Imaging Recommendations for Diagnosis, Staging, and Management of Hepatic and Biliary Tract Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 241-250
DOI: DOI: 10.1055/s-0042-1760320
Abstract
Major hepatobiliary cancers include hepatocellular carcinoma, gallbladder carcinoma, and cholangiocarcinoma. There are multiple guidelines and recommendations for the imaging evaluation of these cancers. This article reviews and summarizes principles and recommendations of imaging in hepatobiliary cancers. The cross-sectional imaging protocol is similar among these lesions and is discussed at first followed by the separate discussion of each cancer.
Keywords
cholangiocarcinoma - computed tomography - gallbladder carcinoma - hepatocellular carcinoma - magnetic resonance imagingPublication History
Article published online:
01 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Major hepatobiliary cancers include hepatocellular carcinoma, gallbladder carcinoma, and cholangiocarcinoma. There are multiple guidelines and recommendations for the imaging evaluation of these cancers. This article reviews and summarizes principles and recommendations of imaging in hepatobiliary cancers. The cross-sectional imaging protocol is similar among these lesions and is discussed at first followed by the separate discussion of each cancer.
Keywords
cholangiocarcinoma - computed tomography - gallbladder carcinoma - hepatocellular carcinoma - magnetic resonance imagingIntroduction
Major hepatobiliary cancers include hepatocellular carcinoma (HCC), gallbladder carcinoma, and cholangiocarcinoma (CCA). There are multiple guidelines and recommendations for the imaging evaluation of these cancers. This article reviews and summarizes principles and recommendations of imaging in hepatobiliary cancers. The cross-sectional imaging protocol is similar among these lesions and is discussed at first followed by the separate discussion of each cancer. Authors have reviewed existing international and Indian guidelines including but not limited to National Comprehensive Cancer Network (NCCN), American Society of Clinical Oncology (ASCO), American College of Radiology (ACR), Indian Council for Medical Research (ICMR), and Indian College of Radiology and Imaging (ICRI). Imaging recommendations that are unequivocally mentioned in the majority of guidelines are included in this article. In the presence of conflicting or absent recommendations, authors have reviewed the existing literature, achieved a consensus regarding the issue in question, and, based on current evidence and their experience, provided recommendations following a short discussion.
Imaging Protocol in Hepatobiliary Cancer
Ultrasound of Whole Abdomen
Ultrasound of the upper abdomen is the modality of choice for screening of at-risk patients for liver cancer. Ultrasound does not have any role in diagnosis of liver lesions except as a guide to biopsy and other interventional procedures.
Computed Tomography Scan of the Whole Abdomen with Contrast
Noncontrast phase: For identification of fat, iron, calcification, blood products, and iodized oil (after chemoembolization). Necessary for generation of subtraction images.
Early arterial phase (15–20 seconds, for all cases): Identified by complete enhancement of aorta and hepatic arteries and no or minimal enhancement of portal vein. This is important for arterial anatomy that is essential for surgical planning.
Late arterial phase (35–40 seconds, for suspected hepatocellular cancers): Identified by complete enhancement of hepatic arteries and streaky partial enhancement of portal vein. Early arterial enhancement of HCC is best visualized in this phase.
Portal venous phase (75–90 seconds): Identified by complete enhancement of portal vein and partial enhancement of hepatic veins. Some enhancement of hepatic veins is essential for surgical planning and volumetry.
Delayed phase (120–300 seconds): Only necessary for hepatic venous anatomy and to rule out suspected hemangioma.
Contrast dose: A total of 1.8 to 2 mL/kg of 300 to 350 mg iodine/mL contrast through antecubital vein with a flow rate of at least 3 mL/s (Optimal contrast flow rate is crucial for arterial phase images. One may consider lower flow rate if the disease is already metastatic and information regarding vascular anatomy and vascular involvement is not critical).
Coverage of the whole abdomen from diaphragm to symphysis pubis is recommended to screen for peritoneal metastasis.
Slice thickness: 2/3 mm, slice interval: 0 mm.
Neutral oral contrast with 350 to 500 mL of water immediately to 15 minutes before computed tomography (CT) (optional). Positive oral contrast is discouraged.
Multiplanar reformat in coronal and sagittal is critical (thickness: 2 mm, interval: 0 mm).
