How I Treat Neuroendocrine Tumors
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2021; 42(05): 470-478
DOI: DOI: 10.1055/s-0041-1732833
How I Treat
How I Treat Neuroendocrine Tumors
Financial Disclosures?A.C. receives research support from BMS, Clovis Oncology, EMD Serono, Nanopharmaceuticals, Tersera, ECS Progastrin. Advisor: Novartis, Ipsen, TerSera, Lexicon, Seneca Therapeutics, and ECS Progastrin. R.A.R. serves as a speaker for Merck & Co., Inc., Advanced Accelerator Applications, Genentech, Inc., AstraZeneca, and Ipsen Biopharmaceuticals, Inc. Consultant for Curium Pharma, Advanced Accelerator Applications, AstraZeneca, Ipsen Biopharmaceuticals and Novartis Pharmaceuticals, Corp. Research funding from Aadi Biosciences, and Merck & Co., Inc. Other coauthors do not have relevant disclosures.
Introduction
Neuroendocrine tumors (NETs) originate from diffuse neuroendocrine cell system and can develop in many organs. Gastroenteropancreatic (GEP) NETs account for approximately 70%, followed by bronchopulmonary and thymic NETs.[1] The World Health Organization (WHO) classification divides GEP NETs into well-differentiated NETs and poorly differentiated neuroendocrine carcinoma (NEC). Well-differentiated NETs can be grade 1 (G1; mitotic count <2 xss=removed>i-67 < 3 xss=removed>i-67: 3?20%) tumors, and G3 (mitotic count >20, Ki-67 > 20%).[2] Poorly differentiated NECs are always G3 tumors with >20 mitotic count and Ki-67 index >20% and include small- and large-cell NECs.[2] A total of 10 to 13% of NETs do not have a primary site identified at the time of diagnosis and are called NETs of unknown primary.[1] [3] NETs can also be differentiated based on the secretion of vasoactive amines and hormones into functional (30%) and nonfunctional NETs (70%).[1] This article focuses on the management of well-differentiated NETs with attention to systemic therapy. Factors influencing initial medical decision-making in NET management include functional status, stage, and grade, burden of metastatic disease, and symptoms at presentation.
Publication History
Publication Date:
23 September 2021 (online)
? 2021. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
How I Treat Neuroendocrine Tumors
Introduction
Neuroendocrine tumors (NETs) originate from diffuse neuroendocrine cell system and can develop in many organs. Gastroenteropancreatic (GEP) NETs account for approximately 70%, followed by bronchopulmonary and thymic NETs.[1] The World Health Organization (WHO) classification divides GEP NETs into well-differentiated NETs and poorly differentiated neuroendocrine carcinoma (NEC). Well-differentiated NETs can be grade 1 (G1; mitotic count <2 xss=removed>i-67 < 3 xss=removed>i-67: 3?20%) tumors, and G3 (mitotic count >20, Ki-67 > 20%).[2] Poorly differentiated NECs are always G3 tumors with >20 mitotic count and Ki-67 index >20% and include small- and large-cell NECs.[2] A total of 10 to 13% of NETs do not have a primary site identified at the time of diagnosis and are called NETs of unknown primary.[1] [3] NETs can also be differentiated based on the secretion of vasoactive amines and hormones into functional (30%) and nonfunctional NETs (70%).[1] This article focuses on the management of well-differentiated NETs with attention to systemic therapy. Factors influencing initial medical decision-making in NET management include functional status, stage, and grade, burden of metastatic disease, and symptoms at presentation.
Case 1
A 61-year-old female presented with right hip pain, diarrhea, and weight loss over the past few months. Pelvic X-ray showed lytic lesions in right femoral acetabulum. Computerized tomography (CT) of abdomen showed multiple liver lesions and additional bony metastasis in ribs. Biopsy of a liver lesion showed a metastatic G2 well-differentiated NET with Ki-67 of 10%.
Diagnosis
CT and/or magnetic resonance (MR) scans are the commonly utilized imaging modalities for initial evaluation. Multiphasic CT/MRI is helpful in evaluating liver metastasis since NETs are highly vascular and can appear isodense on conventional scans. NETs with unknown primary site should be additionally evaluated with upper and lower endoscopy with attention to the terminal ileum or by CT enterography. Evaluation with somatostatin receptor (SSR)-based imaging, like 68-Ga-DOTATATE positron emission tomography (PET), or Cu-64-DOTATATE (preferred over Indium-111-pentetreotide SPECT), is utilized to assess receptor status for determining benefit of SSR-directed therapy and evaluate suspected metastasis if unclear on initial imaging.
When symptoms are suggestive of carcinoid syndrome (unclear in this case), the initial biochemical evaluation of choice is 24-hour urinary excretion of 5-hydroxyindoleacetic acid (5-HIAA). The test has 90% sensitivity and 90% specificity to detect carcinoid syndrome.[4] Urinary 5-HIAA level in carcinoid syndrome was found to range between 99 and 2,070 mg/day; however, lower levels may be present with foregut and hindgut tumors. Chromogranin and serotonin levels lack sensitivity and specificity; however, it is useful in foregut, rectal, and pancreatic NETs where 5-HIAA level is not usually elevated.[5] Plasma 5-HIAA level has not been well validated. Serum VIP (VIPoma), glucagon (glucagonoma), gastrin (gastrinoma), and insulin/pro-insulin/C-peptide (insulinoma) levels are helpful in functional pancreatic NETs.
