBV) infection,[4] smoking, and exposure to salt-cured preserved foods.[5] Usage of certain Chinese medical herbs, smoking, and nitrosamines present in preserved foods act by causing a reactivation of EBV in nasal mucosa causing NPC. Genetic polymorphisms in CYP2A6 and certain HLA haplotypes have also been studied in detail as causations in NPC.[6] NPC has three distinct histopathological subtypes—keratinizing squamous cell (World Health Organization [WHO] type I, sporadic form contributing to less than 20%-cases globally), nonkeratinizing (WHO types II and III, contributing to more than 95%-cases in endemic areas), and least commonly, the basaloid squamous cell type. The nonkeratinizing type is strongly associated with EBV infection and has more favorable prognosis than the other histological subtypes.[7] [8]
Epidemiology
NPC accounts for 0.7%-of all newly diagnosed cancer cases worldwide with more than 130,000 new reported cases and 80,000 deaths in global cancer registry. More than 70%-new cases are reported from Southeast China, with remainder being accounted predominantly in Southeast Asia and Northeast Africa regions. India accounts for 4%-of total new NPC cases with a high-ethnic incidence in Nagas community with more than 16 per 100,000 person-years incidence.[5]
Clinical Profile and Diagnostic Workup
Patient presentation is varied and the tumor may go undetected from unawareness about the symptoms of the disease and relatively “clinically occult” site of origin of the carcinoma at nasopharynx. In the early stages of cancer, patients are asymptomatic or have mild symptoms such as nasal stuffiness. Local symptoms such as epistaxis, headache, hearing defects, otalgia, or neuropathies may occur from involvement of adjacent cranial nerves. Overall, the patients usually directly present when there is cervical lymph node enlargement (in more than 70–90%-cases at initial presentation) or from symptoms at sites where distant metastasis (bones, liver, lungs, and distant nodes) has already occurred.[1] A clinical triad of nasal obstruction with epistaxis, neck mass, and serous otitis media occurs infrequently. With high clinical suspicion and genetic/environmental history, an early detection on endoscopy can be rewarding, however, often difficult as the tumor at the pharyngeal recess is hidden from the endoscopic view. This could also be hindered by the presence of a coexisting benign hyperplasia. Endoscopic biopsy plays a significant role in definitive diagnosis of the disease. Patient's initial diagnostic workup should also include a pretreatment EBV DNA levels which have been shown to influence survival outcomes, besides investigations such as blood counts, liver function tests, cross-sectional imaging, and chest radiographs.[8] [9] The role of EBV DNA levels is also evidenced by its incorporation in the American Joint Committee on Cancer TNM staging ([Table 1]). It is important to note that incisional neck biopsies or nodal dissections should be avoided as these procedures negatively impact subsequent treatment.
|