Multiphasic Magnetic Resonance Imaging with Contrast
It is used as a problem solving tool in biliary tract cancers and can be used as a primary diagnostic modality in suspected HCC or intrahepatic cholangiocarcinoma (IHCC).
Minimum required sequences:
Axial and coronal T2-weighted.
Axial diffusion-weighted imaging.
Axial T1-weighted in and opposed phase images: for intralesional fat.
Axial and coronal T1-weighted (VIBE/LAVA) precontrast, multiphasic arterial, portal venous, and delayed phase. In magnetic resonance imaging (MRI), multiple arterial phases are acquired in tandem. The timing of the rest of the phases is similar to that of CT. It is very important to generate subtraction images from postcontrast images to look for residual enhancement in treated liver nodules.
Thin-slab and thick-slab magnetic resonance cholangiography (MRCP) (HASTE/SSFSE/RARE) and three-dimensional MRCP (for biliary tract tumors).
If gadobenate dimeglumine is used as an intravenous contrast, then a hepatobiliary phase taken at 45 to 90 minutes is useful.
Technical requirements:
High field strength magnet (> 1.5 Tesla).
Phased array multichannel torso coil.
Contrast: Both extracellular contrast agent (e.g., gadovist, gadobutrol, gadoterate, etc.) or hepatobiliary contrast agent (gadobenate dimeglumine) are acceptable. Contrast dose 0.1 mmol/kg.
Hepatocellular Carcinoma
Risk Factors and Etiopathogenesis
Cirrhosis of the liver is the single most important risk factor for HCC. In India, 70 to 97%-of patients with HCC at the time of diagnosis had underlying cirrhosis of the liver.[1] Other important risk factors are chronic hepatitis B and C infection, alcoholic liver disease, and nonalcoholic fatty liver disease.[2] In India, hepatitis B virus (HBV), particularly genotype D is most commonly implicated in chronic hepatitis-related HCC.[3]
Epidemiology, Clinical Presentation in India and Global
Worldwide, HCC accounts for 90%-of cancers of the liver. It is the third leading cause of cancer-related death annually and constitutes the fifth most common cancer globally.[4] In India, age-adjusted incidence rate of liver cancer 0.7 to 7.5 in men and 0.2 to 2.2 in women per 100,000 population per year.[1] Based on a prospective observational study from North India, the annual incidence rate of HCC in cirrhotic patients is 1.6%.[5] The incidence of liver cancer in India is increasing.[6] The age standardized mortality rate is reported to be 6.8/100,000 population in men and 5.1/100,000 population in women.[7] The incidence of liver cancer increased with increasing age, with a median age at presentation of 40 to 70 years. It is four times more common in men.
Imaging Referral Guidelines
The imaging referral algorithm for HCC in at-risk patients is demonstrated in [Fig. 1].
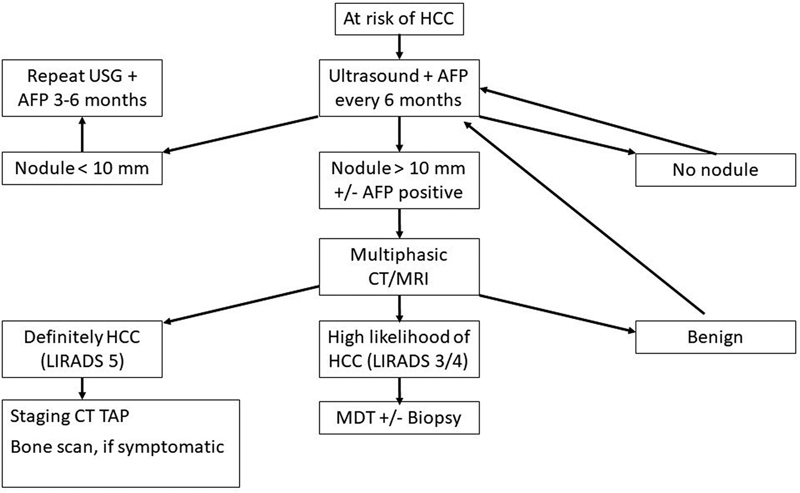
| Fig 1 :Imaging referral algorithm in hepatocellular carcinoma (HCC) in at-risk patients. Modified from National Comprehensive Cancer Network (NCCN) guidelines.
