Imaging Recommendations for Diagnosis, Staging, and Management of Hematological Malignancies
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(03): 302-307
DOI: DOI: 10.1055/s-0042-1760327
Abstract
The NIC defines hematological cancers as those that begin in blood forming tissues such as bone marrow or cells of the immune system and these broadly include three groups: leukemias, lymphomas, and myelomas. The role of imaging is also fundamentally different between the three main groups of hematological malignancies. While imaging is the main tool for staging as well as treatment response assessment in lymphoma, it represents one of several key criteria for the diagnosis and follow-up of myeloma; whereas in leukemia, imaging has a role to play in the detection and management of treatment-related complications which is a crucial part of post-transplant treatment.
Disclaimer
This article is not an original paper and is only a compilation of imaging guidelines from various sources, which have been cited appropriately.
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The NIC defines hematological cancers as those that begin in blood forming tissues such as bone marrow or cells of the immune system and these broadly include three groups: leukemias, lymphomas, and myelomas. The role of imaging is also fundamentally different between the three main groups of hematological malignancies. While imaging is the main tool for staging as well as treatment response assessment in lymphoma, it represents one of several key criteria for the diagnosis and follow-up of myeloma; whereas in leukemia, imaging has a role to play in the detection and management of treatment-related complications which is a crucial part of post-transplant treatment.
Introduction
The NIC defines hematological cancers as those that begin in blood forming tissue such as bone marrow or cells of the immune system and these broadly include three groups leukemias, lymphomas, and myelomas.[1] The role of imaging is also fundamentally different between the three main groups of hematological malignancies. While imaging is the main tool for staging as well as treatment response assessment in lymphoma,[2] [3] it represents one of several key criteria for the diagnosis and follow-up of myeloma[4]; whereas in leukemia, imaging has a role to play in the detection and management of treatment-related complications which is a crucial part of post-transplant treatment.
In myeloma, whole-body magnetic resonance imaging (WB-MRI) is recognized as a highly sensitive test for the assessment of myeloma, and is also endorsed by clinical guidelines, especially for detection and staging. In lymphoma, WB-MRI is presently not recommended, and merely serves as an alternative technique to the current standard imaging, Flourine-18 fluorodeoxyglucose positron emission tomography/computed tomography ([18F]FDG-PET/CT), especially in pediatric patients.[5]
Even for lymphomas with variable FDG avidity, such as extranodal mucosa-associated lymphoid tissue lymphoma (MALT), contrast-enhanced CT, but not WB-MRI, is presently recommended, despite the high sensitivity of diffusion-weighted MRI and its ability to capture treatment response that has been reported in the literature.[5] In leukemia, neither MRI nor any other cross-sectional imaging test (including PET) is currently recommended outside of clinical trials.[5]
Epidemiology, Clinical Presentation in India and Global
Almost all of these cancers occur almost a decade earlier in India compared with the West. Possible reasons proposed have included the demographics of the Indian population (largely younger), increased incidence of chronic infections and antigenic stimulation, genetics, and socioeconomic status. The average ASR for multiple myeloma (MM) is 0.1 to 1.9 in India, and around 2.8 to 3.9 in the US, with similar figures for Hodgkin's lymphoma (HL).[6] Incidence of leukemias is between 2.4 and 4.6 per 100,000 when compared with 9.6 to 11 in Canada.[6]
Imaging Guidelines
Lymphomas