Primary tumor (T) TX—Primary tumor cannot be assessed; T0—EBV-positive cervical node(s), no tumor identified; Tis—tumor in situ; T1—nasopharynx, nasal cavity, oropharynx without parapharyngeal extension; T2—parapharyngeal extension, adjacent soft tissue involvement (prevertebral muscles, medial pterygoid, lateral pterygoid); T3–Bony structures (skull base, pterygoid, cervical vertebra) with or without paranasal sinuses involvement; T4—cranial nerves, intracranial extension, orbit, hypopharynx, extensive, soft tissue involvement beyond lateral surface of lateral pterygoid |
|
Regional lymph nodes (N) NX—regional nodes cannot be assessed; N0—no regional nodal metastasis; N1—unilateral (U/L) cervical, U/L or bilateral (B/L) retropharyngeal lymph nodes ≤ 6 cm; N2—B/L metastasis in lymph nodes, ≤ 6 cm in greatest dimension, above the caudal border of the cricoid cartilage; N3— > 6 cm and/or extension below the caudal border of the cricoid cartilage |
|
Distant metastasis (M) M0—no distant metastasis, M1—distant metastasis |
|
Stage group classification I—T1 N0 M0 II—T2 N0–1 M0, T0 1 N1 M0 III—T3 N0–2 M0, T0 2 N2 M0 IVA—T4 or N3 M0 IVB—Any T, any N, M1 |
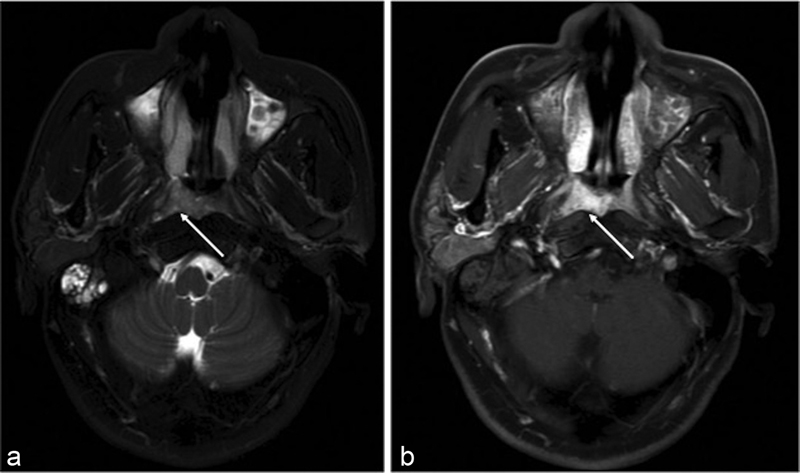
| Figure 1:(a) Axial T2W MR image showing hyperintense poorly defined mass involving the right side of nasopharynx in the fossa of Rosenmuller (white arrow) with associated fluid in the mastoid air cells. (b) Axial postgadolinium T1W MR image showing enhancement of the lesion (white arrow). MR, magnetic resonance; T2W, T2-weighted.
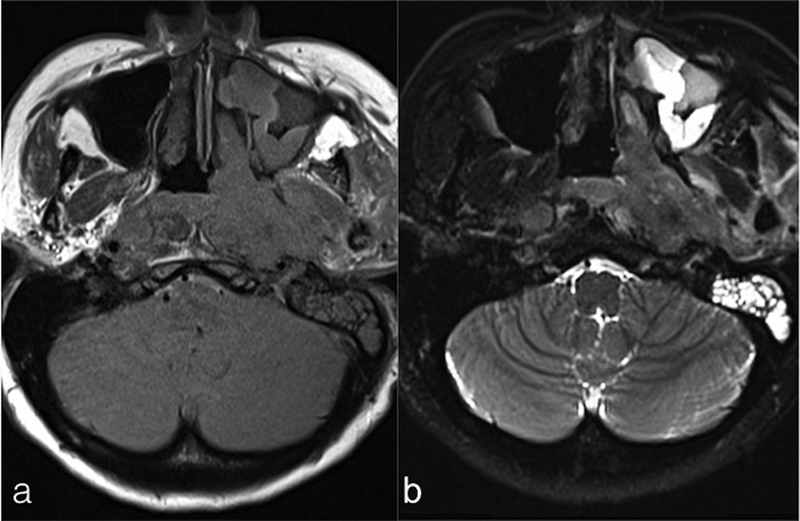
| Figure 2:Axial T1WI (a) and T2WI and (b) fat sat images showing a large mass involving the left side of nasopharynx with extension into left parapharyngeal space and involvement of pterygoid muscles. Enlarged retropharyngeal nodes and left-sided mastoiditis are also seen. T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
Nodal Involvement
Imaging can detect nodal spread of NPC in 60 to 90%-of cases.[20] Nodal spread usually starts at retropharyngeal nodes (RPNs) followed by involvement of nodes at levels II, III, and IV.[21] Skip metastases to lymph nodes at level VI, supraclavicular fossa, and distant spread to thoracic and abdominal nodes can be seen in about 5%-cases. However, the absence of RPN involvement is seen in up to 1/3 of patients.[22] Some studies have suggested that level IIb nodes may be the first echelon nodes.[22] Lymphadenopathy is often bilateral in NPC. On imaging, supraclavicular nodes include levels IV and Vb nodes and are seen on the axial imaging section with a portion of the clavicle. Shortest axial diameter is used to measure the involved nodes. Normal size nodes may also harbor metastatic disease.[23] Loss of normal fatty hilum with change in the shape of the node from oval to round leading to increase in axial dimension and the longest longitudinal to axial dimension ratio <2 href="https://www.thieme-connect.com/products/ejournals/html/10.1055/s-0042-1760309#BR22970764-23" xss=removed>23] Clustered nodes >3 in the drainage area of primary tumor suggests metastatic involvement.[24] The presence of necrosis, seen as hyperintense signal on T2WI with peripheral postcontrast enhancement, is very specific for metastatic disease. Extranodal spread beyond the capsule can be seen in up to 23%-of cases.[25] It is seen as contour irregularity of the nodal margins with stranding in the adjacent fat.
