Imaging Recommendations for Diagnosis, Staging, and Management of Gastric Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(01): 066-070
DOI: DOI: 10.1055/s-0042-1759715
Abstract
Gastric cancer is the second most common cause of cancer-related death in Indian men and women aged between 15 and 44 years. Most patients present at an advanced stage of disease. Surgically resectable disease usually requires a standard gastric resection and D2 lymphadenectomy. Imaging, especially with computed tomography scan of abdomen as well as thorax, is necessary for localization, nodal mapping, and metastatic workup of gastric cancer. In this review, we discuss current imaging recommendations for gastric carcinoma.
Publication History
Article published online:
06 March 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Gastric cancer is the second most common cause of cancer-related death in Indian men and women aged between 15 and 44 years. Most patients present at an advanced stage of disease. Surgically resectable disease usually requires a standard gastric resection and D2 lymphadenectomy. Imaging, especially with computed tomography scan of abdomen as well as thorax, is necessary for localization, nodal mapping, and metastatic workup of gastric cancer. In this review, we discuss current imaging recommendations for gastric carcinoma.
Introduction
Gastric cancer is the second most common cause of cancer-related death in Indian men and women aged between 15 and 44 years.[1] Most patients present at an advanced stage of disease. Surgically resectable disease usually requires a standard gastric resection and D2 lymphadenectomy. Imaging, especially with computed tomography (CT) scan of abdomen as well as thorax, is necessary for localization, nodal mapping, and metastatic workup of gastric cancer.
Risk Factors and Etiopathogenesis
Risk factors may differ for proximal and distal gastric cancers. The important risk factors include gastric adenomas or dysplasia, chronic atrophic gastritis, previous gastric surgery, Helicobacter pylori infection, high intake of pickled, smoked, salted, or preserved foods, smoking and alcohol consumption, obesity, and family history.[2] [3] [4]
Epidemiology and Clinical Presentation—India and Global
Gastric carcinoma is currently the fifth most common cancer worldwide and accounts for 8.2%-of all cancer-related deaths globally.[5] There is substantial geographic variation in gastric cancer incidence. High age-standardized incidence rate is seen in the high-income Asia Pacific region (Japan, South Korea), with incidences of 29.5 per thousand population, followed by Eastern Europe and Andean Latin America. In contrast, India has relatively low rates of gastric carcinoma, with an age-standardized incidence rate of 7.5 per 100,000 population.[6] Gastric cancer is the second most common cause of cancer-related death in Indian men and women aged between 15 and 44 years.[1] Highest incidence is reported from north-eastern and southern parts of India.[7] Most patients present at an advanced stage of disease. Standard gastric resection and D2 lymphadenectomy offer the best chance of survival. The overall survival for gastric carcinoma is poor and the 5-year survival rate with surgical treatment alone ranges between 23 and 25%.[8]
Clinical features of gastric carcinoma include weight loss, persistent abdominal pain, dysphagia (proximal tumors), gastric outlet obstruction and/or vomiting (distal tumors), occult gastrointestinal bleeding with or without iron deficiency anemia, and signs or symptoms of distant metastases that include palpable nodes such as left supraclavicular node (Virchow's node), periumbilical nodes (Sister Mary Joseph node), and left axillary node (Irish node). Patients may also present with ascites from peritoneal spread.
Diagnostic Workup
Other than history, physical examination, and cross-sectional imaging, the diagnostic workup of suspected gastric cancer includes:
Complete blood count and comprehensive chemistry profile.
Endoscopy and biopsy. In case of metastatic disease, human epidermal growth factor receptor 2 (HER-2), Programmed cell death protein 1 (PD-1) and Microsatellite instability (MSI) testing are recommended. Histology should be reported according to the World Health Organization criteria. A histopathology confirmation is mandatory before definitive treatment.
Biopsy of metastatic disease, as clinically indicated.
Staging laparoscopy: Staging laparoscopy can upstage up to 30%-of tumors. It is indicated for stage IB to III gastric cancer (as assessed by clinicoradiological examination) to determine treatment intent before commencement of neoadjuvant therapy.[9] It is desirable to collect peritoneal washings during laparoscopy.[10]
Imaging Guidelines
Screening
Some countries with high incidence of gastric carcinoma (such as Japan) have national screening programs. These programs allow early diagnosis of gastric carcinoma when the disease is potentially curable.[11] Japanese guidelines recommend screening endoscopy for adults more than 50 years of age.[12]
Diagnosis and Staging
Goal of Imaging
Identify resectable disease.