PET-CT is recommended for the routine staging of FDG-avid, nodal lymphomas (essentially all histologies except chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL), lymphoplasmacytic lymphoma/Waldenström's macroglobulinemia, mycosis fungoides, and marginal zone non-Hodgkin's lymphomas (NHLs), unless there is a suspicion of aggressive transformation) as the gold standard.[7]
CT scan is preferred in the other lymphomas. A chest X-ray is no longer required in lymphoma staging because it less accurate than CT.[8] Moreover, CT identifies more hilar nodes and may better discriminate between a single large nodal mass and an aggregate of individual nodes.[3]
Definition of bulky disease: A single nodal mass, in contrast to multiple smaller nodes, of 10 cm or greater than a third of the transthoracic diameter at any level of thoracic vertebrae as determined by CT is retained as the definition of bulky disease for HL.[9] A chest X-ray is not required to determine bulk because of its high concordance with CT.[8] However, a variety of sizes have been suggested for NHL[10] with limited evidence suggesting 6 cm as best for follicular lymphoma15 and 6 to 10 cm in the rituximab era for diffuse large-B-cell lymphoma (DLBCL).[11] However, none of the proposed sizes have been validated in the current therapeutic era. Therefore, the recommendation for HL and NHL is to record the longest measurement by CT scan, with the term X no longer necessary.[3]
If a PET-CT is performed, a bone marrow aspirate/biopsy is no longer required for the routine evaluation of patients with HL. In DLBCL, PET-CT is also more sensitive than bone marrow biopsy (BMB) but has been reported to miss low-volume diffuse involvement of 10 to 20%-of the marrow.[12] If the scan is negative, a BMB is indicated to identify involvement by discordant histology if relevant for a clinical trial or patient management.[13]
Response Assessment
Lugano's criteria are used for response assessment as summarized in [Table 1].[18] End-of-treatment assessment is more accurate with PET-CT, especially for patients with radiologic (CT) CRu or partial response (PR) in HL, DLBCL, and follicular lymphoma.[3] PET-CT-based criteria eliminate CRu and improve the prognostic value of PR. In early- and advanced-stage patients with HL, a negative predictive value of 95 to 100%-and a positive predictive value of more than 90%- have been reported.[14] [15] In aggressive NHL, studies have reported a negative predictive value of 80 to 100%, but a lower positive predictive value, ranging from 50 to 100%.[16]
|
Modality |
Complete response |
Partial response |
Stable disease |
Progressive disease |
|---|---|---|---|---|
|
CT |
Lymph modes ≤ 1.5 cm in Ldi Complete disappearance of radiologic evidence of disease |
Single lesion: ↓ ≥ 50%-in PPD Multiple lesions: ↓ ≥ 50%-in SPD of up to six lymph nodes or extranodal sites |
↓ ≤ 50%-in SPD of up to six lymph nodes or extranodal sites (no criteria for progressive disease are met) |
1) New lymphadenopathy or ↑; single node must be abnormal with: a) Ldi > 1.5 cm and b) PPD ≥ 50%-and c) Ldi or Sdi ↑ 0.5 cm if ≤ 2.0 cm and ↑ 1.0 cm if > 2.0 cm |
|
2) ↑ splenic volume: a) with prior splenomegaly: ↑ > 50%-of its prior ↑ beyond baseline b) without prior splenomegaly: ↑> 2.0 cm c) New or recurrent splenomegaly |
||||
|
3)New or Larger non measured lesions |
||||
|
4) Recurrent previously resolved lesions |
||||
|
5) New extranodal lesion > 1.0 cm in any axis (new lesions < 1.0 cm in any axis are included if attributable to lymphoma) |
||||
|
FDG-PET-CT |
Scores 1, 2, 3 in nodal or extra nodal sites with or without a residual mass |
Scores 4 or 5 with ↓ uptake compared with baseline and residual mass(es) |
Scores 4 or 5 with no obvious change in FDG uptake |
Scores 4 or 5 any lesion with ↑ uptake from baseline and /or new FDG-avid foci |
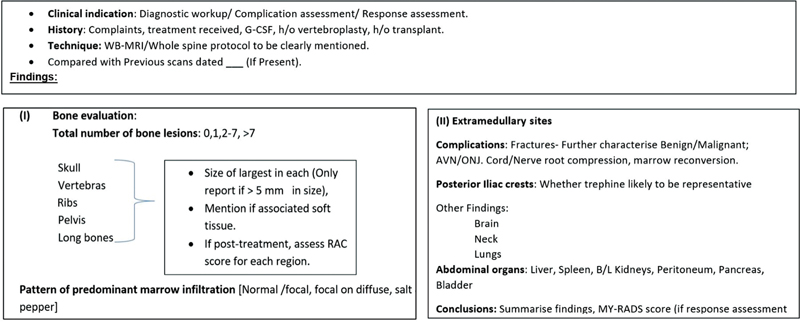
| Fig 1 :Structured reporting format of myelomas.[27] AVN,—; G-CSF,—; MY-RADS, Myeloma Response Assessment and Diagnosis System; ONJ,—; RAC,—; WB-MRI, whole body magnetic resonance imaging.