? 24-hour urine 5-HIAA level was 928 mg/day. While awaiting further workup, what treatment should be given for her symptomatic disease?
Management of Symptoms of Hormone Secretion
The most common symptoms from functional NETs are flushing and diarrhea and are associated with elevated urinary 5-HIAA. Since 80% of well-differentiated gastrointestinal (GI)-NETs express SSR, somatostatin analogs (SSA)-like octreotide and lanreotide are highly effective in controlling the symptoms. Initial therapy should be with octreotide of 50 to 750 ?g/day, two to four times a day subcutaneously (typically started at 100?150 ?g thrice a day with some patients requiring up to 1,500 ?g/day, although data limited). This not only provides rapid symptomatic relief but also acts as test dose before initiating long-acting depot. After 1 to 2 weeks on short-acting SSA confirming symptomatic relief and absence of adverse reactions, we initiate long-acting depot injections starting with Octreotide intramuscular (IM) depot injection (octreotide long acting release - LAR) 20 to 30 mg at every 4 weeks. Although dose can be decreased to 10-mg IM at every 4 weeks depending on the response, in our practice, we start at 30-mg IM every 4 weekly dose and usually do not deescalate due to its benefit in tumor stabilization as seen in PROMID randomized clinical trial.[6] Continue short-acting SSA for the first 2 weeks to maintain therapeutic levels. Temporary exacerbation of symptoms can be treated with additional subcutaneous injections. Lanreotide given 120-mg subcutaneous at every 4 weeks has similar efficacy and tolerability with additional progression-free survival (PFS) benefit and carcinoid syndrome control as noted in CLARINET and ELECT studies.[7] Phase-II randomized trials have not shown benefit of adding pasireotide, a second-generation SSA along to everolimus.[8] [9]
Telotristat ethyl, a serotonin synthesis inhibitor is Food and Drug Administration (FDA)-approved for the management of carcinoid syndrome diarrhea refractory to SSA and is usually very well tolerated.[10] Low-dose interferon alfa can improve symptoms in patients refractory to SSA.[11] However, the treatment is rarely used due to its high toxicity profile of fatigue, depression, and flu-like symptoms. Antidiarrheal therapy with loperamide and/or diphenoxylate-atropine should also be considered. Reducing the SSA intervals, and increasing the dose may offer some benefit.
Initial therapy for insulinomas is carbohydrates and diazoxide which inhibit hormone release. For gastrinomas, oral proton pump inhibitors should be considered. SSA can be used in all refractory disease.[12]
? CT chest showed no disease. Upper and lower GI endoscopic evaluation was unremarkable. What is the initial treatment for this patient?
Treatment
Surgery remains the mainstay for local or locoregional resectable NETs. With selective low surgical risk patients, early surgical exploration can be considered even in the setting of unknown primary.[13] There is no clear role of adjuvant radiation or chemotherapy and observation is the preferred approach. Surgical resection can also be considered in metastatic disease with refractory symptoms from hormone secretion. Resection of an asymptomatic primary site in the setting of unresectable metastases is generally not recommended.[14]
For low-volume and asymptomatic unresectable disease, watchful waiting until symptomatic disease or radiological progression is reasonable and acceptable. Consider CT scan in 3 to 4 months to assess tumor growth rate. Low-grade well-differentiated NETs with very low burden of metastatic disease which is stable on the repeat scan can be watched closely without initiating further systemic therapy and extending the scanning interval to 6 months. However, in symptomatic, functional, moderate-to-high-volume tumors, or tumors with documented radiological growth, treatment initiation should be considered.
SSAs have also been found to provide disease stabilization and PFS benefit.[6] [7] These should be considered as first-line therapy due to their favorable side-effect profile and proven benefit in randomized controlled trials. GETNE-TRASGU nomograms could be used to estimate PFS in patient receiving SSA based on other factors, like tumor location, Ki-67 index, and symptoms; however, it is not widely used in the United States.[15] Benefit of lanreotide and octreotide LAR in advanced well-differentiated nonfunctioning GEP NET was shown in randomized controlled trials (RCTs).[16] [Table 1] summarizes some key clinical trials in NETs.