Plan resection:
Siewert classification (Location of midpoint of the tumor in relation to the gastroesophageal junction).
Determine nodal involvement (N+ or not) and nonregional, metastatic nodes.
Vascular and root of mesentery encasement. Encasement of aorta or its major branches, except splenic artery, is a contraindication to curative surgery.
Identify and assess the burden of metastases.
Response assessment (following neoadjuvant chemotherapy or palliative chemotherapy)
Identify postoperative complications.
Imaging Methods
Endoscopic ultrasound is the most accurate preoperative staging modality of early gastric carcinoma with an accuracy ranging between 78 and 94%.[13]
Method of choice of cross-sectional imaging for staging of gastric cancer is contrast-enhanced CT (CECT) scan thorax including lower neck, abdomen, and pelvis (CT TAP). CT scans perform better at T-staging of advanced (T3 and T4) carcinomas versus early (T1 and T2) carcinomas.
Positron emission tomography (PET-CT) evaluation from skull base to midthigh is recommended in locally advanced gastric cancer for metastatic workup especially if metastases are not evident on conventional CECT. Routine use of 18F-fluorodeoxyglucose (FDG) PET-CT offers no significant incremental value over and above CECT as up to one third of cases of gastric cancer are not FDG avid.[7] In one retrospective study, only a small percentage of nodes were spotted in PET-CT that were not identified by conventional staging CT.[14] Some studies support the use of PET in gastric cancer staging, particularly in characterizing distant metastases or lymphatic metastases beyond D1 or D2 compartment. In postoperative cases with suspected recurrence, equivocal findings on CECT can be better characterized with the added metabolic information of FDG-PET as disease recurrence may be difficult to identify in some cases due to altered anatomy.
Technique of CECT
CECT for gastric cancer is done in two phases: noncontrast and portal venous phase ([Table 1]). An additional arterial phase is optional and may be helpful in the evaluation of arterial anatomy and detection of very early lesions. Iodinated contrast media (Iodine concentration 300/320/350) is usually given as intravenous (IV) contrast at a dose of 1.5 mL/ kg body weight through the antecubital vein at a rate of 3 mL/s. Neutral or negative oral contrast is preferred for optimal distension of stomach and duodenum. Approximately 1,000 to 1,200 mL of plain water usually provide sufficient distension. Injection Buscopan is not recommended.
|
Noncontrast |
Arterial |
Portal venous |
|
|---|---|---|---|
|
Purpose |
Baseline |
Vascular anatomy for surgical planning. Delineation of early tumors |
Extent of tumor, liver metastases, other metastases |
|
Area covered |
Xiphisternum to symphysis pubis |
Xiphisternum to iliac crest |
Xiphisternum to symphysis pubis |
|
FOV (mm) |
422–500 |
380 |
450 |
|
kV |
100 |
100 |
100 |
|
mAs |
Auto |
Auto |
Auto |
|
Slice thickness (mm) |
5 |
5 |
5 |
|
Interslice gap (mm) |
5 |
5 |
5 |
|
Reconstruction thickness (mm) |
1 |
1 |
1 |
|
Reconstruction interval (mm) |
0.5 |
0.5 |
0.5 |
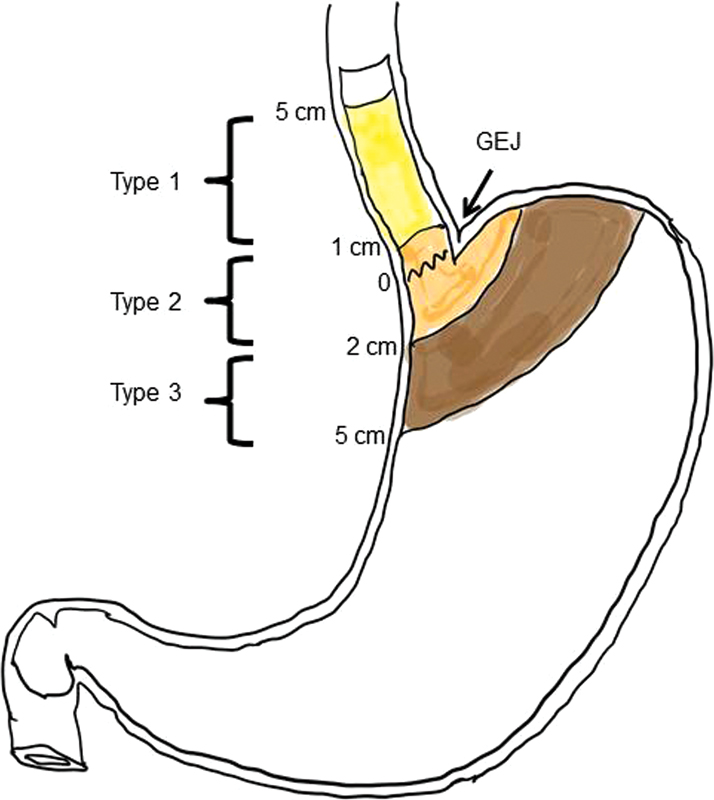
| Figure 1:Siewert classification of gastroesophageal junction (GEJ) carcinoma. Type I, epicenter of the lesion 1 to 5 cm above the GEJ; type II, epicenter of the lesion within a point 1 cm above to a point 2 cm below the GEJ; and, type III, epicenter of the lesion is 2 to 5 cm below GEJ (arrow).
