Imaging Recommendations for Diagnosis, Staging, and Management of Esophageal Cancer
CC BY 4.0 · Indian J Med Paediatr Oncol 2023; 44(02): 229-240
DOI: DOI: 10.1055/s-0042-1760324
Abstract
Early staging and treatment initiation affect prognosis of patients with esophageal and esophagogastric junction cancer; hence, it is imperative to have knowledge of proper choice of imaging modality for staging of these patients, to effectively convey relevant imaging findings to the treating physician/surgeon. It is also essential to be aware of pertinent imaging findings that need to be conveyed to the treating physician/surgeon at staging, and after treatment, including post-therapy complications (if any), so as to provide timely management to such patients. In this article, we have provided imaging guidelines for diagnosis, staging, post-therapy response evaluation, follow-up, and assessment of post-therapy complications of esophageal and esophagogastric junction cancer in a systematic manner. Besides, risk factors and clinical workup have also been elucidated. We have also attached comprehensive staging and post-therapy contrast-enhanced computed tomography and fluorodeoxyglucose-positron emission tomography/computed tomography-based synoptic reporting formats “ECI-RADS” and “pECI-RADS,” respectively, for esophageal and esophagogastric junction cancer in the supplement, for effective communication of imaging findings between a radiologist and the treating physician/surgeon.
Keywords
esophageal cancer - imaging guidelines - staging - post-therapy - synoptic reporting formatsAuthor's Contributions
Nivedita Chakrabarty was involved in conceptualization, designing, definition of intellectual content, literature search, manuscript preparation, manuscript editing Abhishek Mahajan was involved in manuscript editing and review. Kumar Prabhash, Prachi Patil, Manoranjan Chowhan, Naveen Munmmudi, Devayani Niyogi, Deepak Dabkara, Suryaveer Singh, Ajaykumar Singh, Sanjana Devarmani, and Varun Singh Dhull contributed to manuscript preparation.
Ethical Committee Clearance
Not required as patient data not revealed.
Publication History
Article published online:
10 February 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Early staging and treatment initiation affect prognosis of patients with esophageal and esophagogastric junction cancer; hence, it is imperative to have knowledge of proper choice of imaging modality for staging of these patients, to effectively convey relevant imaging findings to the treating physician/surgeon. It is also essential to be aware of pertinent imaging findings that need to be conveyed to the treating physician/surgeon at staging, and after treatment, including post-therapy complications (if any), so as to provide timely management to such patients. In this article, we have provided imaging guidelines for diagnosis, staging, post-therapy response evaluation, follow-up, and assessment of post-therapy complications of esophageal and esophagogastric junction cancer in a systematic manner. Besides, risk factors and clinical workup have also been elucidated. We have also attached comprehensive staging and post-therapy contrast-enhanced computed tomography and fluorodeoxyglucose-positron emission tomography/computed tomography-based synoptic reporting formats “ECI-RADS” and “pECI-RADS,” respectively, for esophageal and esophagogastric junction cancer in the supplement, for effective communication of imaging findings between a radiologist and the treating physician/surgeon.
Keywords
esophageal cancer - imaging guidelines - staging - post-therapy - synoptic reporting formatsIntroduction
Esophageal cancer (EC) is the seventh most common cancer in the world (GLOBOCAN 2020).[1] [2] As per American Joint Committee on Cancer (AJCC), ECs have been divided into those located in the cervical, upper thoracic, middle thoracic, and lower thoracic esophagus (including the esophagogastric junction and up to 2 cm of gastric cardia).[3] [4] Squamous cell carcinoma (SCC) is the predominant EC subtype usually seen in the middle third and lower third of esophagus, while most of the adenocarcinomas (ACs) occur in the distal esophagus.[3] [5] Two to ten percent of all the cancers are located in the cervical esophagus and are of SCC subtype.[6] [7]
Risk Factors and Etiopathogenesis
Major risk factors for SCC include tobacco chewing and smoking and alcohol consumption. Achalasia and consumption of hot beverages are other predisposing factors. Main risk factors for AC include gastroesophageal reflux disease, Barrett's esophagus, obesity, and tobacco. Poor nutrition, mineral and vitamin deficiencies due to low intake of fruits and vegetables, radiotherapy for thoracic malignancies, and caustic ingestion predispose to both SCC and AC.[8] [9] [10] Various somatic mutations implicated in the pathogenesis of EC have been identified, and most notable of them being mutations of TP53, a major tumor-suppressor gene, and PIK3CA.[10]
Epidemiology and Clinical Presentation
EC peaks in the seventh and eighth decades with 70%-cases occurring in men.[1] [11] In the West, the incidence of SCC has declined, with AC now being the dominant subtype.[12] As per GLOBOCAN 2020 data, EC is the fifth most common cancer in terms of incidence and mortality in India, and ranks seventh and sixth, respectively, in terms of incidence and mortality worldwide.[13] [14] Patients are asymptomatic in early stages, in advanced stage, present with progressive dysphagia (solids then liquids), weight loss, hematemesis, melena, and hoarseness from recurrent laryngeal nerve involvement.[15]
Imaging Referral Guidelines
National Comprehensive Cancer Network (NCCN), European Society of Medical Oncology, AJCC, National Cancer Grid, and Indian College of Radiology and Imaging (ICRI) recommend endoscopic ultrasound (EUS) and contrast-enhanced computed tomography (CECT) with oral contrast for locoregional staging and fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) for distant metastatic workup.[4] [16] [17] [18] [19] ICRI specifically recommends the use of EUS only in early stage primary tumor evaluation of the middle third EC.[19] In addition, American College of Radiology recommends the use of single water-soluble contrast esophagogram on fluoroscopy in the immediate postoperative period to detect fistula or leaks, and suggests CT scan for negative esophagogram when clinical suspicion is high.[20] Imaging-based clinical (cTNM) staging proposed by 8th edition AJCC is followed for esophagus and gastroesophageal junction (GEJ) epithelial cancers.[4] [Fig. 1] shows the NCCN imaging algorithm and [Table 1] shows the 8th edition AJCC staging for EC.[4] [16]
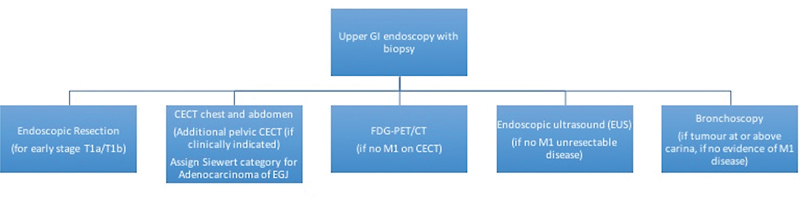
| Fig 1 :Imaging guidelines for esophageal and esophagogastric junction tumors (adapted from NCCN guidelines version 2.2022).[16] CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; GI, gastrointestinal.