Metastatic Spread
Distant metastases at initial diagnosis can be seen in up to 11%-patients.[26] Most common sites are bones, chest (pulmonary lesions and mediastinal lymph nodes), liver, and distant lymph nodes other than in the mediastinum. Hypertrophic pulmonary osteoarthropathy can be rarely seen in patients with intrathoracic metastatic disease.[26] FDG PET/CT is preferred modality to evaluate distant metastasis, mapping/characterizing cervical/distant lymph nodes and for work up of residual and recurrent disease.[27]
Imaging for Response Assessment and Follow-up
Follow-up assessment of patients after treatment for NPC includes evaluation of the nasopharynx and neck, cranial nerves, and clinical work up/imaging to detect distant metastasis. A baseline scan within 6 months of completion of chemoradiotherapy in T3/4-N2/3 NPC is recommended by National Comprehensive Cancer Network. Comprehensive clinical evaluation/endoscopy guides further imaging “as indicated based on signs/symptoms.”[28] Both MRI or PET/CT can be used for response assessment.[29] CT scans are considered much inferior to contrast-enhanced MRI in differentiating residual/recurrent disease from post-RT–chemotherapy changes. A baseline MRI scan is usually performed at 3 months posttreatment to detect subclinical residual lymphadenopathy.[29] Due to high soft tissue resolution, it may also serve as a baseline scan to detect future early recurrences. Diffusion-weighted imaging sequence with diffusion kurtosis imaging has been recently utilized to differentiate recurrent neoplasm from posttreatment changes. Increase in the apparent diffusion coefficient values after initiation of chemotherapy when compared with pretreatment scans indicates favorable response. No residual enlarged lymph nodes should be seen; otherwise, a neck dissection is necessary. On MRI, nonenhancing tissue with dark signal on T2WI represents mature fibrosis. Immature fibrosis (being cellular with fluid content) is seen as intermediate to high signal on T2WI and may sometimes enhance making it difficult to distinguish it from residual/recurrent disease.[30] Post-RT changes can appear as asymmetry of the nasopharynx with loss of normal contours. Also, distortion and partial effacement of the fossa of Rosenmuller (primary site) due to fibrosis can present difficulties for the radiologist to distinguish from residual/recurrent disease.
PET/CT is considered helpful in such cases and has a high negative predictive value of 95%-when done after 12 weeks posttherapy.[29] Recurrent/residual tumor has bulging contours with enhancement that can be detected with certainty on PET/CT as an FDG avid lesion. Regression of abnormal tissue on follow-up scans may indicate post-RT changes, but unchanged or growing lesions may suggest recurrent disease. Progressing masses or the appearance of a new node suggest recurrence. Clinical assessment, endoscopy, and EBV DNA levels can be helpful guide for initial evaluation, which can be followed by either PET/CT or MRI in positive cases to detect recurrence of NPC.[14] Isolated meningeal thickening in the posterior fossa at the jugular foramen or foramen magnum without any visible nasopharyngeal component can be seen in recurrent cases.[31]
Principles of Management
Management, as in any other cancer, is directed toward providing the best chance of cure/remission with good long-term outcomes and minimal adverse effects. The treatment is multidisciplinary with RT forming the mainstay for patients with early and locoregionally advanced NPC ([Supplementary Table S1]). Concurrent chemoradiotherapy is preferred in patients beyond Stage II disease. Induction chemotherapy with cisplatin and gemcitabine has been shown to provide benefit in Stages III and IVA disease, which is followed by concurrent chemoradiation. Bilateral neck dissection is often performed in all patients due to early involvement of lymph nodes in the disease. Patients with advanced stage disease are less responsive to therapy and also have higher chances of recurrence.
Follow-up Imaging and Management of Recurrent Disease
Follow-up MRI of the neck including the skull base and whole-body PET-CT is usually recommended after 3 to 4 months of treatment completion. An earlier imaging may create problems in distinguishing residual tumor from posttherapy changes. Posttreatment surveillance with endoscopy and a general clinical examination is recommended at 3 monthly intervals for the first 2 years followed by 6 monthly for the next 3 to 4 years. Imaging is performed in cases of clinical suspicion of recurrence.