|
Drug |
Indication |
Trial |
Comparison |
n |
PFS |
OS |
Comments (reference) |
MCBS score[a] |
|---|---|---|---|---|---|---|---|---|
|
Octreotide (LAR 30-mg IM q28d) |
Midgut or UP NF G1, G2 NET |
PROMID, phase III |
Placebo |
85 (1:1) |
14.3 vs. 6 mo (HR: 0.34, 95% CI: 0.20?0.59) |
84.7 vs. 83.7 mo (p = NS,107.6 mo if low tumor load) |
Did not evaluate SSR positivity. No QoL benefit[6] |
2 (3?1) |
|
Lanreotide (120-mg deep sc q28d) |
Midgut, pancreatic or UP NF NET and Ki-67 <10> |
CLARINET, phase III |
Placebo |
204 (1:1) |
NR vs. 18 mo (HR: 0.47, 95% CI: 0.30?0.73) |
No mature data |
96% patients had stable disease for 3?6 mo prior to enrollment. PFS estimate 32.8 mo. No QoL benefit[7] |
3 |
|
177Lu-DOTATATE |
Midgut NET with SSTR+, PD |
NETTER-1, phase III |
Octreotide LAR 60mg |
229 (1:1) |
NR vs. 8.4 mo (HR: 0.21, 95% CI: 0.13?0.33) |
Interim analysis HR for death 0.40, p = 0.0004 |
Can be considered for any type of SSR+ disease, improved QoL[26] [28] |
4 (3 + 1) |
|
Everolimus + octreotide LAR |
Advanced G1, G2 NET |
RADIANT-2, phase III |
Placebo + octreotide LAR |
429 (1:1) |
16.4 vs. 11.3 m (HR: 0.77, 95% CI: 0.59?1.0) |
Open-label extension (29.2 vs. 35.2 mo) |
||
|
Everolimus |
Pancreatic NET, G1 or 2 PD |
RADIANT-3, phase III |
Placebo |
407 (1:1) |
11 versus 4.6 mo (HR 0.35, 95% CI 0.27?0.45) |
44 vs. 37.7 mo (p = NS) |
85% cross-over limits OS data. No QoL data[53] |
3 |
|
Everolimus + BSC |
Midgut and lung NF NET, PD |
RADIANT-4, phase III |
Placebo + BSC |
302 (2:1) |
11 vs. 3.9 mo (HR: 0.48, 95% CI: 0.35?0.67) |
No mature data |
Preserved QoL[23] |
3 |
|
Sunitinib |
Pancreatic NET, PD |
Phase III |
Placebo |
171 (1:1) |
11.4 vs. 5.5 mo (HR: 0.42, 95% CI: 0.26?0.66) |
38.6 vs. 29.1 mo (p = NS) |
69% cross-over limits OS data[30] |
3 |
|
Pasireotide + everolimus |
Lung and thymus carcinoids, PD |
LUNA, phase II |
Everolimus |
124 (1:1:1) |
11.8 mo PFS gain |
No QoL data[8] |
2 (3?1) |
|
|
Everolimus |
Lung and thymus carcinoids, PD |
LUNA, phase II |
Pasireotide |
124 (1:1:1) |
12.5 mo PFS gain |
No QoL data[8] |
3 |
|
|
Pasireotide |
Lung and thymus carcinoids, PD |
LUNA, phase II |
Everolimus |
124 (1:1:1) |
8.5 mo PFS gain |
No QoL data[8] |
3 |
|
|
Pasireotide+ everolimus |
Well-differentiated pancreatic NET, PD |
COOPERATE-2, phase II |
Everolimus |
160 (1:1) |
16.8 vs. 16.6 mo (HR: 0.99, 95% CI: 0.64?1.54) |
No difference at 22.6 mo |
G 3/4 hyperglycemia 37% patients[9] |
|
|
Octreotide + bevacizumab |
Advanced G1, G2 NET |
SWOG S0518, phase III |
Octreotide + interferon |
427 (1:1) |
16.6 vs. 15.4 mo (HR: 0.90, 95% CI: 0.72?1.12) |
Radiologic response rate higher in Bev arm 12 vs. 4%[34] |
||
|
Pazopanib |
Well-differentiated non-pancreatic NET |
ALLIANCE A021202, phase III |
Placebo |
97:74 |
11.6 vs. 8.5 mo (HR: 0.53, p = 0.0005) |
41 vs. 42 mo (HR: 1.13, p = 0.70) |
Only abstract available[31] |
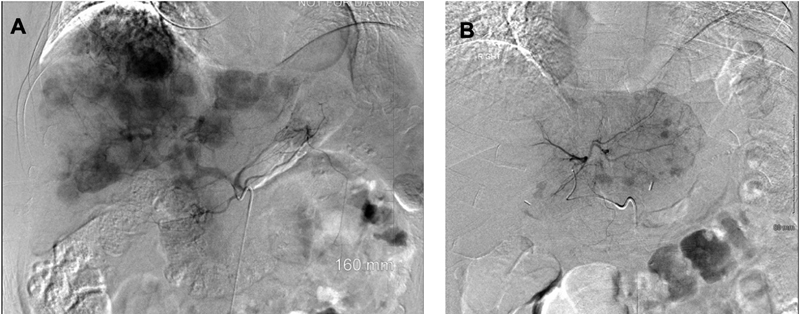
|?Fig. 1 (A) ? Celiac artery angiogram demonstrating multiple enhancing metastatic NET throughout the liver. (B) Left hepatic artery angiogram demonstrating enhancing metastatic NET in the left lobe of the liver (representative images; source: Department of Radiology, University of Kentucky). NET, neuroendocrine tumors.|
? She underwent right and left lobe liver bland embolization without complication. Also received palliative radiation therapy to the right hip. What systemic therapy is ideal now?
