Histiocytoid Sweet Syndrome: A Rare Variant of Classic Paraneoplastic Dermatosis in Relation to Myelodysplastic Syndrome - A Case Report
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(05): 748-751
DOI: DOI: 10.4103/ijmpo.ijmpo_100_20
Abstract
Histiocytoid Sweet syndrome is a distinctive variant of Sweet syndrome, which is more frequently associated with myelodysplastic syndromes (MDS) than the latter. We describe a 59-year-old woman, diagnosed with MDS 4 months back, who developed sudden onset of rapidly progressing multiple painful erythematous papules and plaques on bilateral forearms, without any systemic complaints. The biopsy revealed interstitial and perivascular infiltrate of immature histiocytoid cells (positive for CD 68, myeloperoxidase, lysozyme, and CD15), along with papillary dermal edema. The eruption resolved with topical steroids in 3 weeks.
Keywords
Histiocytoid Sweet syndrome - myelodysplastic syndrome - paraneoplastic dermatosesPublication History
Received: 19 March 2020
Accepted: 14 May 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Histiocytoid Sweet syndrome is a distinctive variant of Sweet syndrome, which is more frequently associated with myelodysplastic syndromes (MDS) than the latter. We describe a 59-year-old woman, diagnosed with MDS 4 months back, who developed sudden onset of rapidly progressing multiple painful erythematous papules and plaques on bilateral forearms, without any systemic complaints. The biopsy revealed interstitial and perivascular infiltrate of immature histiocytoid cells (positive for CD 68, myeloperoxidase, lysozyme, and CD15), along with papillary dermal edema. The eruption resolved with topical steroids in 3 weeks.
Keywords
Histiocytoid Sweet syndrome - myelodysplastic syndrome - paraneoplastic dermatosesIntroduction
Classical Sweet syndrome (SS) was described in women in the age group of 30–60 years by Dr. Robert Douglas Sweet in 1964 as acute febrile illness associated with peripheral neutrophilia and multiple erythematous, tender skin nodules and plaques showing a dermal infiltrate of mature neutrophils.[1] It is classified into three etiological subtypes: idiopathic, drug induced, or associated with malignancies, mainly hematologic.[2]
In 2005, Requena et al. described histiocytoid SS (HSS) in a series of 41 patients with typical lesions of SS, but histopathology demonstrated an infiltrate composed of histiocytoid mononuclear cells with intense myeloperoxidase (MPO) reactivity. These were interpreted as immature mononuclear myeloid cells.[3]
Myelodysplastic syndrome (MDS) is a heterogeneous myeloproliferative disorder characterized by dysplastic and inadequate blood cell production, with a variable risk of progressing to acute leukemia.[4] Rarely, cases of HSS have been described in association with MDS.[3]
Case Report
A 59-year-old woman admitted in the hematology ward was seen on dermatology consultation with a 1-week history of the sudden eruption of erythematous lesions localized to bilateral forearms. There was no organomegaly. Complete blood count demonstrated pancytopenia: hemoglobin – 5.9 g/dl, total leukocyte count – 2270/mm3, differential leukocyte count – N40, L54, M04, and Blasts 02, and platelet count – 75,000/mm3. Liver and renal function tests and serum electrolytes were normal. Bone marrow biopsy revealed paucicellular marrow (cellularity: 15%–25%) displaying a mild diffuse interstitial increase in medium-sized immature cells with round-to-oval nuclei, delicate chromatin, and scant cytoplasm with an increase in blasts (15%–18%) with no dyserythropoiesis. Immunohistochemistry revealed a diffuse interstitial increase in CD34 immunoreactive blasts (15%–18% blasts of all nucleated cells). These blasts were focally positive for CD 117 and KP1. She was diagnosed to have MDS with excess blasts (MDS-EB2). The differential of a hypocellular variant of acute myelogenous leukemia was not considered, as the percentage of the blast cell population was less than 20% of all nucleated cells on bone marrow biopsy. The Revised International Prognostic Scoring System (IPSS-R) for MDS risk assessment calculator score was at least 5 in the absence of cytogenetics with the IPSS-R category designated as high.
She was initiated on azacytidine (75mg/m2, subcutaneous, monthly, 5-day cycle). Nine days after receiving the fourth cycle of chemotherapy, she was admitted with complaints of generalized weakness, dry cough, and dyspnea on exertion. High-resolution computed tomography of the chest revealed evidence of bronchiolitis, which was successfully managed with antibiotics (amoxicillin-clavulanic acid 625mg TDS for 5 days) and supportive treatment.