Recurrent disease following treatment is difficult to manage. However, chemotherapy with cisplatin and gemcitabine has been shown to be of some benefit than other agents. Targeted therapy including vascular endothelial growth factor receptor and epidermal growth factor receptor inhibitors has not shown to be much useful. Reirradiation strategies using IMRT and immunotherapy are being used for treatment of a subset of recurrent NPC patients and have shown some promise; however, a lot still needs to be done for the management of this patient cohort.
Summary of Recommendations
Endoscopic nasopharyngeal biopsy is recommended in all clinically suspected cases of NPC, as evidenced by swelling in the nasopharynx, combined with EBV-encoded small RNAs in situ hybridization examination.
For assessment of the locoregional extent of the disease, imaging using MRI of nasopharynx, skull base, and neck until upper mediastinum is suggested. CT scan can be used alternatively; however, it is inferior to MRI in detecting the extent of cranial nerve, osseous, and intracranial involvement.
Additional imaging of metastasis using PET scan may be done, and if the same is not available, combination of a bone scan and a CT of the chest and abdomen may be helpful. Pure tone audiometry are optimal recommendations.
For patient follow-up, PET-CT/MRI is used for response evaluation with examination of nasopharynx and neck, cranial nerve function, and evaluation of systemic complaints. For T3 and T4 tumors, PET-CT/MRI is used yearly for at least 5 years and thyroid function tests are recommended at 1, 2, and 5 years.
Synoptic Reporting Format is given in [Supplementary Table S2].
Conflict of Interest
None declared.
Financial Disclosure
None.
Supplementary Material
References
- Abdel Khalek Abdel Razek A, King A. MRI and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol 2012; 198 (01) 11-18
- Chen Y-P, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019; 394 (10192): 64-80
- Raghupathy R, Hui EP, Chan AT. Epstein-Barr virus as a paradigm in nasopharyngeal cancer: from lab to clinic. Am Soc Clin Oncol Educ Book 2014; 34 (01) 149-153
- Ung A, Chen CJ, Levine PH. et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res 1999; 19 (1B): 661-665
- Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet 2016; 387 (10022): 1012-1024
- Hildesheim A, Apple RJ, Chen CJ. et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst 2002; 94 (23) 1780-1789
- Barnes L, Eveson JW, Sidransky D, Reichart P. eds. Pathology and Genetics of Head and Neck Tumours. Geneva, Switzerland: : IARC; 2005
- Leung SF, Zee B, Ma BB. et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24 (34) 5414-5418
- Lee AWM, Lydiatt WM, Colevas AD. et al. Nasopharynx. In: Amin MB. ed. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017: 103
- Saba NF, Salama JK, Beitler JJ. et al; Expert Panel on Radiation Oncology-Head and Neck Cancer. ACR appropriateness criteria® for nasopharyngeal carcinoma. Head Neck 2016; 38 (07) 979-986
- NCG Guidelines Manual. Accessed March 20, 2022 https://tmc.gov.in/ncg/index.php/guidelines/draft-guidelines-2020
- Colevas AD, Yom SS, Pfister DG. et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw 2018; 16 (05) 479-490
- NCCN Guidelines for patients Nasopharyngeal Cancer. Accessed March 20, 2022 https://www.nccn.org/patients/guidelines/content/PDF/hn-nasopharynx-patient.pdf
- Chan KCA, Hung ECW, Woo JKS. et al. Early detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA analysis in a surveillance program. Cancer 2013; 119 (10) 1838-1844
- Lee AWM, Ng WT, Chan LLK. et al. Evolution of treatment for nasopharyngeal cancer–success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014; 110 (03) 377-384
- King AD, Vlantis AC, Bhatia KSS. et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 2011; 258 (02) 531-537