EUS can differentiate the various layers of esophageal wall and has a sensitivity of 92 and 82%, respectively, for cT4 tumors and cT1 tumors.[16] [21] CECT best detects invasion of adjacent structures (pleura, pericardium, azygous vein, diaphragm, peritoneum, aorta, trachea, and vertebra).[2] [21] [22] CT has a low accuracy for N staging showing a sensitivity of 30 to 60%, specificity of 60 to 80%, and accuracy 27 to 86%-for lymph node more than 1cm.[21] Whereas the sensitivity of PET/CT for detection of locoregional nodal involvement is also low (51%).[16] EUS has a better sensitivity of 85%-than either CT or PET/CT for the detection of nodal involvement.[16] Metastasis that is occult on CT can be detected on FDG-PET/CT.[16] PET/CT has a sensitivity and specificity of 69 and 93%, respectively, for M stage.[4]
Clinical/Diagnostic Workup (Excluding Imaging)
All patients should have a thorough history and clinical examination with particular reference to weight loss. Grade III/IV dysphagia points toward bulky primary and probably T3/T4 stage. Upper gastrointestinal (GI) endoscopy with multiple biopsies is required to have sufficient tissue for histopathological examination and biomarker testing.[23] Differentiation between squamous and AC histology has prognostic and therapeutic implication.[24] In case of esophageal SCC, ear nose throat (ENT) examination to evaluate oral cavity, oropharynx, and hypopharynx should be done by an ENT specialist. In case of tumors located at or above tracheal bifurcation, a tracheobronchoscopy should be done to rule out tracheal invasion and a synchronous cancer in aerodigestive tract.[17]
In locally advanced AC of GEJ, approximately 15%-patients have peritoneal metastasis. Laparoscopy is advised in locally advanced GEJ AC to prevent futile surgeries.[25] For patients with metastatic disease, human epidermal growth factor receptor 2 testing by immunohistochemistry or fluorescence in situ hybridization for AC, mismatch repair deficiency/microsatellite instability, program death-ligand 1 (PD-L1) expression, and neurotrophic-tropomyosin receptor kinase fusion are advised to decide for targeted therapy/immunotherapy.[26]
Imaging Guidelines
a) Screening: Routine screening for EC is not recommended.
b) Diagnosis: Upper GI endoscopy-guided biopsy is used for the diagnosis of primary tumor.[23] There are two types of echoendoscopes available: radial and linear array. EUS using linear array echoendoscopes have a limited field but have the important ability to visualize a needle in real time and hence are used for tissue sampling. Endoscopy also helps in guiding nasogastric tube insertion for feeding when needed. In patients having clinically palpable and significant appearing supraclavicular node or liver metastasis, tissue diagnosis can be obtained from node or liver metastasis, when planned for palliative therapy.
c) Staging
Role of EUS
On EUS, five layers of the esophageal wall described are layer 1 (hyperechoic) superficial mucosa, layer 2 (hypoechoic) deep mucosa, layer 3 (hyperechoic) submucosa, layer 4 (hypoechoic) muscularis propria, and layer 5 (hyperechoic) adventitia. Tumors appear as hypoechoic lesions involving the wall layers, and malignant lymph nodes are typically seen in the vicinity of the tumor, appearing hypoechoic and round with smooth borders, and may be more than 10 mm in size.[27] The standard 7.5 to 12 MHz frequency transducers do not have adequate resolution to accurately “T” stage very early stage disease with mucosal involvement or superficial submucosal involvement, and a 20 MHz radial mini-probe may be needed for scanning in such cases. The accuracy of EUS increases with increasing T stage. EUS can be performed in patients with early-stage EC prior to endoscopic resection (ER) or in patients planned for upfront surgery, mainly to rule out lymph-node metastases in selected high-risk cases. Although EUS is the only modality for distinguishing different layers of esophageal wall, ER is more accurate than EUS for the staging of T1a/T1b EC and may also be therapeutic in some cases.[16] When high-definition white-light or image-enhanced endoscopy is suggestive of a small nodular lesion with high grade dysplasia or early-stage EC, a staging ER is encouraged.[16] [28] An EUS-guided fine-needle aspiration can be performed for suspicious lymph nodes if they can be sampled without traversing the tumor or major blood vessels and if the result will change management.[16] Most patients with stenotic tumors are likely to have locally advanced disease where an EUS may be unnecessary.[29] If at all an EUS is indicated in stenotic tumors, a smaller caliber wire-guided probe or mini-probe can be used; however, availability and cost may be an issue. [Fig. 2(A–C)] shows EC staging on EUS with malignant regional node.