Extent
Focal, segmental, or diffuse.
Size
Three dimensions of focal lesions; maximum length of the involved segment in segmental lesion.
Relationship with adjacent structures: Involvement of surrounding structures especially gastrohepatic ligament, duodenum, pancreas, left adrenal, and colon.
Identify periarterial cuffing /thickening along celiac axis and its branches/identify small perigastric veins and extramural venous invasion; optional).[15]
Nodal Status
Lymphatic spread is found in 74 to 88%-of gastric cancers at diagnosis.[16] Presence of nodes in preoperative staging warrants perioperative chemotherapy and indicates higher chance of local recurrence. In staging CT, nodes larger than 6 to 8 mm in the short axis are considered significant.[17]
Radiology report should mention the location and approximate number of significant nodes. Dimensions of the largest node are mentioned in two axes. Nodes can be described in two large groups.
Regional (D1 and D2 nodes): Perigastric, along left gastric artery, common hepatic artery, celiac artery, splenic artery, splenic hilar and hepatoduodenal nodes. Superior mesenteric vein nodes are also considered regional node.[17]
Nonregional nodes: para-aortic, aortocaval, mediastinal, and left supraclavicular nodes.
Metastasis
Liver, omentum, peritoneum, lungs, bone, ovaries, and rectovesical pouch.
Presence of ascites and if present, its predominant location and nature (attenuation and internal septations).
Synchronous primary lesion elsewhere in the esophagus or stomach.
Arterial Anatomy
Celiac artery and its branches and any anatomic arterial variation thereof is desirable to be mentioned in the preoperative evaluation.
Chemotherapy Response Assessment
Assessment of response following perioperative chemotherapy is currently performed with multidetector computed tomography (MDCT) and/or FDG-PET/CT. On CT, the Response Evaluation Criteria in Solid Tumors (RECIST) criteria is considered the method of choice in the assessment of response; however, the primary gastric tumor has been considered unmeasurable according to RECIST. Response assessment focuses on short axis measurement of involved lymph nodes and exclusion of disease progression. CT tumor volumetry (TV) to accurately measure the primary tumor is shown to be useful. A 15%-reduction in tumor volume evaluated with MDCT has been shown to correlate with histologic response.[18] FDG-PET/CT is not routinely used for treatment response. More evidence is needed to use Positron Emission Tomography (PET) Response Criteria in Solid Tumors criteria in chemotherapy follow-up.[19]
Postoperative Imaging
Most advanced gastric carcinoma requires neoadjuvant chemotherapy followed by proximal, distal, subtotal or total gastrectomy, depending upon site and location of malignancy. Other than response evaluation following surgery and subsequent chemotherapy, if any, imaging is necessary in cases in immediate or early postoperative period.
Gastrectomy with D2 lymph node clearance is associated with postoperative complications such as bleeding, anastomotic leak, sepsis, duodenal blow out, intestinal obstruction, and pulmonary complications. If the patient deviates from the normal recovery pathway in the postoperative period, then the patient may require imaging.
Often a noncontrast CT of abdomen and pelvis is sufficient. Oral contrast is given when obstruction or leak is suspected. IV contrast is given when bleeding is suspected.