Progression
In functional NETs, SSAs may be continued for symptom management. Systemic therapy options on progression include everolimus, sunitinib, or Lu-DOTATATE radionuclide therapy ([Table 1]).
Everolimus improves PFS without overall survival (OS) benefit in advanced nonfunctional GI and lung NETs. Grade 1/2 skin, GI side effects, and fatigue were mainly reported.[23] In advanced G1/2 NETs, treatment with octreotide plus everolimus showed clinically meaningful prolongation of PFS but only trended toward statistical significance.[24] An open-label extension of the study failed to show any OS benefit as many patients received everolimus off-study.[25] In our experience, everolimus is active in NETs and is able to stabilize disease in most patients for several months. Oral mucositis can be prevented with prophylactic steroid mouth wash, especially during first 4 to 6 weeks of therapy and dose adjustments are often required.
Peptide receptor radionuclide therapy: The evidence of PFS benefit from radiolabeled SSA, Lu-DOTATATE was demonstrated in NETTER-1 trial with an interim analysis, also demonstrating OS benefit.[26] Objective response rate was higher among patients on peptide receptor radionuclide therapy PRRT (18 vs. 3%). The most common side effect was nausea (59%) from the amino acid infusions administered during treatment for renal protection. Mild cytopenias were also common with nadir counts expected around 4 to 6 weeks after infusion and resolved by 8 weeks.[27] Later studies also showed improved quality of life.[28] Patients with G1/2 inoperable and metastatic SSR + NET, with expected survival of >3 months, Karnofsky?s performance status >50, sufficient bone marrow reserve, and creatinine clearance >50 mL/min were included in the clinical trial and should be the ideal candidates for this treatment. The Lu-DOTATATE is acceptable as second- or third-line treatment option for metastatic progressive midgut and pancreatic NET with SSR expression, although pancreatic NETs were not studied in NETTER-1 phase-III clinical trial.[28] In our practice, we often consider?177Lu-DOTATATE as second line if patient has symptomatic disease, bone metastasis, or need for cytoreduction. Lu-DOTATATE has the most superior PFS and overall recurrence rate (ORR) data among the approved therapies for NETs. It can be potentially deferred to third- or fourth-line treatment option in patients with mesenteric disease, as PRRT often does not work well for peritoneal metastasis and in young patients due to concern for long-term myelotoxicity and less than 5% long-term risk of myelodysplasias or leukemia.[29]
The presence of SSR can be determined by diagnostic imaging using a radiolabeled SSA Indium-111 pentetreotide (OctreoScan) or PET scan using Gallium-68 DOTATATE. The higher sensitivity of the later one makes it the preferred option, especially in patients with low tumor volume. Uptake of radiolabeled isotope is predictive of response to therapy.
Tyrosine kinase inhibitors: Although sunitinib, sorafenib, surufatinib, pazopanib, lenvatinib, and cabozantinib have been evaluated in advanced GI-NET in phase-2 and -3 trials, only sunitinib is currently FDA approved for metastatic progressive pancreatic NETs.[30] [31] [32] In lung and GI-NETs, pazopanib showed PFS improvement compared with placebo without OS benefit. Nintedanib, was evaluated in a phase-2 trial for G1/2 NET and was associated with disease stabilization and delayed deterioration of quality of life.[33]
Bevacizumab had a PFS benefit when given with octreotide compared to interferon alfa in a phase-II trial.[34] However, the confirmatory larger randomized trial with 427 patients failed to show any PFS benefit.[35] We do not recommend use of bevacizumab in the management of NETs.
Interferon alfa (IFNa) is recommended only if other treatment options are unavailable due to the relatively low level of evidence of benefit and significant side-effect profile.[36] [37] Low-dose IFNa can reduce symptoms of hormonal hypersecretion and result in tumor stabilization in some patients, but tumor regression is rare.[34] [38] [39] IFNa is dosed at 3 to 5 MU three times weekly and dose should be titrated to a leukocyte count of 3,000/?L. Pegylated IFN (80?150 ?g per week subcutaneous) has better tolerability compared with IFNa.