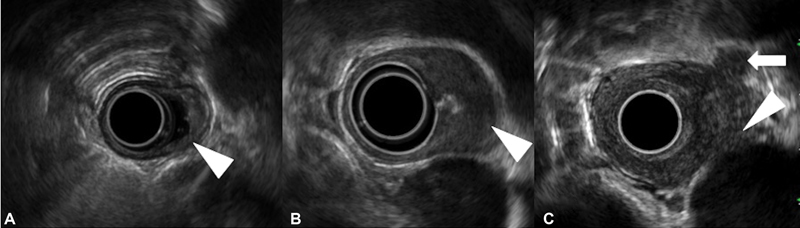
| Figure 2:Staging of esophageal cancer on endoscopic ultrasonography. (A) T1 tumor invading the submucosa (arrowhead). (B) T2 tumor invading the muscularis propria (arrowhead). (C) T4 tumor involving the aorta (arrowhead). A malignant regional lymph node is also seen (arrow).|
Role of CT
CECT thorax and abdomen with oral contrast is recommended for EC staging. CT of the pelvic region should be included for esophagogastric junction tumors or if clinically indicated. Upper third EC including cervical EC requires additional evaluation with CECT neck. CT thorax protocol for EC is shown in [Table 2].[19]
|
Modality |
Typical protocol |
|---|---|
|
CT thorax |
-Noncontrast and CECT are performed -On table positive oral contrast (500 mL water + 30 mL nonionic contrast), or plain water, to distend the esophagus. -For CECT, 100 cc of intravenous nonionic contrast (300 mg iodine per mL) at 2.5–3 mL/sec |
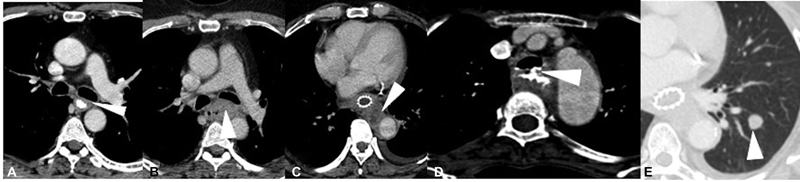
| Figure 3:Esophageal carcinoma staging on axial contrast-enhanced computed tomography. (A) Mild asymmetric mid-esophageal wall thickening (arrowhead), with no peri-adventitial infiltration and maintained fat planes with the adjacent structures (T2). (B) Mid-esophageal growth with periadventitial fat infiltration (T3) without any tracheobronchial or aortic invasion. The mass is seen to indent upon posterior wall of left mainstem bronchus (arrowhead); however, invasion was ruled out by bronchoscopy. (C) More than 90-degree arc of contact between the esophageal mass and aorta with involvement of aortic wall (arrowhead), along with soft tissue extension into the triangular fat pad indicating aortic invasion (T4b). (D) Irregular circumferential thickening involving the upper thoracic esophagus with frank fistulous communication with the tracheal lumen (arrowhead), and passage of oral positive contrast into the trachea (T4b). (E) Well defined left lower lobe nodule (arrowhead) on lung window, in a case of esophageal cancer, suggestive of lung metastasis (M1).
Role of FDG-PET/CT
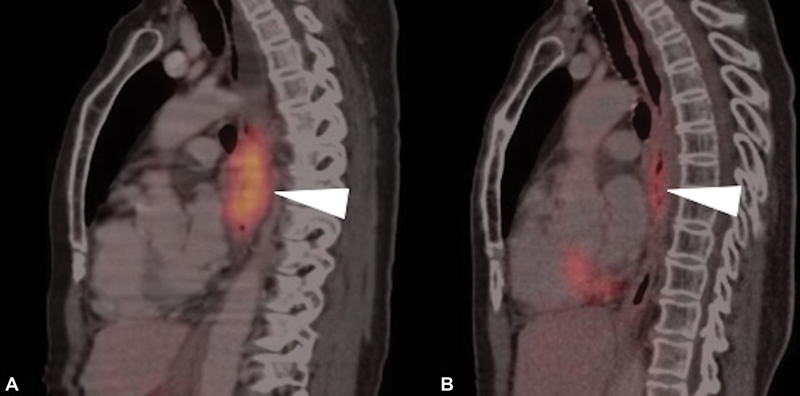
Following intravenous injection of 18F-FDG-PET-CT images are acquired from skull base to mid-thigh. Both oral and intravenous contrast is used for the acquisition of CT images. The predominant role of FDG-PET/CT is to detect distant metastases including metastasis to bones and distant nodal metastasis when no metastasis is detected on CECT scan.[16] PET-CT is the modality of choice to diagnose occult metastasis to various organs that thus help in avoiding futile surgeries.[30] [31] [32] Besides, PET-CT is also useful to detect second primary tumor and studies have indicated that FDG-PET/CT is superior to conventional anatomical imaging to evaluate synchronous tumors (especially head and neck cancers and colon neoplasm) during primary staging of esophageal SCC.[33] PET/CT also plays an important role in radiation therapy treatment planning of EC.[34] [35] PET-CT-based intensity-modulated radiation therapy offers several advantages that includes (1) dose escalation to target (tumor), (2) minimizes dose delivery to normal tissue, (3) decrease acute toxicity, (4) lessens long-term toxicity by optimizing treatment delivery to target tissue, thereby achieving better therapy outcome. [Fig. 4 (A] and [B)] shows EC staging on FDG-PET/CT.