Clinical/Diagnostic Workup Excluding Imaging
Along with estimation of functional status of the patient and staging of the tumor, the following workup is recommended:
Serology for HBV and hepatitis C virus (HCV) (hepatitis B surface antigen [HBsAg], immunoglobulin G [IgG]/total-HBc, anti-HBs, anti-HCV). If HBsAg is positive, then hepatitis B e-antigen, anti-HBe antibody, and HBV-deoxyribonucleic acid levels are estimated. If anti-HCV is positive, then HCV genotype and HCV-ribonucleic acid levels are estimated.
Liver function test (LFT), for assessment liver functional reserve (Child–Pugh score or Model for End-Stage Liver Disease score) and surgical planning.
Assessment of portal hypertension (upper gastrointestinal endoscopy for varices and hepatic venous pressure gradient measurement).
Chest CT, with or without contrast.
Bone scan in presence of bone symptoms.
Positron emission tomography (PET)-CT is not recommended.
Imaging Guidelines
Screening
ICMR has recommended surveillance with a 6-monthly ultrasound examination of the liver by an experienced radiologist. Doppler ultrasound may be added for better detection of new thrombus in hepatic or portal vein. Candidates for surveillance include those with Child A and B cirrhosis of any etiology and Child C patients on the waiting list for a liver transplantation. Non-cirrhotic patients with chronic hepatitis B (males > 40 years and females > 50 years), chronic HBV infection of any age with family history of HCC, or chronic HCV with advanced fibrosis are candidates for surveillance[8] (ICMR 2019). Although ICMR does not recommend serum alpha-fetoprotein (AFP) as a screening method, addition of serum AFP has been shown to increase sensitivity of HCC detection in at-risk patients and NCCN has recently recommended addition of AFP in their 2021 guidelines.[9] [10] ACR recommends use of a standard Ultrasound Liver Reporting and Data System (US-LIRADS) for the reporting of surveillance ultrasound examination. Quality or adequacy of the diagnostic ultrasound examination can be reported with a visualization score ([Chart 1]). If an observation of size > 10 mm is visualized on ultrasound, further evaluation with multiphasic CT or MRI is warranted. Final ultrasound can be reported with an US-LIRADS score ([Chart 2]).[11]
|
Category |
Description |
Recommendation |
|---|---|---|
|
US-LIRADS 1, negative |
No or benign observation |
Routine follow-up |
|
US-LIRADS 2, subthreshold |
Observation < 10 mm |
Ultrasound at 3 mo |
|
US-LIRADS 3, positive |
Observation > 10 mm or new thrombus in a vein |
Multiphasic CT or MRI |
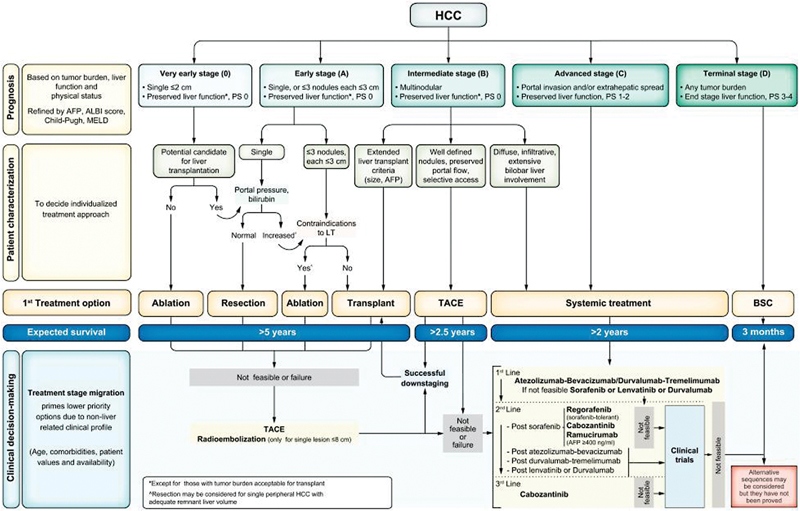
| Figure 2:Barcelona Clinic Liver Cancer (BCLC) staging system and treatment strategy.