Reporting checklist of a postoperative CT in gastric cancer includes:
Pleural effusion or basal atelectasis.
Any collection at perioperative site or anastomotic site.
Status of bowel (small/large bowel) dilatation/narrowing.
Area of stenosis or narrowing or abnormal wall thickening in anastomotic site or bowel and to look for normal passage of oral contrast.
Look for a stoma site, if any.
Look for intra-abdominal drains and their position.
Ascites.
Principles of Management
Surgery
Resectable Lesions
The standard oncological resection for gastric cancer involves resection of at least two-thirds of the stomach along with D2 lymph node clearance. The type of resection depends on the location of the tumor, which includes total gastrectomy (includes cardia and pylorus), distal gastrectomy (two-thirds of distal stomach), and proximal gastrectomy (including gastro-esophageal junction).[20] A proximal resection margin of at least 3 cm is recommended for mass-forming and ulcerative lesions and of at least 5 cm of the same for infiltrative lesions. En bloc resection of the gastric cancer along with resection of left lateral section of liver, spleen, tail of pancreas, diaphragm can be done to achieve R0 resection. However, in cases of involvement of the second portion of duodenum, an extended resection of the head or body of the pancreas or the hepatoduodenal ligament is not recommended.
Nodal resection: D1: perigastric nodes; D2: nodes along left gastric artery (LGA), common hepatic artery (CHA), splenic artery, and celiac axis.
Splenectomy is indicated when there is direct splenic involvement from a greater curvature tumor.
For selected cases of peritoneal carcinomatosis (low volume peritoneal metastases or isolated cytology positive), a multimodal and aggressive treatment, including neoadjuvant chemotherapy (systemic, intraperitoneal, or a combination of these), curative gastrectomy, D2 lymphadenectomy along hyperthermic intraperitoneal chemotherapy can be beneficial.[21]
Palliative Surgery
Palliative surgery is only indicated for relieving symptoms like bleeding or obstruction in presence of metastases. There is no advantage of cytoreductive surgery over palliative chemotherapy in the presence of metastatic disease.[22] Gastrojejunostomy is preferred over stenting in cases of obstruction, if surgery can be done, and prognosis is reasonable. Nodal resection not indicated.
Interventional Radiology
Role of interventional radiology is limited to embolization for bleeding that is not controlled by endoscopic methods or relieving of obstruction by placement of a stent or gastrostomy tube.
Chemotherapy and Immunotherapy
In case of localized or locally advanced disease, the treatment intent becomes curative. The current standard of care is FLOT regime (fluorouracil, leucovorin, oxaliplatin, and docetaxel), which comprises four cycles of neoadjuvant FLOT chemotherapy followed by surgery and another four cycles of FLOT. FLOT has shown to increase overall survival compared with the ECF/ECX (epirubicin-cisplatin-capecitabine), the previous standard of care (hazard ratio: 0·77; 95%-confidence interval: 0.63–0·94].[23] In case of metastatic cancer, the treatment depends on the combined positive score. If it is high, there is a role of immunotherapies like pembrolizumab/nivolumab in combination with a standard CAPOX/FOLFOX-based regimen to improve overall survival. Treatment depends on PD-L1 combined positive score. In case of low immune score, the standard of care remains chemotherapy alone.[24] [25] In the case of HER 2 positive tumors, adding trastuzumab with standard chemotherapy regimens has shown to improve survival.[26] [27]
Follow-Up Imaging
For early disease (pTi, pT1) treated by endoscopic resection, CT TAP is indicated only when there is clinical concern for recurrence. In cases of pathological (Yp) stage I to III cases, CT TAP every 6 to 12 months is indicated for 2 years, then annually for up to 5 years.
Summary of Recommendations
Diagnosis of gastric carcinoma is done by endoscopy and biopsy.
For primary staging of early lesions, endoscopic ultrasound and/or endoscopic resection is necessary.
For primary staging of other lesions, CT TAP is recommended. PET has a limited role.
For T3 and above lesions, diagnostic laparoscopy with peritoneal washing is recommended.
In follow-up after chemotherapy and postoperative cases, the imaging method of choice is CT.
No conflict of interest has been declared by the author(s).