During admission, prior to starting of antibiotic, she developed sudden onset of rapidly progressing multiple painful erythematous papules and plaques on bilateral forearms, without concurrent fever, arthralgia, or systemic complaints. Physical examination revealed multiple shiny, edematous, erythematous 0.3 cm × 0.3 cm to 3 cm × 3 cm sized soft papules and plaques involving bilateral forearms only [Figure 1]. Clinical differentials of SS, leukemia cutis, urticarial vasculitis, and palisaded neutrophilic granulomatous dermatitis (PNGD) were considered.
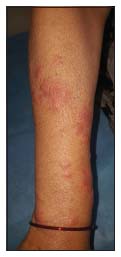
| Figure 1: Multiple shiny, edematous, erythematous 0.3 cm × 0.3 cm to 3 cm × 3 cm sized soft papules and plaques involving right forearm
A 4 mm punch biopsy from a lesion showed marked papillary dermal edema with a predominantly interstitial and mild perivascular infiltrate of predominantly histiocytoid mononuclear cells and lymphocytes along with some karyorrhectic debris [Figure 2a and b]. These histiocytoid cells had a large vesicular nucleus with conspicuous nucleoli and pale abundant cytoplasm. Neutrophils were absent in the infiltrate. The histiocytoid cells showed occasional mitotic figures and were immunopositive for CD68, MPO, lysozyme, and CD 15 and negative for CD117 and CD34 [Figure 3a,b,c,d,e,f]. Leukemia cutis was ruled out, as no blast cells were seen. A diagnosis of HSS was made. We did not consider possibility of vasculitis due to the lack of fibrinoid necrosis, extravasation of erythrocytes, and prominent angiocentric infiltrate. PNGD was also ruled out due to the absence of a combination of leukocytoclastic vasculitis and interstitial and palisaded infiltrate of histiocytes/granulomas.
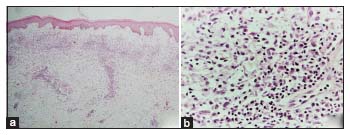
| Figure 2: (a and b) Photomicrograph showing marked papillary dermal edema with an interstitial and perivascular infiltrate of predominantly histiocytoid mononuclear cells and lymphocytes along with some karyorrhectic debris in the upper and mid dermis (H and E, ×20 [a], and × 100 [b])
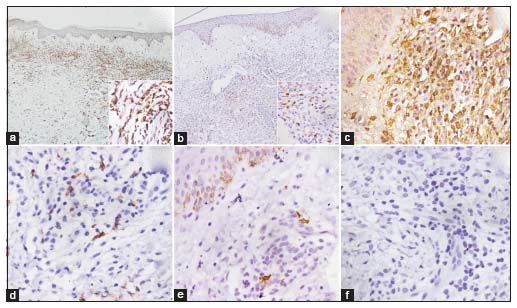
| Figure 3: (a) Photomicrograph showing CD68 immunopositivity (×100); inset showing strong cytoplasmic granular positivity (×400). (b) Photomicrograph showing myeloperoxidase immunopositivity (×100); inset showing focal cytoplasmic granular positivity (×400). (c) Photomicrograph showing immunopositivity to lysozyme (×400). (d) Photomicrograph showing focal cytoplasmic granular immunopositivity for CD15 (×400). (e) Photomicrograph showing CD117 immunopositivity in melanocytes of the epidermis and dermis, but lesional cells were immunonegative for CD117 (×400). (f) Photomicrograph showing immunonegativity to CD34 (×400)
The patient was treated with a once-daily application of topical super-potent steroid ointment and oral antihistamine. She reported substantial improvement within a few days, and all lesions resolved in 3 weeks. No recurrence was noted after 3 months of follow-up.