Management—Role of Interventional Radiology
Role of interventional radiology (IR) is increased in the 2022 update of the BCLC recommendations. In BCLC 0, ablation is the treatment of choice. Resection should be considered only when ablation is not feasible. Transarterial chemoembolization (TACE) and transarterial radioembolization (TARE) can also be considered. TARE is to be used in single lesions that are less than 8 cm.[14] In BCLC-A with single lesions, resection is favored in tumors up to 2 cm in size. For larger lesions, TARE can be considered in case the remnant liver is small. In multiple lesions with increased portal pressure or high bilirubin, ablation is recommended. For patients with > 3 lesions, TACE and TARE may be indicated. In LT candidates with > 6 months waiting time, ablation, TACE, or TARE can be used for bridging. BCLC-B patients with preserved portal flow and well-defined lesions TACE is recommended. Some cases of BCLC-B can still be considered for LT if they meet local practice guidelines. Some advanced cases of stage B may be downstaged to become eligible for LT through TACE or TARE and systemic therapies.[15]
Response Assessment
For follow-up of locoregional and systemic therapy in HCC, modified Response Evaluation Criteria in Solid Tumors (mRECIST) is recommended over RECIST 1.1 guidelines in early and intermediate cases. In advanced cases, both methods can be used.[16] mRECIST takes into account the concept of “viable” tumor. After locoregional and systemic therapy in HCC, many lesions do not show tumor shrinkage but show necrosis which is defined by loss of enhancement. mRECIST advocates measurement of enhancing or “viable” tumors only. Furthermore, enlargement of portal nodes and ascites/pleural effusion can also be a part of the chronic liver disease process and adjustments are made in this criteria to prevent overdiagnosis of disease progression.
mRECIST:
Selection and measurement of target lesions:
○ Typical intrahepatic target lesion: ≥ 1 cm, intratumoral arterial phase enhancement.
○ Atypical intrahepatic target lesion: ≥ 1 cm, no arterial phase enhancement.
○ Extrahepatic target lesion, non-nodal: ≥ 1 cm longest axis.
○ Extrahepatic target lesion, nodal: ≥ 2 cm short axis if portal node, 1.5 cm short axis for nodes elsewhere.
○ Target lesions should be suitable for accurate and repeat measurement. Total number of target lesions should not exceed five with no more than two lesions per organ.
○ For hepatic targets, the longest diameter of the viable (arterially enhancing) tumor is measured. For extrahepatic non-nodal targets, the longest diameter is measured and for nodal lesions, the short axis diameter is measured.
For baseline assessment sum of diameters of all target lesions are measured. The disease status on follow-up images is assessed using criteria similar to RECIST 1.1 ([Table 1]). Follow-up is usually done at 6 to 8 weeks intervals.
For immunotherapy, mRECIST criteria can be applied with a longer follow-up interval of 8 to 12 weeks since immunomodulating agents take longer to show tumor response.
|
Complete response |
No intraluminal arterial enhancement in all hepatic typical target and disappearance of atypical hepatic target and all extrahepatic target lesions |
|
Partial response |
≥ 30%-reduction in sum of diameters of all viable (arterially enhancing) target lesions |
|
Stable disease |
Not classifiable as partial response or progressive disease |
|
Progressive disease |
≥ 20%-increase in sum of diameters of all viable (arterially enhancing) target lesions |
|
Category |
Subcategory |
Description |
|
|---|---|---|---|
|
T |
Tis |
Carcinoma in situ |
|
|
T1 |
T1a |
Limited to lamina propria |
|
|
T1b |
Invasion of muscular layer |
||
|
T2 |
T2a |
Invasion of perimuscular connective tissue on peritoneal side |
|
|
T2b |
Invasion of perimuscular connective tissue on liver side |
||
|
T3 |
Perforates serosa and/or invades liver and/or one other adjacent [stomach, duodenum, colon, pancreas, omentum, extrahepatic bile ducts |
||
|
T4 |
Invades main portal vein or hepatic artery or two or more extrahepatic organs |
||
|
N |
N0 |
No regional node metastases |
|
|
N1 |
Metastasis in 1–3 regional nodes |
||
|
N2 |
Metastasis in ≥ 4 regional nodes |
||
|
M |
M0 |
No distant metastases |
|
|
M1 |
Distant metastases |
||
|
Classification |
Biliary involvement |
Portal vein invasion |
Liver lobe atrophy |
|---|---|---|---|
|
T1 |
Hilum ± unilateral second order bile ducts |
No |
No |
|
T2 |
Hilum ± unilateral second order bile ducts |
Ipsilateral |
Ipsilateral |
|
T3 |
Hilum ± bilateral second order bile ducts |
Any |
Any |
|
Hilum ± unilateral second order bile ducts |
Contralateral |
Any |
|
|
Hilum ± unilateral second order bile ducts |
Any |
Contralateral |
|
|
Hilum ± unilateral second order bile ducts |
Bilateral |