References
- Servarayan Murugesan C, Manickavasagam K, Chandramohan A. et al. Gastric cancer in India: epidemiology and standard of treatment. Updates Surg 2018; 70 (02) 233-239
- Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci 2010; 14 (04) 302-308
- Fock KM, Talley N, Moayyedi P. et al; Asia-Pacific Gastric Cancer Consensus Conference. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol 2008; 23 (03) 351-365
- Ramakrishna BS. Helicobacter pylori infection in India: the case against eradication. Indian J Gastroenterol 2006; 25 (01) 25-28
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020; 5 (01) 42-54
- ICMR Subcommittee on Gastric Cancer. ICMR: Consensus Document for Management of Gastric Cancer. Indian Council of Medical Research, editor. ICMR Subcommittee on Gastric Cancer. Indian Council of Medical Research; 2014;1–75
- Awiwi MO, Ramanan RV, Elshikh M, Vikram R. Imaging of gastric carcinoma. part one: diagnosis and staging. J Gastrointest Abdom Radiol. 2021; 4 (03) 194-205
- Leake PA, Cardoso R, Seevaratnam R. et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012; 15 (Suppl 1): S38-S47
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology [Internet]. [cited 2020 Nov 6]. Accessed November 17, 2022, at: https://www.nccn.org/professionals/physician_gls
- Leung WK, Wu MS, Kakugawa Y. et al; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008; 9 (03) 279-287
- Hamashima C. Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018; 48 (07) 673-683
- Kelly S, Harris KM, Berry E. et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001; 49 (04) 534-539
- Findlay JM, Antonowicz S, Segaran A. et al. Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol 2019; 29 (05) 2490-2498
- Yang YT, Dong SY, Zhao J, Wang WT, Zeng MS, Rao SX. CT-detected extramural venous invasion is corelated with presence of lymph node metastasis and progression-free survival in gastric cancer. Br J Radiol 2020; 93 (1116): 20200673
- Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136 (05) E359-E386
- Maru P, Roy B, Sen S, Chatterjee A. Imaging of gastric carcinoma. part two: lymph node mapping in gastric carcinoma. J Gastrointest Abdomin Radiol 2021; 4 (03) 206-213
- Beer AJ, Wieder HA, Lordick F. et al. Adenocarcinomas of esophagogastric junction: multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology 2006; 239 (02) 472-480
- Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol 2011; 2 (01) 39-44
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021; 24 (01) 1-21
- Sugarbaker PH, Van der Speeten K. Adjuvant HIPEC for gastric cancer. J Gastrointest Oncol 2021; 12 (Suppl 1): S18-S19
- Fujitani K, Yang HK, Mizusawa J. et al; REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016; 17 (03) 309-318
- Al-Batran SE, Homann N, Pauligk C. et al; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393 (10184): 1948-1957
- Janjigian YY, Kawazoe A, Yanez PE. et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Orthod 2021; 39 (15, suppl): 4013-4013
- Janjigian YY, Shitara K, Moehler M. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398 (10294): 27-40
- Bang YJ, Van Cutsem E, Feyereislova A. et al; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376 (9742): 687-697
- Janjigian YY, Maron
SB, Chatila WK. et al. First-line
pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an
open-label, single-arm, phase 2 trial. Lancet Oncol 2020; 21 (06) 821-831
Address for correspondence
Argha Chatterjee, MDDepartment of Radiology and Imaging, Tata Medical CenterKolkata, West Bengal 700156IndiaEmail: arghachat84@gmail.comPublication History
Article published online:
06 March 2023© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:Siewert classification of gastroesophageal junction (GEJ) carcinoma. Type I, epicenter of the lesion 1 to 5 cm above the GEJ; type II, epicenter of the lesion within a point 1 cm above to a point 2 cm below the GEJ; and, type III, epicenter of the lesion is 2 to 5 cm below GEJ (arrow).