| Figure 4:(A and B) Staging of esophageal cancer on fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) images reveals moderately FDG-avid primary esophageal mass lesion (arrowhead) with extensive FDG-avid metastatic abdominal nodes (curved arrow) and multifocal hypermetabolic hepatic (metastatic) lesions (arrow) implying M1
Role of Other Modalities
Magnetic resonance imaging (MRI) plays a pertinent role in detecting the status of spinal cord, when there is intraspinal extension of the tumor with involvement of vertebrae. Also, when there is doubt on CT regarding pericardial and aortic wall involvement, MRI can come to the rescue. One of the studies has shown that PET-MR has comparable sensitivity with EUS in staging primary esophageal involvement, and offers higher diagnostic accuracy compared with EUS and PET/CT.[36] However, due to limited availability and cost factor, PET-MR is not routinely used.
[Table 3] enlists the role of each modality in staging of EC.[2] [3] [4] [16] [22] [30] [31] [32]
|
Modality |
Role in staging |
|---|---|
|
Endoscopic resection |
-DifferentiatingT1a from T1b -Biopsy from lesion |
|
EUS |
-T staging -N staging -EUS-guided FNA of regional nodes |
|
CECT thorax and abdomen with oral contrast -Additional CECT neck if upper third (including cervical) EC -Additional CECT pelvis (if pelvic symptoms or EGJ tumors) |
-T3 stage -T4a and T4b stages -Craniocaudal dimension of the tumor -Helps to assign Siewert category for adenocarcinoma of EGJ as follows: a. Siewert type I: tumor epicenter within 1 to 5 cm above EGJ b. Siewert type II: tumor epicenter within 1 cm above and 2 cm below EGJ c. Siewert type III: tumor epicenter between 2 and 5 cm below EGJ (included under gastric carcinoma) -Metastasis to liver and lungs |
|
FDG-PET/CT |
-Distant metastasis (including bone and distant nodal metastasis) when CECT is negative for metastasis -Occult metastasis to various organs -Synchronous tumors |
|
MRI |
-Status of spinal cord in case of intraspinal extension of the tumor with involvement of vertebrae. -Pericardial involvement when doubtful on CT -Aortic involvement when doubtful on CT |
| Figure 5:(A and B) Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) images of a 50-year-old patient diagnosed as metastatic carcinoma esophagus treated with radiotherapy (RT). (A) Sagittal pretreatment PET/CT image depicts the metabolically active primary neoplastic mass lesion (arrowhead). (B) Sagittal post therapy PET/CT image after 3 months of RT reveals significant reduction in metabolic activity of primary lesion, suggestive of significant metabolic remission.
|
Modality |
Response assessment after therapy |
|---|---|
|
CECT |
≥ 30%-decrease in esophageal mural thickening (short axis) or the lymph node (short axis), post-therapy suggests partial response ≥ 20%-increase in esophageal mural thickening (short axis) or the lymph node (short axis), post-therapy suggests disease progression |
|
FDG-PET/CT |
SUVmax and MTV after concurrent chemoradiation therapy help in assessing response as well as prognosis. No definite cutoff value has been mentioned |
|
Follow-up method |
Frequency |
|---|---|
|
CECT thorax and abdomen |
• First 2 years: Every 6 months • 2–5 years: Annual • After 5 years: Only if indicated |
|
Upper GI endoscopy |
• At 3 months • 1 year • Then 3 yearly |
|
Clinical (history and physical examination) |
• First 2 years: 3 monthly • 2–5 years: 6 monthly • After 5 years: Annual |

| Figure 6:Algorithm for the management of esophageal carcinoma. NACTRT, neoadjuvant chemoradiotherapy; Periop, perioperative; SCC: Squamous cell carcinoma.
|
Location/stage of EC |
Treatment |
|---|---|
|
Upper third EC (including cervical) |
Definitive chemoradiation |
|
Tis, T1a |
Endoscopic mucosal resection |
|
Middle and lower third T1b, T2N0 or less |
Upfront surgery |
|
Middle and lower third localized disease greater than T3N0 |
NACTRT with CROSS protocol for SCC and perioperative chemotherapy with FLOT regimen for AC |
|
T4b stage |
Definitive chemoradiation |
|
Involvement of trachea, great vessels, vertebra or heart |
Palliative chemotherapy |
|
Metastatic disease |
Systemic therapy (chemotherapy and/or immunotherapy) |
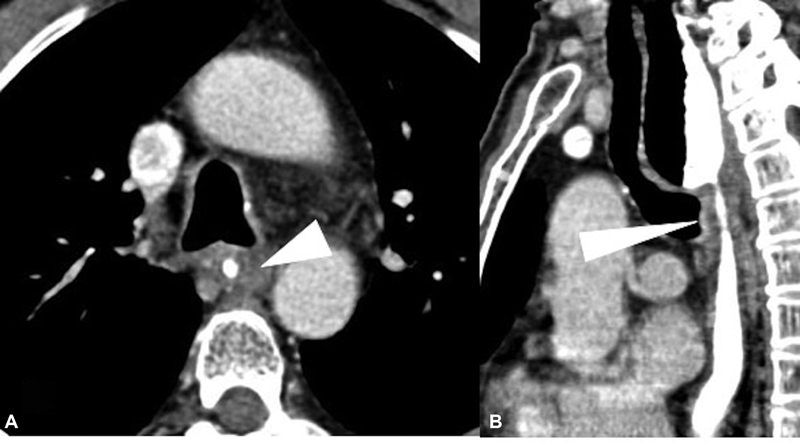
| Fig 7:(A and B) Case of squamous cell carcinoma of the middle third of the esophagus, postneoadjuvant chemoradiotherapy, presented with increasing dysphagia. Brush cytology was negative for malignancy. Axial (A) and sagittal (B) computed tomographic images show a short segment smooth circumferential wall thickening (arrowhead), with proximal esophageal dilatation.