Conflict of Interest
None declared.
Disclaimer
This article is not an original paper and is only a compilation of imaging guidelines from various sources, which have been cited appropriately.
References
- Definition of hematologic cancer - NCI Dictionary of Cancer Terms - National Cancer Institute. Accessed December 21, 2022, at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/hematologic-cancer
- Younes A, Hilden P, Coiffier B. et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017; 28 (07) 1436-1447
- Cheson BD, Fisher RI, Barrington SF. et al; Alliance, Australasian Leukaemia and Lymphoma Group, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin's Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, Southwest Oncology Group, United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32 (27) 3059-3068
- Chantry A, Kazmi M, Barrington S. et al; British Society for Haematology Guidelines. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 2017; 178 (03) 380-393
- Mayerhoefer ME, Archibald SJ, Messiou C, Staudenherz A, Berzaczy D, Schöder H. MRI and PET/MRI in hematologic malignancies. J Magn Reson Imaging 2020; 51 (05) 1325-1335
- Bhutani M, Vora A, Kumar L, Kochupillai V. Lympho-hemopoietic malignancies in India. Med Oncol 2002; 19 (03) 141-150
- Barrington SF, Mikhaeel NG, Kostakoglu L. et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32 (27) 3048-3058
- Bradley AJ, Carrington BM, Lawrance JAL, Ryder WDJ, Radford JA. Assessment and significance of mediastinal bulk in Hodgkin's disease: comparison between computed tomography and chest radiography. Citeseer. 17:2493–2498. Accessed December 21, 2022, at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.982.5563&rep=rep1&type=pdf
- Lister TA, Crowther D, Sutcliffe SB. et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989; 7 (11) 1630-1636
- Federico M, Bellei M, Marcheselli L. et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009; 27 (27) 4555-4562
- Pfreundschuh M, Ho A, Cavallin-Stahl E. oncology MW-T lancet, 2008 undefined. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like. Elsevier. Accessed December 21, 2022, at: https://www.sciencedirect.com/science/article/pii/S1470204508700780
- Pelosi E, Penna D, Douroukas A. et al. MB-TQJ, 2010 undefined. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. europepmc.org. Accessed December 21, 2022, at: https://europepmc.org/article/med/21150862
- Paone G, Itti E, Haioun C. et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging 2009; 36 (05) 745-750
- Cerci JJ, Pracchia LF, Linardi CCG. et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med 2010; 51 (09) 1337-1343
- Engert A, Haverkamp H, Kobe C. et al; German Hodgkin Study Group, Swiss Group for Clinical Cancer Research, Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 2012; 379 (9828): 1791-1799
- Mikhaeel NG, Timothy AR, Hain SF, O'Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol 2000; 11 (Suppl 1): 147-150
- Conte MJ, Bowen DA, Wiseman GA. et al. Use of positron emission tomography-computed tomography in the management of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma 2014; 55 (09) 2079-2084
- Mauro FR, Chauvie S, Paoloni F. et al. Diagnostic and prognostic role of PET/CT in patients with chronic lymphocytic leukemia and progressive disease. Leukemia 2015; 29 (06) 1360-1365
- Guha A, Vijan A, Agarwal U. et al. Imaging for plasma cell dyscrasias: what, when, and how?. Front Oncol 2022; 12: 825394 DOI: 10.3389/FONC.2022.825394.
- Hillengass J, Usmani S, Rajkumar SV. et al. International Myeloma Working Group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol 2019; 20 (06) e302-e312
- Ormond Filho AG, Carneiro BC, Pastore D. et al. Whole-body imaging of multiple myeloma: diagnostic criteria. Radiographics 2019; 39 (04) 1077-1097
- Lecouvet FE. Whole-body MR imaging: musculoskeletal applications. Radiology 2016; 279 (02) 345-365
- Rajkumar S, Dimopoulos M, Palumbo A. oncology JB-T lancet, 2014 undefined. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Elsevier. Accessed December 21, 2022, at: https://www.sciencedirect.com/science/article/pii/S1470204514704425
- Koutoulidis V, Fontara S, Terpos E. et al. Quantitative diffusion-weighted imaging of the bone marrow: an adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma. Radiology 2017; 282 (02) 484-493
- Bergstrom DJ, Kotb R, Louzada ML, Sutherland HJ, Tavoularis S, Venner CP. Myeloma Canada Research Network Consensus Guideline Consortium. Consensus guidelines on the diagnosis of multiple myeloma and related disorders: recommendations of the myeloma Canada research network consensus guideline consortium. Clin Lymphoma Myeloma Leuk 2020; 20 (07) e352-e367
- Nanni C. PET/CT with standard non-FDG tracers in multiple myeloma. Mol Imaging Mult Myeloma 2019; 93–97 DOI: 10.1007/978-3-030-19019-4_7.