Any |
References
- Acharya SK. Epidemiology of hepatocellular carcinoma in India. J Clin Exp Hepatol 2014; 4 (Suppl. 03) S27-S33
- Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2013; 1 (06) 180-182
- Asim M, Sarma MP, Kar P. Etiological and molecular profile of hepatocellular cancer from India. Int J Cancer 2013; 133 (02) 437-445
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56 (04) 908-943
- Paul SB, Sreenivas V, Gulati MS. et al. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from a tertiary care center in northern India. Indian J Gastroenterol 2007; 26 (06) 274-278
- Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev 2008; 9 (01) 97-100
- Dikshit R, Gupta PC, Ramasundarahettige C. et al; Million Death Study Collaborators. Cancer mortality in India: a nationally representative survey. Lancet 2012; 379 (9828): 1807-1816
- Singal AG, Zhang E, Narasimman M. et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a systematic review and meta-analysis. J Hepatol 2022; 77 (01) 128-139
- Tzartzeva K, Obi J, Rich NE. et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018; 154 (06) 1706-1718.e1
- Benson AB, D'Angelica MI, Abbott DE. et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (05) 541-565
- American College of Radiology. Ultrasound LI-RADS v2017. Accessed December 23, 2022 https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/LI-RADS-Ultrasound-v2017
- Marrero JA, Kulik LM, Sirlin CB. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018; 68 (02) 723-750
- American College of Radiology. Liver imaging reporting and data system. Accessed December 23, 2022. https://www.acr.org/Quality-Safety/Resources/LIRADS
- Salem R, Johnson GE, Kim E. et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 2021; 74 (05) 2342-2352
- Mazzaferro V, Citterio D, Bhoori S. et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020; 21 (07) 947-956
- Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020; 72 (02) 288-306
- Gordan JD, Kennedy EB, Abou-Alfa GK. et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020; 38 (36) 4317-4345
- Akinyemiju T, Abera S, Ahmed M. et al; Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3 (12) 1683-1691
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6 (02) 172-187
- Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol 2019; 5 (02) 93-102
- Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Linchuang Zhongliuxue Zazhi 2019; 8 (04) 33
- Rathanaswamy S, Misra S, Kumar V. et al. Incidentally detected gallbladder cancer- the controversies and algorithmic approach to management. Indian J Surg 2012; 74 (03) 248-254
- Rodríguez-Fernández A, Gómez-Río M, Medina-Benítez A. et al. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol 2006; 93 (08) 650-664
- Akiba J, Nakashima O, Hattori S. et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 2013; 37 (04) 496-505
- DeOliveira ML, Cunningham SC, Cameron JL. et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007; 245 (05) 755-762
- Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol 2007; 19 (08) 615-617
- Chatterjee A, Lopes Vendrami C, Nikolaidis P. et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics 2019; 39 (02) 388-412
- Ainechi S, Lee H. Updates on precancerous lesions of the biliary tract: biliary precancerous lesion. Arch Pathol Lab Med 2016; 140 (11) 1285-1289
- Lamarca A, Barriuso J, Chander A. et al. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol 2019; 71 (01) 115-129
- Ahn JC, Yang JD. Screening indications and treatments for cholangiocarcinoma. Curr Hepatol Rep 2019; 18: 408-416
- Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol 2003; 181 (03) 819-827
- Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012; 264 (03) 751-760
- Watkinson AF, Adam A. Interventional Radiology: A Practical Guide. New York, USA:: Radcliffe Publishing; 1996
- Jung G-S, Huh J-D, Lee SU, Han BH, Chang H-K, Cho YD. Bile duct: analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology 2002; 224 (03) 725-730
- Ahrar K, Gupta S. Percutaneous Image-Guided Biopsy. New York, USA:: Springer Science & Business Media; 2013
- Brugge WR, De Witt J, Klapman JB. et al. Techniques for cytologic sampling of pancreatic and bile duct lesions: the Papanicolaou Society of Cytopathology guidelines. Cytojournal 2014; 11 (Suppl. 01) 2
- Madhusudhan KS, Gamanagatti S, Gupta AK. Imaging and interventions in hilar cholangiocarcinoma: a review. World J Radiol 2015; 7 (02) 28-44
- Nimura Y, Kamiya J, Kondo S. et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 2000; 7 (02) 155-162
Address for correspondence
Publication History
Article published online:
01 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :Imaging referral algorithm in hepatocellular carcinoma (HCC) in at-risk patients. Modified from National Comprehensive Cancer Network (NCCN) guidelines.