Localized recurrences after surgery are best treated with CTRT. Residual or recurrent disease after CTRT is considered for salvage surgery. Various systemic therapy options exist for both histologies. For palliation of dysphagia, local radiation (external beam or brachytherapy), feeding procedures (nasojejunal/gastric tube), or esophageal or airway stenting (for tracheobronchial infiltration) are feasible options.
Summary of Recommendations
Differentiation of T1a and T1b is best achieved by ER.[16]
EUS is the modality of choice for T staging of EC and for regional lymph node assessment.[16]
Invasion of surrounding structures (T4 stage) is best depicted on CECT scan. Metastasis to liver and lungs and post-therapy complications are well visualized on CECT scan.[2] [3] [4] [22] [46]
FDG-PET/CT is the modality of choice for detecting distant metastasis, occult metastasis, and synchronous tumors. Besides, detection of post-therapy recurrence and response evaluation is best achieved with FDG-PET/CT.[16] [30] [31] [32] [33]
Synoptic reporting formats for pre-treatment (ECI-RADS) and post-therapy assessment (pECI-RADS), [49] along with EUS staging reporting format, are provided in the supplement.
The manuscript has been read and approved by all the authors and the requirements for authorship have been met, and each author believes that the manuscript represents honest work.
Conflicts of Interest
None declared.
Author's Contributions
Nivedita Chakrabarty was involved in conceptualization, designing, definition of intellectual content, literature search, manuscript preparation, manuscript editing Abhishek Mahajan was involved in manuscript editing and review. Kumar Prabhash, Prachi Patil, Manoranjan Chowhan, Naveen Munmmudi, Devayani Niyogi, Deepak Dabkara, Suryaveer Singh, Ajaykumar Singh, Sanjana Devarmani, and Varun Singh Dhull contributed to manuscript preparation.
Ethical Committee Clearance
Not required as patient data not revealed.
References
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Jayaprakasam VS, Yeh R, Ku GY. et al. Role of imaging in esophageal cancer management in 2020: update for radiologists. AJR Am J Roentgenol 2020; 215 (05) 1072-1084
- Kim TJ, Kim HY, Lee KW, Kim MS. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics 2009; 29 (02) 403-421
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017; 6 (02) 119-130
- Daly JM, Fry WA, Little AG. et al. Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 2000; 190 (05) 562-572 , discussion 572–573
- Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol 2016; 27 (09) 1664-1674
- Lee DJ, Harris A, Gillette A, Munoz L, Kashima H. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J 1984; 77 (11) 1365-1367
- Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21 (26) 7933-7943
- Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 2018; 41 (03) 210-215
- Watanabe M. Risk factors and molecular mechanisms of esophageal cancer: differences between the histologic subtypes. J Cancer Metastasis Treat 2015; 1: 1-7
- Choksi D, Kolhe KM, Ingle M. et al. Esophageal carcinoma: an epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J Family Med Prim Care 2020; 9 (03) 1695-1699
- Sekar A, Rajendra A, Noronha V. et al. The epidemiological trend of esophageal cancer in Mumbai, India over the past two decades. Journal of Clinical Oncology 2021; 39 (15_suppl) e16095-e16095
- Accessed December 10, 2022, at: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf
- Accessed December 10, 2022, at: https://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf
- Accessed December 10, 2022, at: https://www.msdmanuals.com/en-in/professional/gastrointestinal-disorders/tumors-of-the-gastrointestinal-tract/esophageal-cancer
- Accessed December 10, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D. ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (suppl 5): v50-v57
- Accessed December 10, 2022, at: https://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/ThoacicGuidelinesAndResearch/NCG_Esophageal_Cancer_Guidelines.pdf
- Accessed December 10, 2022, at: https://iria.org.in/oncologic-imaging-protocols-and-reporting-checklist/
- Accessed December 10, 2022, at: https://acsearch.acr.org/docs/69471/narrative/
- Elsherif SB, Andreou S, Virarkar M. et al. Role of precision imaging in esophageal cancer. J Thorac Dis 2020; 12 (09) 5159-5176
- Iyer R, Dubrow R. Imaging of esophageal cancer. Cancer Imaging 2004; 4 (02) 125-132
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349 (23) 2241-2252
- Shah MA, Kennedy EB, Catenacci DV. et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol 2020; 38 (23) 2677-2694
- Convie L, Thompson RJ, Kennedy R, Clements WD, Carey PD, Kennedy JA. The current role of staging laparoscopy in oesophagogastric cancer. Ann R Coll Surg Engl 2015; 97 (02) 146-150
- Dhakras P, Uboha N, Horner V, Reinig E, Matkowskyj KA. Gastrointestinal cancers: current biomarkers in esophageal and gastric adenocarcinoma. Transl Gastroenterol Hepatol 2020; 5: 55
- DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer?. World J Gastroenterol 2017; 23 (05) 751-762
- Mannath J, Ragunath K. Role of endoscopy in early oesophageal cancer. Nat Rev Gastroenterol Hepatol 2016; 13 (12) 720-730
- Bang JY, Ramesh J, Hasan M. et al. Endoscopic ultrasonography is not required for staging malignant esophageal strictures that preclude the passage of a diagnostic gastroscope. Dig Endosc 2016; 28 (06) 650-656
- Erasmus JJ, Munden RF. The role of integrated computed tomography positron-emission tomography in esophageal cancer: staging and assessment of therapeutic response. Semin Radiat Oncol 2007; 17 (01) 29-37 s.