Cytotoxic chemotherapy ([Table 2]) should be considered in patients with progressive metastatic disease with no standard approved treatment options. Regimens that have shown evidence of activity include capecitabine plus temozolomide (CAPTEM) and short-term infusional 5 FU with leucovorin plus oxaliplatin (FOLFOX); however. confirmatory studies are needed.[40] [41] Based on emerging data and toxicity profile, we consider CAPTEM as the initial chemotherapy regimen especially for G2/3 well-differentiated midgut and pancreatic NET after progression on other treatments. The poor PFS from Eastern Cooperative Oncology Group (ECOG) E1281 trial and toxicity of the drugs have questioned the use of 5 FU, streptozocin, and doxorubicin in the treatment of NET.[42]
Regimen |
Trial (year) |
n |
CR (%) |
PR (%) |
SD (%) |
mPFS (mo) |
mOS (mo) |
Comments (ref.) |
|---|---|---|---|---|---|---|---|---|
|
Capecitabine |
Phase II (2011) |
19 |
? |
? |
68 |
9.9 |
36.5 |
[54] |
|
Capecitabine + oxaliplatin |
Phase II (2007) |
27 |
? |
30 |
48 |
18 |
32 |
[55] |
|
Capecitabine + Bev |
Phase II (2014) |
49 |
? |
18 |
70 |
23.4 |
NR |
2-year OS: 85%; 82% ileal primary[56] |
|
5 FU + streptozocin 5 FU + doxorubicin 9 PD: dacarbazine |
Phase II/III ECOG E1281 (2005) |
78 85 |
2.4 |
16 13.5 8 |
15.4 15.4 |
4.5 5.3 |
24.3 11.9 |
Grades 1, 2 renal toxicity 35% with STZ[42] |
|
FOLFOX + Bev CAPEOX + Bev |
Phase II (2016) |
36 40 |
? |
25 18 |
69.4 60 |
21 19.1 |
31 42.2 |
Included 6 NEC. Did not meet 1-degree endpoint.[41] |
|
TMZ |
Retrospective (2007) |
36 |
? |
14 |
53 |
7 |
16 |
[57] |
|
TMZ |
Retrospective (2013) |
31 |
? |
14 |
52 |
5.3 |
23.2 |
Only bronchial carcinoids[58] |
|
Capecitabine + TMZ |
Retrospective (2013) |
18 |
5.5 |
55.5 |
22.2 |
14 |
83 |
Only patients with liver metastasis[59] |
|
Capecitabine + TMZ |
Phase II (2014) |
28 |
11 |
32 |
54 |
>20 |
NR |
Abstract only[60] |
|
Capecitabine + TMZ |
Retrospective (2011) |
30 |
? |
70 |
27 |
18 |
? |
2-year OS: 92%. Only pancreatic NET[61] |
|
TMZ + Bev |
Phase II (2012) |
34 |
? |
15 |
65 |
11 |
33.3 |
Most of the benefit seen in pancreatic NET[62] |
|
Pembrolizumab |
Phase I KEYNOTE-028 (2020) |
41 |
? |
9.7 |
70.7 |
5.6/4.5[a] |
? |
PDL1 positive tumors only[63] |
|
Pembrolizumab |
Phase II KEYNOTE -158 (2020) |
107 |
? |
3.7 |
56.1 |
4.1 |
24.2 |
All PR in PDL1 negative tumors.[64] |
|
Ipilimumab + Nivolumab |
Phase II DART SWOG 1609 (2020) |
32 |
3 |
22 |
41 |
4 |
11 |
Only nonpancreatic NET. 56% NEC[44] |
- Yao JC, Hassan M, Phan A. et al.?One hundred years after ?carcinoid?: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26 (18) 3063-3072
- Nagtegaal ID, Odze RD, Klimstra D. et al.?WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76 (02) 182-188
- Catena L, Bichisao E, Milione M. et al.?Neuroendocrine tumors of unknown primary site: gold dust or misdiagnosed neoplasms?. Tumori 2011; 97 (05) 564-567
- Sj?blom SM.?Clinical presentation and prognosis of gastrointestinal carcinoid tumours. Scand J Gastroenterol 1988; 23 (07) 779-787
- Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E.?Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol 2001; 12 (Suppl. 02) S69-S72
- Rinke A, Wittenberg M, Schade-Brittinger C. et al.?PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology 2017; 104 (01) 26-32
- Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA. ELECT Study Group.?Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (elect): a randomized, double-blind, placebo-controlled trial. Endocr Pract 2016; 22 (09) 1068-1080
- Ferolla P, Brizzi MP, Meyer T. et al.?Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2017; 18 (12) 1652-1664
- Kulke MH, Ruszniewski P, Van Cutsem E. et al.?A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol 2019; 30 (11) 1846
- Kulke MH, H?rsch D, Caplin ME. et al.?Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol 2017; 35 (01) 14-23
- Frank M, Klose KJ, Wied M, Ishaque N, Schade-Brittinger C, Arnold R.?Combination therapy with octreotide and alpha-interferon: effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol 1999; 94 (05) 1381-1387
-
Frankton S, Bloom SR.?