- Messiou C, Hillengass J, Delorme S. et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS). Radiology 2019; 291 (01) 5-13
- Van Heertum RL, Scarimbolo R, Wolodzko JG. et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther 2017; 11: 1719-1728
Address for correspondence
Publication History
Article published online:
12 May 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
References
- Definition of hematologic cancer - NCI Dictionary of Cancer Terms - National Cancer Institute. Accessed December 21, 2022, at: https://www.cancer.gov/publications/dictionaries/cancer-terms/def/hematologic-cancer
- Younes A, Hilden P, Coiffier B. et al. International Working Group consensus response evaluation criteria in lymphoma (RECIL 2017). Ann Oncol 2017; 28 (07) 1436-1447
- Cheson BD, Fisher RI, Barrington SF. et al; Alliance, Australasian Leukaemia and Lymphoma Group, Eastern Cooperative Oncology Group, European Mantle Cell Lymphoma Consortium, Italian Lymphoma Foundation, European Organisation for Research, Treatment of Cancer/Dutch Hemato-Oncology Group, Grupo Español de Médula Ósea, German High-Grade Lymphoma Study Group, German Hodgkin's Study Group, Japanese Lymphorra Study Group, Lymphoma Study Association, NCIC Clinical Trials Group, Nordic Lymphoma Study Group, Southwest Oncology Group, United Kingdom National Cancer Research Institute. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol 2014; 32 (27) 3059-3068
- Chantry A, Kazmi M, Barrington S. et al; British Society for Haematology Guidelines. Guidelines for the use of imaging in the management of patients with myeloma. Br J Haematol 2017; 178 (03) 380-393
- Mayerhoefer ME, Archibald SJ, Messiou C, Staudenherz A, Berzaczy D, Schöder H. MRI and PET/MRI in hematologic malignancies. J Magn Reson Imaging 2020; 51 (05) 1325-1335
- Bhutani M, Vora A, Kumar L, Kochupillai V. Lympho-hemopoietic malignancies in India. Med Oncol 2002; 19 (03) 141-150
- Barrington SF, Mikhaeel NG, Kostakoglu L. et al. Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. J Clin Oncol 2014; 32 (27) 3048-3058
- Bradley AJ, Carrington BM, Lawrance JAL, Ryder WDJ, Radford JA. Assessment and significance of mediastinal bulk in Hodgkin's disease: comparison between computed tomography and chest radiography. Citeseer. 17:2493–2498. Accessed December 21, 2022, at: http://citeseerx.ist.psu.edu/viewdoc/download?doi=10.1.1.982.5563&rep=rep1&type=pdf
- Lister TA, Crowther D, Sutcliffe SB. et al. Report of a committee convened to discuss the evaluation and staging of patients with Hodgkin's disease: Cotswolds meeting. J Clin Oncol 1989; 7 (11) 1630-1636
- Federico M, Bellei M, Marcheselli L. et al. Follicular lymphoma international prognostic index 2: a new prognostic index for follicular lymphoma developed by the international follicular lymphoma prognostic factor project. J Clin Oncol 2009; 27 (27) 4555-4562
- Pfreundschuh M, Ho A, Cavallin-Stahl E. oncology MW-T lancet, 2008 undefined. Prognostic significance of maximum tumour (bulk) diameter in young patients with good-prognosis diffuse large-B-cell lymphoma treated with CHOP-like. Elsevier. Accessed December 21, 2022, at: https://www.sciencedirect.com/science/article/pii/S1470204508700780
- Pelosi E, Penna D, Douroukas A. et al. MB-TQJ, 2010 undefined. Bone marrow disease detection with FDG-PET/CT and bone marrow biopsy during the staging of malignant lymphoma: results from a large multicentre study. europepmc.org. Accessed December 21, 2022, at: https://europepmc.org/article/med/21150862