- King AD, Lam WW, Leung SF, Chan YL, Teo P, Metreweli C. MRI of local disease in nasopharyngeal carcinoma: tumour extent vs tumour stage. Br J Radiol 1999; 72 (860) 734-741
- Chong VF, Fan YF, Khoo JB. Nasopharyngeal carcinoma with intracranial spread: CT and MR characteristics. J Comput Assist Tomogr 1996; 20 (04) 563-569
- King AD, Vlantis AC, Tsang RKY. et al. Magnetic resonance imaging for the detection of nasopharyngeal carcinoma. AJNR Am J Neuroradiol 2006; 27 (06) 1288-1291
- King AD, Ahuja AT, Leung SF. et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 2000; 22 (03) 275-281
- Ng SH, Chang JT, Chan SC. et al. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging 2004; 31 (08) 1073-1080
- Chong VF, Fan YF, Khoo JB. Retropharyngeal lymphadenopathy in nasopharyngeal carcinoma. Eur J Radiol 1995; 21 (02) 100-105
- Som PM, Brandwein MS. Lymph nodes. In: Som PM, Curtin HD. eds. Head and Neck Imaging. 4th ed. Vol. 2. Missouri, USA: : Mosby Inc; 2003: 1910-1911
- Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol 1992; 158 (05) 961-969
- King AD, Tse GM, Ahuja AT. et al. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology 2004; 230 (03) 720-726
- Teo PM, Kwan WH, Lee WY, Leung SF, Johnson PJ. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 1996; 77 (12) 2423-2431
- Chang JT, Chan SC, Yen TC. et al. Nasopharyngeal carcinoma staging by (18)F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 2005; 62 (02) 501-507
- Pfister DG, Spencer S, Adelstein D. et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020; 18 (07) 873-898
- Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology 2008; 249 (01) 203-211
- Offiah C, Hall E. Post-treatment imaging appearances in head and neck cancer patients. Clin Radiol 2011; 66 (01) 13-24
- Chong VF, Fan
YF. Meningeal
infiltration
in recurrent nasopharyngeal carcinoma. Australas Radiol 2000; 44 (01) 23-27
Address for correspondence
Chirag Kamal Ahuja, DMDivision of Neuroimaging and Interventional Neuroradiology, Department of Radiodiagnosis and ImagingPostgraduate Institute of Medical Education and ResearchChandigarh 160012IndiaEmail: chiragkahuja@gmail.comPublication History
Article published online:
01 March 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:(a) Axial T2W MR image showing hyperintense poorly defined mass involving the right side of nasopharynx in the fossa of Rosenmuller (white arrow) with associated fluid in the mastoid air cells. (b) Axial postgadolinium T1W MR image showing enhancement of the lesion (white arrow). MR, magnetic resonance; T2W, T2-weighted.

| Figure 2:Axial T1WI (a) and T2WI and (b) fat sat images showing a large mass involving the left side of nasopharynx with extension into left parapharyngeal space and involvement of pterygoid muscles. Enlarged retropharyngeal nodes and left-sided mastoiditis are also seen. T1WI, T1-weighted imaging; T2WI, T2-weighted imaging.
References
- Abdel Khalek Abdel Razek A, King A. MRI and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol 2012; 198 (01) 11-18
- Chen Y-P, Chan ATC, Le QT, Blanchard P, Sun Y, Ma J. Nasopharyngeal carcinoma. Lancet 2019; 394 (10192): 64-80
- Raghupathy R, Hui EP, Chan AT. Epstein-Barr virus as a paradigm in nasopharyngeal cancer: from lab to clinic. Am Soc Clin Oncol Educ Book 2014; 34 (01) 149-153
- Ung A, Chen CJ, Levine PH. et al. Familial and sporadic cases of nasopharyngeal carcinoma in Taiwan. Anticancer Res 1999; 19 (1B): 661-665
- Chua MLK, Wee JTS, Hui EP, Chan ATC. Nasopharyngeal carcinoma. Lancet 2016; 387 (10022): 1012-1024
- Hildesheim A, Apple RJ, Chen CJ. et al. Association of HLA class I and II alleles and extended haplotypes with nasopharyngeal carcinoma in Taiwan. J Natl Cancer Inst 2002; 94 (23) 1780-1789
- Barnes L, Eveson JW, Sidransky D, Reichart P. eds. Pathology and Genetics of Head and Neck Tumours. Geneva, Switzerland: : IARC; 2005