Discussion
HSS clinically differs from classic SS in terms of lack of fever and constitutional symptoms. Peripheral neutrophilia occurs in about 20% of HSS cases in contrast to more than 80% of cases of classic SS. Histologically, it consists of superficial and deep dermal infiltrate of mononuclear cells, with a large, eccentric kidney-shaped nucleus, conspicuous nucleolus, and eosinophilic cytoplasm.[5] HSS has multiple associations; rarely, it is described with MDS. However, HSS occurs more frequently with MDS than classic SS.[6]
Magro et al. described clinical and pathological findings of 13 HSS cases (5 females and 8 males with a mean age of 60 years – range: 23–80 years), with more than half the patients (7 cases) with underlying myeloproliferative disorder and majority (5 cases) having MDS.[7] While one patient had no comorbidity, others were associated with familial Mediterranean fever, concomitant administration of bortezomib in one patient of multiple myeloma, endometrioid adenocarcinoma in one case each, and therapy with COX-2 inhibitors in two patients. In most cases, the skin eruption was localized to the extremities with 9 of 13 patients having the involvement of the upper extremity. The eruption was mostly asymptomatic, with few patients reporting tenderness and pruritus and three patients complaining of arthralgia. The lesions resolved either spontaneously or with a short course of oral corticosteroid within days to a few weeks. Except for one case, the eruption was not associated with worsening of the underlying myeloproliferative disorder. Light microscopy revealed a superficial and deep mononuclear infiltrate which was both angiocentric and interstitial and also noted around eccrine coils and nerves. In few cases, the infiltrate extended into the subcutis. In contrast to classic SS, papillary dermal edema was noted only in 4/13 (31%) cases. Unlike the previous hypothesis that considered the histiocytoid mononuclear cells to be precursors of neutrophils based on MPO positivity, Magro et al. proposed that the main implicated cell in HSS is a macrophage. The mononuclear cells were immunopositive for monocytic markers including CD163, CD68, CD14, and CD4 and also positive for either CD16 or MPO. The authors noted that extensive expression of CD68 is strongly suggestive of HSS. Apalla et al. and others have also reported similar phenotypic expression of mononuclear cells showing positivity for lysozyme and CD68.[8]
Our case showed the immunoreactivity pattern of myeloid lineage, i.e., positivity to CD68, MPO (focal), lysozyme, and CD15. MPO is a constituent of primary granules expressed in the cytoplasm of both mature and immature stages of neutrophils and eosinophils. Cells of monocyte lineage react variably and are usually weakly reactive or nonreactive, but it is certainly not expressed in the resident macrophages. CD68 is a glycoprotein that is co-expressed with lysozymes in the cytoplasm of monocyte/macrophage lineage.[9],[10] Recently, CD68 has been identified as a marker for neutrophils as well.[11] CD15 is expressed in both neutrophils and monocytes but is variably expressed in the precursor cells.[12]
Peroni et al. described a series of 12 HSS patients (mean age 48.4 years) of which four patients had an underlying hematological disorder (three had MDS and one had monoclonal gammopathy), another four patients had no comorbidity, one was suspected of Kawasaki disease, and the remaining had upper respiratory tract infection. The eruption was noted on the trunk and upper and lower extremities. Histopathology revealed upper dermal characteristic histiocytoid cells, which were abundant in 75% of cases and discrete in the rest. Upper dermal edema was variable in intensity. Immunohistochemistry tested positive for CD68, CD163, and MPO. The eruption lasted for a few weeks to months in most cases, except two cases who had a remitting and relapsing course, over the next 2 years.[10]
In our patient, in the background of a hematological anomaly, differentials of classic SS and leukemia cutis were considered. The presence of mononuclear histiocytoid cells which stained positively with CD68 lysozyme and MPO and negative for CD117 and 34 ruled out leukemic or highly undifferentiated cells and favored the diagnosis of HSS.
SS is known to be triggered by certain medications, and in the given context, filgrastim and azacytidine are known to be associated.[13] Our patient did not receive filgrastim. She developed the first episode of SS, 9 days after the fourth cycle of azacytidine. Interestingly, there are reports of HSS after administration of azacytidine.[14] The algorithm by Naranjo et al. [15] and others was applied, and a score of 4 was obtained that suggested a lesser possibility of drug-induced SS in our case; however, the patient will be followed up for subsequent cycles of chemotherapy to look into the temporal association of SS and azacytidine administration.