- Paone G, Itti E, Haioun C. et al. Bone marrow involvement in diffuse large B-cell lymphoma: correlation between FDG-PET uptake and type of cellular infiltrate. Eur J Nucl Med Mol Imaging 2009; 36 (05) 745-750
- Cerci JJ, Pracchia LF, Linardi CCG. et al. 18F-FDG PET after 2 cycles of ABVD predicts event-free survival in early and advanced Hodgkin lymphoma. J Nucl Med 2010; 51 (09) 1337-1343
- Engert A, Haverkamp H, Kobe C. et al; German Hodgkin Study Group, Swiss Group for Clinical Cancer Research, Arbeitsgemeinschaft Medikamentöse Tumortherapie. Reduced-intensity chemotherapy and PET-guided radiotherapy in patients with advanced stage Hodgkin's lymphoma (HD15 trial): a randomised, open-label, phase 3 non-inferiority trial. Lancet 2012; 379 (9828): 1791-1799
- Mikhaeel NG, Timothy AR, Hain SF, O'Doherty MJ. 18-FDG-PET for the assessment of residual masses on CT following treatment of lymphomas. Ann Oncol 2000; 11 (Suppl 1): 147-150
- Conte MJ, Bowen DA, Wiseman GA. et al. Use of positron emission tomography-computed tomography in the management of patients with chronic lymphocytic leukemia/small lymphocytic lymphoma. Leuk Lymphoma 2014; 55 (09) 2079-2084
- Mauro FR, Chauvie S, Paoloni F. et al. Diagnostic and prognostic role of PET/CT in patients with chronic lymphocytic leukemia and progressive disease. Leukemia 2015; 29 (06) 1360-1365
- Guha A, Vijan A, Agarwal U. et al. Imaging for plasma cell dyscrasias: what, when, and how?. Front Oncol 2022; 12: 825394 DOI: 10.3389/FONC.2022.825394.
- Hillengass J, Usmani S, Rajkumar SV. et al. International Myeloma Working Group consensus recommendations on imaging in monoclonal plasma cell disorders. Lancet Oncol 2019; 20 (06) e302-e312
- Ormond Filho AG, Carneiro BC, Pastore D. et al. Whole-body imaging of multiple myeloma: diagnostic criteria. Radiographics 2019; 39 (04) 1077-1097
- Lecouvet FE. Whole-body MR imaging: musculoskeletal applications. Radiology 2016; 279 (02) 345-365
- Rajkumar S, Dimopoulos M, Palumbo A. oncology JB-T lancet, 2014 undefined. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Elsevier. Accessed December 21, 2022, at: https://www.sciencedirect.com/science/article/pii/S1470204514704425
- Koutoulidis V, Fontara S, Terpos E. et al. Quantitative diffusion-weighted imaging of the bone marrow: an adjunct tool for the diagnosis of a diffuse MR imaging pattern in patients with multiple myeloma. Radiology 2017; 282 (02) 484-493
- Bergstrom DJ, Kotb R, Louzada ML, Sutherland HJ, Tavoularis S, Venner CP. Myeloma Canada Research Network Consensus Guideline Consortium. Consensus guidelines on the diagnosis of multiple myeloma and related disorders: recommendations of the myeloma Canada research network consensus guideline consortium. Clin Lymphoma Myeloma Leuk 2020; 20 (07) e352-e367
- Nanni C. PET/CT with standard non-FDG tracers in multiple myeloma. Mol Imaging Mult Myeloma 2019; 93–97 DOI: 10.1007/978-3-030-19019-4_7.
- Messiou C, Hillengass J, Delorme S. et al. Guidelines for acquisition, interpretation, and reporting of whole-body MRI in myeloma: myeloma response assessment and diagnosis system (MY-RADS). Radiology 2019; 291 (01) 5-13
- Van Heertum RL, Scarimbolo R, Wolodzko JG. et al. Lugano 2014 criteria for assessing FDG-PET/CT in lymphoma: an operational approach for clinical trials. Drug Des Devel Ther 2017; 11: 1719-1728


 PDF
PDF  Views
Views  Share
Share