- Mamede M, Abreu-E-Lima P, Oliva MR, Nosé V, Mamon H, Gerbaudo VH. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: correlation with histopathology results. Am J Clin Oncol 2007; 30 (04) 377-388
- Rustgi AK, El-Serag HB. Esophageal Carcinoma. New England Journal of Medicine 2014; 371 (26) 2499-2509
- Chen SH, Chan SC, Chao YK. et al. Detection of synchronous cancer by flurodeoxy glucose positron emission tomography/CT during primary staging work up for esophageal squamous cell carcinoma in Taiwan. PLoS One 2013; 8 (11) e82812
- Metzger JC, Wollschläger D, Miederer M. et al. Inclusion of PET-CT into planning of primary or neoadjuvant chemoradiotherapy of esophageal cancer improves prognosis. Strahlenther Onkol 2017; 193 (10) 791-799
- Zhong X, Yu J, Zhang B. et al. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2009; 73 (01) 136-141
- Lee G, Hoseok I, Seong-Jung K. et al. Clinical implication of PET-CT, MR in preoperative esophageal carcinoma staging compared with PETCT, EUS and CT. JNM 2014; 55 (08) 1242-1247
- Taniyama Y, Murakami K, Yoshida N. et al. Evaluating the effect of Neoadjuvant chemotherapy for esophageal cancer using the RECIST system with shorter-axis measurements: a retrospective multicenter study. BMC Cancer 2021; 21 (01) 1008 DOI: 10.1186/s12885-021-08747-y.
- Lee S, Choi Y, Park G. et al. 18F-FDG PET/CT parameters for predicting prognosis in esophageal cancer patients treated with concurrent chemoradiotherapy. Technol Cancer Res Treat 2021 Jan-Dec;20–15330338211024655
- Hodi FS, Ballinger M, Lyons B. et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36 (09) 850-858
- Chakrabarty N, Mahajan A, Baheti AD. et al. A radiologist's perspective on treatment-related pseudo progression: clues and hues. Indian J Med Paediatr Oncol 2022; 43 (01) 52-59
- Sudo K, Xiao L, Wadhwa R. et al. Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol 2014; 32 (30) 3400-3405
- Malik S, Sharma G, Sanaka MR, Thota PN. Role of endoscopic therapy in early esophageal cancer. World J Gastroenterol 2018; 24 (35) 3965-3973 cited 2022Mar2 [Internet]
- Al-Batran SE, Homann N, Pauligk C. et al; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393(10184):1948–1957 cited 2022Feb11 [Internet]
- Allum WH, Bonavina L, Cassivi SD. et al. Surgical treatments for esophageal cancers. Ann N Y Acad Sci 2014; 1325 (01) 242-268 cited 2022Mar2 [Internet]
- Cancer E. Statistics | Cancer.Net [Internet]. [cited 2022 Mar 2]. Accessed December 10, 2022, at: Available from: https://www.cancer.net/cancer-types/esophageal-cancer/statistics
- Zeng C, Zhai T, Chen J. et al. Imaging biomarkers of contrast-enhanced computed tomography predict survival in oesophageal cancer after definitive concurrent chemoradiotherapy. Radiat Oncol 2021; 16 (01) 8
- Mantziari S, Pomoni A, Prior JO. et al. 18F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med Imaging 2020; 20 (01) 7
- Park J-H, Kim KY, Song H-Y. et al. Radiation-induced esophageal strictures treated with fluoroscopic balloon dilation: clinical outcomes and factors influencing recurrence in 62 patients. Acta Radiol 2018; 59 (03) 313-321
- Chakrabarty N, Mahajan A. Esophageal cancer imaging - reporting and data system (ECI-RADS) and post-therapy ECI-RADS (pECI-RADS): Comprehensive synoptic reporting formats for esophageal cancer imaging: A narrative review. Cancer Research, Statistics, and Treatment 2022;5(03)
Address for correspondence
Publication History
Article published online:
10 February 2023
© 2023. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig 1 :Imaging guidelines for esophageal and esophagogastric junction tumors (adapted from NCCN guidelines version 2.2022).[16] CECT, contrast-enhanced computed tomography; FDG-PET/CT, fluorodeoxyglucose-positron emission tomography/computed tomography; GI, gastrointestinal.

| Figure 2:Staging of esophageal cancer on endoscopic ultrasonography. (A) T1 tumor invading the submucosa (arrowhead). (B) T2 tumor invading the muscularis propria (arrowhead). (C) T4 tumor involving the aorta (arrowhead). A malignant regional lymph node is also seen (arrow).|

| Figure 3:Esophageal carcinoma staging on axial contrast-enhanced computed tomography. (A) Mild asymmetric mid-esophageal wall thickening (arrowhead), with no peri-adventitial infiltration and maintained fat planes with the adjacent structures (T2). (B) Mid-esophageal growth with periadventitial fat infiltration (T3) without any tracheobronchial or aortic invasion. The mass is seen to indent upon posterior wall of left mainstem bronchus (arrowhead); however, invasion was ruled out by bronchoscopy. (C) More than 90-degree arc of contact between the esophageal mass and aorta with involvement of aortic wall (arrowhead), along with soft tissue extension into the triangular fat pad indicating aortic invasion (T4b). (D) Irregular circumferential thickening involving the upper thoracic esophagus with frank fistulous communication with the tracheal lumen (arrowhead), and passage of oral positive contrast into the trachea (T4b). (E) Well defined left lower lobe nodule (arrowhead) on lung window, in a case of esophageal cancer, suggestive of lung metastasis (M1).