|?Fig. 1 (A) ? Celiac artery angiogram demonstrating multiple enhancing metastatic NET throughout the liver. (B) Left hepatic artery angiogram demonstrating enhancing metastatic NET in the left lobe of the liver (representative images; source: Department of Radiology, University of Kentucky). NET, neuroendocrine tumors.|
References
- Yao JC, Hassan M, Phan A. et al.?One hundred years after ?carcinoid?: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008; 26 (18) 3063-3072
- Nagtegaal ID, Odze RD, Klimstra D. et al.?WHO Classification of Tumours Editorial Board. The 2019 WHO classification of tumours of the digestive system. Histopathology 2020; 76 (02) 182-188
- Catena L, Bichisao E, Milione M. et al.?Neuroendocrine tumors of unknown primary site: gold dust or misdiagnosed neoplasms?. Tumori 2011; 97 (05) 564-567
- Sj?blom SM.?Clinical presentation and prognosis of gastrointestinal carcinoid tumours. Scand J Gastroenterol 1988; 23 (07) 779-787
- Seregni E, Ferrari L, Bajetta E, Martinetti A, Bombardieri E.?Clinical significance of blood chromogranin A measurement in neuroendocrine tumours. Ann Oncol 2001; 12 (Suppl. 02) S69-S72
- Rinke A, Wittenberg M, Schade-Brittinger C. et al.?PROMID Study Group. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors (PROMID): results of long-term survival. Neuroendocrinology 2017; 104 (01) 26-32
- Vinik AI, Wolin EM, Liyanage N, Gomez-Panzani E, Fisher GA. ELECT Study Group.?Evaluation of lanreotide depot/autogel efficacy and safety as a carcinoid syndrome treatment (elect): a randomized, double-blind, placebo-controlled trial. Endocr Pract 2016; 22 (09) 1068-1080
- Ferolla P, Brizzi MP, Meyer T. et al.?Efficacy and safety of long-acting pasireotide or everolimus alone or in combination in patients with advanced carcinoids of the lung and thymus (LUNA): an open-label, multicentre, randomised, phase 2 trial. Lancet Oncol 2017; 18 (12) 1652-1664
- Kulke MH, Ruszniewski P, Van Cutsem E. et al.?A randomized, open-label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well-differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE-2 trial. Ann Oncol 2019; 30 (11) 1846
- Kulke MH, H?rsch D, Caplin ME. et al.?Telotristat ethyl, a tryptophan hydroxylase inhibitor for the treatment of carcinoid syndrome. J Clin Oncol 2017; 35 (01) 14-23
- Frank M, Klose KJ, Wied M, Ishaque N, Schade-Brittinger C, Arnold R.?Combination therapy with octreotide and alpha-interferon: effect on tumor growth in metastatic endocrine gastroenteropancreatic tumors. Am J Gastroenterol 1999; 94 (05) 1381-1387
- Frankton S, Bloom SR.?Gastrointestinal endocrine tumours. Glucagonomas. Baillieres Clin Gastroenterol 1996; 10 (04) 697-705
- Wang YZ, Chauhan A, Rau J. et al.?Neuroendocrine tumors (NETs) of unknown primary: is early surgical exploration and aggressive debulking justifiable?. Linchuang Zhongliuxue Zazhi 2016; 5 (01) 4
- Lesurtel M, Nagorney DM, Mazzaferro V, Jensen RT, Poston GJ.?When should a liver resection be performed in patients with liver metastases from neuroendocrine tumours? A systematic review with practice recommendations. HPB (Oxford 2015; 17 (01) 17-22
- Carmona-Bayonas A, Jim?nez-Fonseca P, Lamarca ?. et al.?Prediction of progression-free survival in patients with advanced, well-differentiated, neuroendocrine tumors being treated with a somatostatin analog: the GETNE-TRASGU study. J Clin Oncol 2019; 37 (28) 2571-2580
- Caplin ME, Pavel M, ?wik?a JB. et al. CLARINET Investigators.?Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med 2014; 371 (03) 224-233
- Glazer ES, Tseng JF, Al-Refaie W. et al.?Long-term survival after surgical management of neuroendocrine hepatic metastases. HPB (Oxford 2010; 12 (06) 427-433
- Wessels FJ, Schell SR.?Radiofrequency ablation treatment of refractory carcinoid hepatic metastases. J Surg Res 2001; 95 (01) 8-12
- Berber E, Flesher N, Siperstein AE.?Laparoscopic radiofrequency ablation of neuroendocrine liver metastases. World J Surg 2002; 26 (08) 985-990
- Kennedy A, Bester L, Salem R, Sharma RA, Parks RW, Ruszniewski P. NET-Liver-Metastases Consensus Conference.?Role of hepatic intra-arterial therapies in metastatic neuroendocrine tumours (NET): guidelines from the NET-Liver-Metastases Consensus Conference. HPB (Oxford 2015; 17 (01) 29-37
- Kennedy AS, Dezarn WA, McNeillie P. et al.?Radioembolization for unresectable neuroendocrine hepatic metastases using resin 90Y-microspheres: early results in 148 patients. Am J Clin Oncol 2008; 31 (03) 271-279
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I. et al.?Liver transplantation in patients with liver metastases from neuroendocrine tumors: a systematic review. Surgery 2017; 162 (03) 525-536