| Figure 2:Barcelona Clinic Liver Cancer (BCLC) staging system and treatment strategy.
References
- Acharya SK. Epidemiology of hepatocellular carcinoma in India. J Clin Exp Hepatol 2014; 4 (Suppl. 03) S27-S33
- Herbst DA, Reddy KR. Risk factors for hepatocellular carcinoma. Clin Liver Dis (Hoboken) 2013; 1 (06) 180-182
- Asim M, Sarma MP, Kar P. Etiological and molecular profile of hepatocellular cancer from India. Int J Cancer 2013; 133 (02) 437-445
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012; 56 (04) 908-943
- Paul SB, Sreenivas V, Gulati MS. et al. Incidence of hepatocellular carcinoma among Indian patients with cirrhosis of liver: an experience from a tertiary care center in northern India. Indian J Gastroenterol 2007; 26 (06) 274-278
- Yeole BB. Trends in cancer incidence in esophagus, stomach, colon, rectum and liver in males in India. Asian Pac J Cancer Prev 2008; 9 (01) 97-100
- Dikshit R, Gupta PC, Ramasundarahettige C. et al; Million Death Study Collaborators. Cancer mortality in India: a nationally representative survey. Lancet 2012; 379 (9828): 1807-1816
- Singal AG, Zhang E, Narasimman M. et al. HCC surveillance improves early detection, curative treatment receipt, and survival in patients with cirrhosis: a systematic review and meta-analysis. J Hepatol 2022; 77 (01) 128-139
- Tzartzeva K, Obi J, Rich NE. et al. Surveillance imaging and alpha fetoprotein for early detection of hepatocellular carcinoma in patients with cirrhosis: a meta-analysis. Gastroenterology 2018; 154 (06) 1706-1718.e1
- Benson AB, D'Angelica MI, Abbott DE. et al. Hepatobiliary Cancers, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021; 19 (05) 541-565
- American College of Radiology. Ultrasound LI-RADS v2017. Accessed December 23, 2022 https://www.acr.org/Clinical-Resources/Reporting-and-Data-Systems/LI-RADS/LI-RADS-Ultrasound-v2017
- Marrero JA, Kulik LM, Sirlin CB. et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018; 68 (02) 723-750
- American College of Radiology. Liver imaging reporting and data system. Accessed December 23, 2022. https://www.acr.org/Quality-Safety/Resources/LIRADS
- Salem R, Johnson GE, Kim E. et al. Yttrium-90 radioembolization for the treatment of solitary, unresectable HCC: the LEGACY study. Hepatology 2021; 74 (05) 2342-2352
- Mazzaferro V, Citterio D, Bhoori S. et al. Liver transplantation in hepatocellular carcinoma after tumour downstaging (XXL): a randomised, controlled, phase 2b/3 trial. Lancet Oncol 2020; 21 (07) 947-956
- Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020; 72 (02) 288-306
- Gordan JD, Kennedy EB, Abou-Alfa GK. et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020; 38 (36) 4317-4345
- Akinyemiju T, Abera S, Ahmed M. et al; Global Burden of Disease Liver Cancer Collaboration. The burden of primary liver cancer and underlying etiologies from 1990 to 2015 at the global, regional, and national level: results from the Global Burden of Disease Study 2015. JAMA Oncol 2017; 3 (12) 1683-1691
- Stinton LM, Shaffer EA. Epidemiology of gallbladder disease: cholelithiasis and cancer. Gut Liver 2012; 6 (02) 172-187