| Figure 4:(A and B) Staging of esophageal cancer on fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) images reveals moderately FDG-avid primary esophageal mass lesion (arrowhead) with extensive FDG-avid metastatic abdominal nodes (curved arrow) and multifocal hypermetabolic hepatic (metastatic) lesions (arrow) implying M1

| Figure 5:(A and B) Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) images of a 50-year-old patient diagnosed as metastatic carcinoma esophagus treated with radiotherapy (RT). (A) Sagittal pretreatment PET/CT image depicts the metabolically active primary neoplastic mass lesion (arrowhead). (B) Sagittal post therapy PET/CT image after 3 months of RT reveals significant reduction in metabolic activity of primary lesion, suggestive of significant metabolic remission.

| Figure 6:Algorithm for the management of esophageal carcinoma. NACTRT, neoadjuvant chemoradiotherapy; Periop, perioperative; SCC: Squamous cell carcinoma.

| Fig 7:(A and B) Case of squamous cell carcinoma of the middle third of the esophagus, postneoadjuvant chemoradiotherapy, presented with increasing dysphagia. Brush cytology was negative for malignancy. Axial (A) and sagittal (B) computed tomographic images show a short segment smooth circumferential wall thickening (arrowhead), with proximal esophageal dilatation.
References
- Sung H, Ferlay J, Siegel RL. et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71 (03) 209-249
- Jayaprakasam VS, Yeh R, Ku GY. et al. Role of imaging in esophageal cancer management in 2020: update for radiologists. AJR Am J Roentgenol 2020; 215 (05) 1072-1084
- Kim TJ, Kim HY, Lee KW, Kim MS. Multimodality assessment of esophageal cancer: preoperative staging and monitoring of response to therapy. Radiographics 2009; 29 (02) 403-421
- Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg 2017; 6 (02) 119-130
- Daly JM, Fry WA, Little AG. et al. Esophageal cancer: results of an American College of Surgeons patient care evaluation study. J Am Coll Surg 2000; 190 (05) 562-572 , discussion 572–573
- Hoeben A, Polak J, Van De Voorde L, Hoebers F, Grabsch HI, de Vos-Geelen J. Cervical esophageal cancer: a gap in cancer knowledge. Ann Oncol 2016; 27 (09) 1664-1674
- Lee DJ, Harris A, Gillette A, Munoz L, Kashima H. Carcinoma of the cervical esophagus: diagnosis, management, and results. South Med J 1984; 77 (11) 1365-1367
- Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol 2015; 21 (26) 7933-7943
- Huang FL, Yu SJ. Esophageal cancer: Risk factors, genetic association, and treatment. Asian J Surg 2018; 41 (03) 210-215
- Watanabe M. Risk factors and molecular mechanisms of esophageal cancer: differences between the histologic subtypes. J Cancer Metastasis Treat 2015; 1: 1-7
- Choksi D, Kolhe KM, Ingle M. et al. Esophageal carcinoma: an epidemiological analysis and study of the time trends over the last 20 years from a single center in India. J Family Med Prim Care 2020; 9 (03) 1695-1699
- Sekar A, Rajendra A, Noronha V. et al. The epidemiological trend of esophageal cancer in Mumbai, India over the past two decades. Journal of Clinical Oncology 2021; 39 (15_suppl) e16095-e16095
- Accessed December 10, 2022, at: https://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf
- Accessed December 10, 2022, at: https://gco.iarc.fr/today/data/factsheets/cancers/6-Oesophagus-fact-sheet.pdf
- Accessed December 10, 2022, at: https://www.msdmanuals.com/en-in/professional/gastrointestinal-disorders/tumors-of-the-gastrointestinal-tract/esophageal-cancer
- Accessed December 10, 2022, at: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Lordick F, Mariette C, Haustermans K, Obermannová R, Arnold D. ESMO Guidelines Committee. Oesophageal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27 (suppl 5): v50-v57
- Accessed December 10, 2022, at: https://tmc.gov.in/ncg/docs/PDF/DraftGuidelines/ThoacicGuidelinesAndResearch/NCG_Esophageal_Cancer_Guidelines.pdf
- Accessed December 10, 2022, at: https://iria.org.in/oncologic-imaging-protocols-and-reporting-checklist/
- Accessed December 10, 2022, at: https://acsearch.acr.org/docs/69471/narrative/
- Elsherif SB, Andreou S, Virarkar M. et al. Role of precision imaging in esophageal cancer. J Thorac Dis 2020; 12 (09) 5159-5176
- Iyer R, Dubrow R. Imaging of esophageal cancer. Cancer Imaging 2004; 4 (02) 125-132
- Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med 2003; 349 (23) 2241-2252
- Shah MA, Kennedy EB, Catenacci DV. et al. Treatment of locally advanced esophageal carcinoma: ASCO guideline. J Clin Oncol 2020; 38 (23) 2677-2694
- Convie L, Thompson RJ, Kennedy R, Clements WD, Carey PD, Kennedy JA. The current role of staging laparoscopy in oesophagogastric cancer. Ann R Coll Surg Engl 2015; 97 (02) 146-150
- Dhakras P, Uboha N, Horner V, Reinig E, Matkowskyj KA. Gastrointestinal cancers: current biomarkers in esophageal and gastric adenocarcinoma. Transl Gastroenterol Hepatol 2020; 5: 55
- DaVee T, Ajani JA, Lee JH. Is endoscopic ultrasound examination necessary in the management of esophageal cancer?. World J Gastroenterol 2017; 23 (05) 751-762
- Mannath J, Ragunath K. Role of endoscopy in early oesophageal cancer. Nat Rev Gastroenterol Hepatol 2016; 13 (12) 720-730
- Bang JY, Ramesh J, Hasan M. et al. Endoscopic ultrasonography is not required for staging malignant esophageal strictures that preclude the passage of a diagnostic gastroscope. Dig Endosc 2016; 28 (06) 650-656
- Erasmus JJ, Munden RF. The role of integrated computed tomography positron-emission tomography in esophageal cancer: staging and assessment of therapeutic response. Semin Radiat Oncol 2007; 17 (01) 29-37 s.