- Yao JC, Fazio N, Singh S. et al.?RAD001 in Advanced Neuroendocrine Tumours, Fourth Trial (RADIANT-4) Study Group. Everolimus for the treatment of advanced, non-functional neuroendocrine tumours of the lung or gastrointestinal tract (RADIANT-4): a randomised, placebo-controlled, phase 3 study. Lancet 2016; 387 (10022) 968-977
- Pavel ME, Hainsworth JD, Baudin E. et al.?RADIANT-2 Study Group. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet 2011; 378 (9808) 2005-2012
- Pavel ME, Baudin E, ?berg KE. et al.?Efficacy of everolimus plus octreotide LAR in patients with advanced neuroendocrine tumor and carcinoid syndrome: final overall survival from the randomized, placebo-controlled phase 3 RADIANT-2 study. Ann Oncol 2017; 28 (07) 1569-1575
- Strosberg J, El-Haddad G, Wolin E. et al. NETTER-1 Trial Investigators.?Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017; 376 (02) 125-135
- Brabander T, van der Zwan WA, Teunissen JJ. et al.?Long-term efficacy, survival, and safety of [177Lu-DOTA0Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017; 23 (16) 4617-4624
- Strosberg J, Wolin E, Chasen B. et al.?NETTER-1 Study Group. Health-related quality of life in patients with progressive midgut neuroendocrine tumors treated with 177Lu-Dotatate in the phase III NETTER-1 trial. J Clin Oncol 2018; 36 (25) 2578-2584
- Sonbol MB, Halfdanarson TR, Hilal T.?Assessment of therapy-related myeloid neoplasms in patients with neuroendocrine tumors after peptide receptor radionuclide therapy: a systematic review. JAMA Oncol 2020; 6 (07) 1086-1092
- Kulke MH, Lenz HJ, Meropol NJ. et al.?Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol 2008; 26 (20) 3403-3410
- Grande E, Capdevila J, Castellano D. et al.?Pazopanib in pretreated advanced neuroendocrine tumors: a phase II, open-label trial of the Spanish Task Force Group for Neuroendocrine Tumors (GETNE. Ann Oncol 2015; 26 (09) 1987-1993
- Capdevila J, Fazio N, Lopez CL. et al.?Final results of the TALENT trial (GETNE1509): a prospective multicohort phase II study of lenvatinib in patients (pts) with G1/G2 advanced pancreatic (panNETs) and gastrointestinal (giNETs) neuroendocrine tumors (NETs. J Clin Oncol 2019; 37 (15suppl) 4106
- Iyer RV, Konda B, Fountzilas C. et al.?Multicenter phase 2 trial of nintedanib in advanced nonpancreatic neuroendocrine tumors. Cancer 2020; 126 (16) 3689-3697
- Yao JC, Phan A, Hoff PM. et al.?Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol 2008; 26 (08) 1316-1323
- Yao JC, Guthrie KA, Moran C. et al.?Phase III prospective randomized comparison trial of depot octreotide plus interferon Alfa-2b versus depot octreotide plus bevacizumab in patients with advanced carcinoid tumors: SWOG S0518. J Clin Oncol 2017; 35 (15) 1695-1703
- Strosberg JR, Halfdanarson TR, Bellizzi AM. et al.?The North American Neuroendocrine Tumor Society Consensus Guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017; 46 (06) 707-714
- Pavel M, O?Toole D, Costa F. et al. Vienna Consensus Conference participants.?ENETS consensus guidelines update for the management of distant metastatic disease of intestinal, pancreatic, bronchial neuroendocrine neoplasms (NEN) and NEN of unknown primary site. Neuroendocrinology 2016; 103 (02) 172-185
- Faiss S, Pape UF, B?hmig M. et al.?International Lanreotide and Interferon Alfa Study Group. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors?the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol 2003; 21 (14) 2689-2696
- Arnold R, Rinke A, Klose KJ. et al.?Octreotide versus octreotide plus interferon-alpha in endocrine gastroenteropancreatic tumors: a randomized trial. Clin Gastroenterol Hepatol 2005; 3 (08) 761-771
- Al-Toubah T, Morse B, Strosberg J.?Capecitabine and temozolomide in advanced lung neuroendocrine neoplasms. Oncologist 2020; 25 (01) e48-e52
- Kunz PL, Balise RR, Fehrenbacher L. et al.?Oxaliplatin-fluoropyrimidine chemotherapy plus bevacizumab in advanced neuroendocrine tumors: an analysis of 2 phase II trials. Pancreas 2016; 45 (10) 1394-1400
- Sun W, Lipsitz S, Catalano P, Mailliard JA, Haller DG. Eastern Cooperative Oncology Group.?Phase II/III study of doxorubicin with fluorouracil compared with streptozocin with fluorouracil or dacarbazine in the treatment of advanced carcinoid tumors: Eastern Cooperative Oncology Group Study E1281. J Clin Oncol 2005; 23 (22) 4897-4904
- Marabelle A, Le DT, Ascierto PA. et al.?Efficacy of pembrolizumab in patients with noncolorectal high microsatellite instability/mismatch repair-deficient cancer: results from the phase II KEYNOTE-158 study. J Clin Oncol 2020; 38 (01) 1-10