- Rawla P, Sunkara T, Thandra KC, Barsouk A. Epidemiology of gallbladder cancer. Clin Exp Hepatol 2019; 5 (02) 93-102
- Dutta U, Bush N, Kalsi D, Popli P, Kapoor VK. Epidemiology of gallbladder cancer in India. Linchuang Zhongliuxue Zazhi 2019; 8 (04) 33
- Rathanaswamy S, Misra S, Kumar V. et al. Incidentally detected gallbladder cancer- the controversies and algorithmic approach to management. Indian J Surg 2012; 74 (03) 248-254
- Rodríguez-Fernández A, Gómez-Río M, Medina-Benítez A. et al. Application of modern imaging methods in diagnosis of gallbladder cancer. J Surg Oncol 2006; 93 (08) 650-664
- Akiba J, Nakashima O, Hattori S. et al. Clinicopathologic analysis of combined hepatocellular-cholangiocarcinoma according to the latest WHO classification. Am J Surg Pathol 2013; 37 (04) 496-505
- DeOliveira ML, Cunningham SC, Cameron JL. et al. Cholangiocarcinoma: thirty-one-year experience with 564 patients at a single institution. Ann Surg 2007; 245 (05) 755-762
- Ben-Menachem T. Risk factors for cholangiocarcinoma. Eur J Gastroenterol Hepatol 2007; 19 (08) 615-617
- Chatterjee A, Lopes Vendrami C, Nikolaidis P. et al. Uncommon intraluminal tumors of the gallbladder and biliary tract: spectrum of imaging appearances. Radiographics 2019; 39 (02) 388-412
- Ainechi S, Lee H. Updates on precancerous lesions of the biliary tract: biliary precancerous lesion. Arch Pathol Lab Med 2016; 140 (11) 1285-1289
- Lamarca A, Barriuso J, Chander A. et al. 18F-fluorodeoxyglucose positron emission tomography (18FDG-PET) for patients with biliary tract cancer: systematic review and meta-analysis. J Hepatol 2019; 71 (01) 115-129
- Ahn JC, Yang JD. Screening indications and treatments for cholangiocarcinoma. Curr Hepatol Rep 2019; 18: 408-416
- Lim JH. Cholangiocarcinoma: morphologic classification according to growth pattern and imaging findings. AJR Am J Roentgenol 2003; 181 (03) 819-827
- Kang Y, Lee JM, Kim SH, Han JK, Choi BI. Intrahepatic mass-forming cholangiocarcinoma: enhancement patterns on gadoxetic acid-enhanced MR images. Radiology 2012; 264 (03) 751-760
- Watkinson AF, Adam A. Interventional Radiology: A Practical Guide. New York, USA:: Radcliffe Publishing; 1996
- Jung G-S, Huh J-D, Lee SU, Han BH, Chang H-K, Cho YD. Bile duct: analysis of percutaneous transluminal forceps biopsy in 130 patients suspected of having malignant biliary obstruction. Radiology 2002; 224 (03) 725-730
- Ahrar K, Gupta S. Percutaneous Image-Guided Biopsy. New York, USA:: Springer Science & Business Media; 2013
- Brugge WR, De Witt J, Klapman JB. et al. Techniques for cytologic sampling of pancreatic and bile duct lesions: the Papanicolaou Society of Cytopathology guidelines. Cytojournal 2014; 11 (Suppl. 01) 2
- Madhusudhan KS, Gamanagatti S, Gupta AK. Imaging and interventions in hilar cholangiocarcinoma: a review. World J Radiol 2015; 7 (02) 28-44
- Nimura Y, Kamiya J, Kondo S. et al. Aggressive preoperative management and extended surgery for hilar cholangiocarcinoma: Nagoya experience. J Hepatobiliary Pancreat Surg 2000; 7 (02) 155-162


 PDF
PDF  Views
Views  Share
Share