- Mamede M, Abreu-E-Lima P, Oliva MR, Nosé V, Mamon H, Gerbaudo VH. FDG-PET/CT tumor segmentation-derived indices of metabolic activity to assess response to neoadjuvant therapy and progression-free survival in esophageal cancer: correlation with histopathology results. Am J Clin Oncol 2007; 30 (04) 377-388
- Rustgi AK, El-Serag HB. Esophageal Carcinoma. New England Journal of Medicine 2014; 371 (26) 2499-2509
- Chen SH, Chan SC, Chao YK. et al. Detection of synchronous cancer by flurodeoxy glucose positron emission tomography/CT during primary staging work up for esophageal squamous cell carcinoma in Taiwan. PLoS One 2013; 8 (11) e82812
- Metzger JC, Wollschläger D, Miederer M. et al. Inclusion of PET-CT into planning of primary or neoadjuvant chemoradiotherapy of esophageal cancer improves prognosis. Strahlenther Onkol 2017; 193 (10) 791-799
- Zhong X, Yu J, Zhang B. et al. Using 18F-fluorodeoxyglucose positron emission tomography to estimate the length of gross tumor in patients with squamous cell carcinoma of the esophagus. Int J Radiat Oncol Biol Phys 2009; 73 (01) 136-141
- Lee G, Hoseok I, Seong-Jung K. et al. Clinical implication of PET-CT, MR in preoperative esophageal carcinoma staging compared with PETCT, EUS and CT. JNM 2014; 55 (08) 1242-1247
- Taniyama Y, Murakami K, Yoshida N. et al. Evaluating the effect of Neoadjuvant chemotherapy for esophageal cancer using the RECIST system with shorter-axis measurements: a retrospective multicenter study. BMC Cancer 2021; 21 (01) 1008 DOI: 10.1186/s12885-021-08747-y.
- Lee S, Choi Y, Park G. et al. 18F-FDG PET/CT parameters for predicting prognosis in esophageal cancer patients treated with concurrent chemoradiotherapy. Technol Cancer Res Treat 2021 Jan-Dec;20–15330338211024655
- Hodi FS, Ballinger M, Lyons B. et al. Immune-modified response evaluation criteria in solid tumors (imRECIST): refining guidelines to assess the clinical benefit of cancer immunotherapy. J Clin Oncol 2018; 36 (09) 850-858
- Chakrabarty N, Mahajan A, Baheti AD. et al. A radiologist's perspective on treatment-related pseudo progression: clues and hues. Indian J Med Paediatr Oncol 2022; 43 (01) 52-59
- Sudo K, Xiao L, Wadhwa R. et al. Importance of surveillance and success of salvage strategies after definitive chemoradiation in patients with esophageal cancer. J Clin Oncol 2014; 32 (30) 3400-3405
- Malik S, Sharma G, Sanaka MR, Thota PN. Role of endoscopic therapy in early esophageal cancer. World J Gastroenterol 2018; 24 (35) 3965-3973 cited 2022Mar2 [Internet]
- Al-Batran SE, Homann N, Pauligk C. et al; FLOT4-AIO Investigators. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet 2019;393(10184):1948–1957 cited 2022Feb11 [Internet]
- Allum WH, Bonavina L, Cassivi SD. et al. Surgical treatments for esophageal cancers. Ann N Y Acad Sci 2014; 1325 (01) 242-268 cited 2022Mar2 [Internet]
- Cancer E. Statistics | Cancer.Net [Internet]. [cited 2022 Mar 2]. Accessed December 10, 2022, at: Available from: https://www.cancer.net/cancer-types/esophageal-cancer/statistics
- Zeng C, Zhai T, Chen J. et al. Imaging biomarkers of contrast-enhanced computed tomography predict survival in oesophageal cancer after definitive concurrent chemoradiotherapy. Radiat Oncol 2021; 16 (01) 8
- Mantziari S, Pomoni A, Prior JO. et al. 18F- FDG PET/CT-derived parameters predict clinical stage and prognosis of esophageal cancer. BMC Med Imaging 2020; 20 (01) 7
- Park J-H, Kim KY, Song H-Y. et al. Radiation-induced esophageal strictures treated with fluoroscopic balloon dilation: clinical outcomes and factors influencing recurrence in 62 patients. Acta Radiol 2018; 59 (03) 313-321
- Chakrabarty N, Mahajan A. Esophageal cancer imaging - reporting and data system (ECI-RADS) and post-therapy ECI-RADS (pECI-RADS): Comprehensive synoptic reporting formats for esophageal cancer imaging: A narrative review. Cancer Research, Statistics, and Treatment 2022;5(03)


 PDF
PDF  Views
Views  Share
Share