- Patel SP, Othus M, Chae YK. et al.?A phase II basket trial of dual anti-CTLA-4 and anti-PD-1 blockade in rare tumors (DART SWOG 1609) in patients with nonpancreatic neuroendocrine tumors. Clin Cancer Res 2020; 26 (10) 2290-2296
- Newman CB, Melmed S, Snyder PJ. et al.?Safety and efficacy of long-term octreotide therapy of acromegaly: results of a multicenter trial in 103 patients?a clinical research center study. J Clin Endocrinol Metab 1995; 80 (09) 2768-2775
- Coriat R, Walter T, Terris B, Couvelard A, Ruszniewski P.?Gastroenteropancreatic Well-differentiated grade 3 neuroendocrine tumors: review and position statement. Oncologist 2016; 21 (10) 1191-1199
- Basturk O, Yang Z, Tang LH. et al.?The high-grade (WHO G3) pancreatic neuroendocrine tumor category is morphologically and biologically heterogenous and includes both well differentiated and poorly differentiated neoplasms. Am J Surg Pathol 2015; 39 (05) 683-690
- Walter T, Tougeron D, Baudin E. et al. CEPD investigators.?Poorly differentiated gastro-entero-pancreatic neuroendocrine carcinomas: Are they really heterogeneous? Insights from the FFCD-GTE national cohort. Eur J Cancer 2017; 79: 158-165
- Heetfeld M, Chougnet CN, Olsen IH. et al. other Knowledge Network members.?Characteristics and treatment of patients with G3 gastroenteropancreatic neuroendocrine neoplasms. Endocr Relat Cancer 2015; 22 (04) 657-664
- Hijioka S, Hosoda W, Matsuo K. et al.?Rb Loss and KRAS mutation are predictors of the response to platinum-based chemotherapy in pancreatic neuroendocrine neoplasm with grade 3: a Japanese multicenter pancreatic NEN-G3 study. Clin Cancer Res 2017; 23 (16) 4625-4632
- Panzuto F, Merola E, Pavel ME. et al.?Stage IV gastro-entero-pancreatic neuroendocrine neoplasms: a risk score to predict clinical outcome. Oncologist 2017; 22 (04) 409-415
- Basu SPR, Ranade R, Thapa P.?Peptide receptor radionuclide therapy in the management of neuroendocrine tumors (Neoplasms)*: Fundamentals and salient clinical practice points for medical oncologists. Indian J Med Paediatr Oncol 2019; 40: 165-167
- Yao JC, Pavel M, Lombard-Bohas C. et al.?Everolimus for the treatment of advanced pancreatic neuroendocrine tumors: overall survival and circulating biomarkers from the randomized, phase III RADIANT-3 study. J Clin Oncol 2016; 34 (32) 3906-3913
- Medley L, Morel AN, Farrugia D. et al.?Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer 2011; 104 (07) 1067-1070
- Bajetta E, Catena L, Procopio G. et al.?Are capecitabine and oxaliplatin (XELOX) suitable treatments for progressing low-grade and high-grade neuroendocrine tumours?. Cancer Chemother Pharmacol 2007; 59 (05) 637-642
- Mitry E, Walter T, Baudin E. et al.?Bevacizumab plus capecitabine in patients with progressive advanced well-differentiated neuroendocrine tumors of the gastro-intestinal (GI-NETs) tract (BETTER trial)?a phase II non-randomised trial. Eur J Cancer 2014; 50 (18) 3107-3115
- Ekeblad S, Sundin A, Janson ET. et al.?Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res 2007; 13 (10) 2986-2991
- Crona J, Fanola I, Lindholm DP. et al.?Effect of temozolomide in patients with metastatic bronchial carcinoids. Neuroendocrinology 2013; 98 (02) 151-155
- Fine RL, Gulati AP, Krantz BA. et al.?Capecitabine and temozolomide (CAPTEM) for metastatic, well-differentiated neuroendocrine cancers: The Pancreas Center at Columbia University experience. Cancer Chemother Pharmacol 2013; 71 (03) 663-670
- Fine RL, Gulati AP, Tsushima D. et al.?Prospective phase II study of capecitabine and temozolomide (CAPTEM) for progressive, moderately, and well-differentiated metastatic neuroendocrine tumors. J Clin Oncol 2014; 32 (Suppl. 03) 179
- Strosberg JR, Fine RL, Choi J. et al.?First-line chemotherapy with capecitabine and temozolomide in patients with metastatic pancreatic endocrine carcinomas. Cancer 2011; 117 (02) 268-275
- Chan JA, Stuart K, Earle CC. et al.?Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol 2012; 30 (24) 2963-2968
- Mehnert JM, Bergsland E, O?Neil BH. et al.?Pembrolizumab for the treatment of programmed death-ligand 1-positive advanced carcinoid or pancreatic neuroendocrine tumors: results from the KEYNOTE-028 study. Cancer 2020; 126 (13) 3021-3030
- Strosberg J, Mizuno N, Doi T. et al.?Efficacy and safety of pembrolizumab in previously treated advanced neuroendocrine tumors: results from the phase II KEYNOTE-158 study. Clin Cancer Res 2020; 26 (09) 2124-2130


 PDF
PDF  Views
Views  Share
Share