References
- Servarayan Murugesan C, Manickavasagam K, Chandramohan A. et al. Gastric cancer in India: epidemiology and standard of treatment. Updates Surg 2018; 70 (02) 233-239
- Compare D, Rocco A, Nardone G. Risk factors in gastric cancer. Eur Rev Med Pharmacol Sci 2010; 14 (04) 302-308
- Fock KM, Talley N, Moayyedi P. et al; Asia-Pacific Gastric Cancer Consensus Conference. Asia-Pacific consensus guidelines on gastric cancer prevention. J Gastroenterol Hepatol 2008; 23 (03) 351-365
- Ramakrishna BS. Helicobacter pylori infection in India: the case against eradication. Indian J Gastroenterol 2006; 25 (01) 25-28
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018; 68 (06) 394-424
- GBD 2017 Stomach Cancer Collaborators. The global, regional, and national burden of stomach cancer in 195 countries, 1990-2017: a systematic analysis for the Global Burden of Disease study 2017. Lancet Gastroenterol Hepatol 2020; 5 (01) 42-54
- ICMR Subcommittee on Gastric Cancer. ICMR: Consensus Document for Management of Gastric Cancer. Indian Council of Medical Research, editor. ICMR Subcommittee on Gastric Cancer. Indian Council of Medical Research; 2014;1–75
- Awiwi MO, Ramanan RV, Elshikh M, Vikram R. Imaging of gastric carcinoma. part one: diagnosis and staging. J Gastrointest Abdom Radiol. 2021; 4 (03) 194-205
- Leake PA, Cardoso R, Seevaratnam R. et al. A systematic review of the accuracy and indications for diagnostic laparoscopy prior to curative-intent resection of gastric cancer. Gastric Cancer 2012; 15 (Suppl 1): S38-S47
- National Comprehensive Cancer Network (NCCN). NCCN clinical practice guidelines in oncology [Internet]. [cited 2020 Nov 6]. Accessed November 17, 2022, at: https://www.nccn.org/professionals/physician_gls
- Leung WK, Wu MS, Kakugawa Y. et al; Asia Pacific Working Group on Gastric Cancer. Screening for gastric cancer in Asia: current evidence and practice. Lancet Oncol 2008; 9 (03) 279-287
- Hamashima C. Systematic Review Group and Guideline Development Group for Gastric Cancer Screening Guidelines. Update version of the Japanese Guidelines for Gastric Cancer Screening. Jpn J Clin Oncol 2018; 48 (07) 673-683
- Kelly S, Harris KM, Berry E. et al. A systematic review of the staging performance of endoscopic ultrasound in gastro-oesophageal carcinoma. Gut 2001; 49 (04) 534-539
- Findlay JM, Antonowicz S, Segaran A. et al. Routinely staging gastric cancer with 18F-FDG PET-CT detects additional metastases and predicts early recurrence and death after surgery. Eur Radiol 2019; 29 (05) 2490-2498
- Yang YT, Dong SY, Zhao J, Wang WT, Zeng MS, Rao SX. CT-detected extramural venous invasion is corelated with presence of lymph node metastasis and progression-free survival in gastric cancer. Br J Radiol 2020; 93 (1116): 20200673
- Ferlay J, Soerjomataram I, Dikshit R. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015; 136 (05) E359-E386
- Maru P, Roy B, Sen S, Chatterjee A. Imaging of gastric carcinoma. part two: lymph node mapping in gastric carcinoma. J Gastrointest Abdomin Radiol 2021; 4 (03) 206-213
- Beer AJ, Wieder HA, Lordick F. et al. Adenocarcinomas of esophagogastric junction: multi-detector row CT to evaluate early response to neoadjuvant chemotherapy. Radiology 2006; 239 (02) 472-480
- Hopkins S, Yang GY. FDG PET imaging in the staging and management of gastric cancer. J Gastrointest Oncol 2011; 2 (01) 39-44
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021; 24 (01) 1-21
- Sugarbaker PH, Van der Speeten K. Adjuvant HIPEC for gastric cancer. J Gastrointest Oncol 2021; 12 (Suppl 1): S18-S19
- Fujitani K, Yang HK, Mizusawa J. et al; REGATTA study investigators. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): a phase 3, randomised controlled trial. Lancet Oncol 2016; 17 (03) 309-318
- Al-Batran SE, Homann N, Pauligk C. et al; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019; 393 (10184): 1948-1957
- Janjigian YY, Kawazoe A, Yanez PE. et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2+ metastatic gastric or gastroesophageal junction (G/GEJ) cancer: Initial findings of the global phase 3 KEYNOTE-811 study. J Clin Orthod 2021; 39 (15, suppl): 4013-4013
- Janjigian YY, Shitara K, Moehler M. et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 2021; 398 (10294): 27-40
- Bang YJ, Van Cutsem E, Feyereislova A. et al; ToGA Trial Investigators. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010; 376 (9742): 687-697
- Janjigian YY, Maron SB, Chatila WK. et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 2020; 21 (06) 821-831


 PDF
PDF  Views
Views  Share
Share