- Leung SF, Zee B, Ma BB. et al. Plasma Epstein-Barr viral deoxyribonucleic acid quantitation complements tumor-node-metastasis staging prognostication in nasopharyngeal carcinoma. J Clin Oncol 2006; 24 (34) 5414-5418
- Lee AWM, Lydiatt WM, Colevas AD. et al. Nasopharynx. In: Amin MB. ed. AJCC Cancer Staging Manual. 8th ed. New York: Springer; 2017: 103
- Saba NF, Salama JK, Beitler JJ. et al; Expert Panel on Radiation Oncology-Head and Neck Cancer. ACR appropriateness criteria® for nasopharyngeal carcinoma. Head Neck 2016; 38 (07) 979-986
- NCG Guidelines Manual. Accessed March 20, 2022 https://tmc.gov.in/ncg/index.php/guidelines/draft-guidelines-2020
- Colevas AD, Yom SS, Pfister DG. et al. NCCN Guidelines Insights: Head and Neck Cancers, Version 1.2018. J Natl Compr Canc Netw 2018; 16 (05) 479-490
- NCCN Guidelines for patients Nasopharyngeal Cancer. Accessed March 20, 2022 https://www.nccn.org/patients/guidelines/content/PDF/hn-nasopharynx-patient.pdf
- Chan KCA, Hung ECW, Woo JKS. et al. Early detection of nasopharyngeal carcinoma by plasma Epstein-Barr virus DNA analysis in a surveillance program. Cancer 2013; 119 (10) 1838-1844
- Lee AWM, Ng WT, Chan LLK. et al. Evolution of treatment for nasopharyngeal cancer–success and setback in the intensity-modulated radiotherapy era. Radiother Oncol 2014; 110 (03) 377-384
- King AD, Vlantis AC, Bhatia KSS. et al. Primary nasopharyngeal carcinoma: diagnostic accuracy of MR imaging versus that of endoscopy and endoscopic biopsy. Radiology 2011; 258 (02) 531-537
- King AD, Lam WW, Leung SF, Chan YL, Teo P, Metreweli C. MRI of local disease in nasopharyngeal carcinoma: tumour extent vs tumour stage. Br J Radiol 1999; 72 (860) 734-741
- Chong VF, Fan YF, Khoo JB. Nasopharyngeal carcinoma with intracranial spread: CT and MR characteristics. J Comput Assist Tomogr 1996; 20 (04) 563-569
- King AD, Vlantis AC, Tsang RKY. et al. Magnetic resonance imaging for the detection of nasopharyngeal carcinoma. AJNR Am J Neuroradiol 2006; 27 (06) 1288-1291
- King AD, Ahuja AT, Leung SF. et al. Neck node metastases from nasopharyngeal carcinoma: MR imaging of patterns of disease. Head Neck 2000; 22 (03) 275-281
- Ng SH, Chang JT, Chan SC. et al. Nodal metastases of nasopharyngeal carcinoma: patterns of disease on MRI and FDG PET. Eur J Nucl Med Mol Imaging 2004; 31 (08) 1073-1080
- Chong VF, Fan YF, Khoo JB. Retropharyngeal lymphadenopathy in nasopharyngeal carcinoma. Eur J Radiol 1995; 21 (02) 100-105
- Som PM, Brandwein MS. Lymph nodes. In: Som PM, Curtin HD. eds. Head and Neck Imaging. 4th ed. Vol. 2. Missouri, USA: : Mosby Inc; 2003: 1910-1911
- Som PM. Detection of metastasis in cervical lymph nodes: CT and MR criteria and differential diagnosis. AJR Am J Roentgenol 1992; 158 (05) 961-969
- King AD, Tse GM, Ahuja AT. et al. Necrosis in metastatic neck nodes: diagnostic accuracy of CT, MR imaging, and US. Radiology 2004; 230 (03) 720-726
- Teo PM, Kwan WH, Lee WY, Leung SF, Johnson PJ. Prognosticators determining survival subsequent to distant metastasis from nasopharyngeal carcinoma. Cancer 1996; 77 (12) 2423-2431
- Chang JT, Chan SC, Yen TC. et al. Nasopharyngeal carcinoma staging by (18)F-fluorodeoxyglucose positron emission tomography. Int J Radiat Oncol Biol Phys 2005; 62 (02) 501-507
- Pfister DG, Spencer S, Adelstein D. et al. Head and Neck Cancers, Version 2.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2020; 18 (07) 873-898
- Comoretto M, Balestreri L, Borsatti E, Cimitan M, Franchin G, Lise M. Detection and restaging of residual and/or recurrent nasopharyngeal carcinoma after chemotherapy and radiation therapy: comparison of MR imaging and FDG PET/CT. Radiology 2008; 249 (01) 203-211
- Offiah C, Hall E. Post-treatment imaging appearances in head and neck cancer patients. Clin Radiol 2011; 66 (01) 13-24
- Chong VF, Fan YF. Meningeal infiltration in recurrent nasopharyngeal carcinoma. Australas Radiol 2000; 44 (01) 23-27


 PDF
PDF  Views
Views  Share
Share