Conclusion
SS is known to occur in association with hematologic malignancies as paraneoplastic dermatosis, related to antineoplastic therapy or as an idiopathic condition. The histiocytoid variant is unique and its association with MDS needs greater recognition. The cell of origin, whether monocytic or neutrophilic precursor, is debatable. Although HSS is a mild self-resolving eruption, whether its association with MDS bears an impact on the prognosis of the disease is still unexplored and needs further studies.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given her consent for her images and other clinical information to be reported in the journal. The patient understands that name and initials will not be published and due efforts will be made to conceal identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- Sweet RD. An acute febrile Neutrophilic dermatosis. Br J Dermatol 1964; 76: 349-56
- Cohen PR, Kurzrock R. Sweet's syndrome revisited: A review of disease concepts. Int J Dermatol 2003; 42: 761-78
- Requena L, Kutzner H, Palmedo G, Pascual M, Fernández-Herrera J, Fraga J. et al. Histiocytoid Sweet syndrome: A dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol 2005; 141: 834-42
- Montalban-Bravo G, Garcia-Manero G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol 2018; 93: 129-47
- Cohen PR. Sweet's syndrome-a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007; 2: 34
- Ghoufi L, Ortonne N, Ingen-Housz-Oro S, Barhoumi W, Begon E, Haioun C. et al. Histiocytoid sweet syndrome is more frequently associated with myelodysplastic syndromes than the classical neutrophilic variant: A comparative series of 62 patients. Medicine (Baltimore) 2016; 95: e3033
- Magro CM, Momtahen S, Nguyen GH, Wang X. Histiocytoid Sweet's Syndrome: A localized cutaneous proliferation of macrophages frequently associated with chronic myeloproliferative disease. Eur J Dermatol 2015; 25: 335-41
- Apalla Z, Kanatli L, Sotiriou E, Manousari A, Papagarifallou I, Calonje E. Histiocytoid Sweet syndrome. Clin Exp Dermatol 2011; 36: 562-3
- Rehg JE, Bush D, Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol 2012; 40: 345-74
- Peroni A, Colato C, Schena D, Rongioletti F, Girolomoni G. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol 2015; 72: 131-9
- Amanzada A, Malik IA, Blaschke M, Khan S, Rahman H, Ramadori G. et al. Identification of CD68(+) neutrophil granulocytes in in vitro model of acute inflammation and inflammatory bowel disease. Int J Clin Exp Pathol 2013; 6: 561-70
- Nakayama F, Nishihara S, Iwasaki H, Kudo T, Okubo R, Kaneko M. et al. CD15 expression in mature granulocytes is determined by α1,3-fucosyltransferase IX, but in promyelocytes and monocytes by α1,3-fucosyltransferase IV. J Biol Chem 2001; 276: 16100-06
- Tintle S, Patel V, Ruskin A, Halasz C. Azacitidine: A new medication associated with Sweet syndrome. J Am Acad Dermatol 2011; 64: e77-9
- Bonazza S, Dalton B, Hardin J, Metelitsa A. Histiocytoid variant of sweet syndrome associated with Azacitidine and recurrence upon rechallenge. Can J Hosp Pharm 2015; 68: 339-41
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA. et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-45
Address for correspondence
Publication History
Received: 19 March 2020
Accepted: 14 May 2020
Article published online:
17 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1: Multiple shiny, edematous, erythematous 0.3 cm × 0.3 cm to 3 cm × 3 cm sized soft papules and plaques involving right forearm

| Figure 2: (a and b) Photomicrograph showing marked papillary dermal edema with an interstitial and perivascular infiltrate of predominantly histiocytoid mononuclear cells and lymphocytes along with some karyorrhectic debris in the upper and mid dermis (H and E, ×20 [a], and × 100 [b])

| Figure 3: (a) Photomicrograph showing CD68 immunopositivity (×100); inset showing strong cytoplasmic granular positivity (×400). (b) Photomicrograph showing myeloperoxidase immunopositivity (×100); inset showing focal cytoplasmic granular positivity (×400). (c) Photomicrograph showing immunopositivity to lysozyme (×400). (d) Photomicrograph showing focal cytoplasmic granular immunopositivity for CD15 (×400). (e) Photomicrograph showing CD117 immunopositivity in melanocytes of the epidermis and dermis, but lesional cells were immunonegative for CD117 (×400). (f) Photomicrograph showing immunonegativity to CD34 (×400)
References
- Sweet RD. An acute febrile Neutrophilic dermatosis. Br J Dermatol 1964; 76: 349-56
- Cohen PR, Kurzrock R. Sweet's syndrome revisited: A review of disease concepts. Int J Dermatol 2003; 42: 761-78
- Requena L, Kutzner H, Palmedo G, Pascual M, Fernández-Herrera J, Fraga J. et al. Histiocytoid Sweet syndrome: A dermal infiltration of immature neutrophilic granulocytes. Arch Dermatol 2005; 141: 834-42
- Montalban-Bravo G, Garcia-Manero G. Myelodysplastic syndromes: 2018 update on diagnosis, risk-stratification and management. Am J Hematol 2018; 93: 129-47
- Cohen PR. Sweet's syndrome-a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis 2007; 2: 34
- Ghoufi L, Ortonne N, Ingen-Housz-Oro S, Barhoumi W, Begon E, Haioun C. et al. Histiocytoid sweet syndrome is more frequently associated with myelodysplastic syndromes than the classical neutrophilic variant: A comparative series of 62 patients. Medicine (Baltimore) 2016; 95: e3033
- Magro CM, Momtahen S, Nguyen GH, Wang X. Histiocytoid Sweet's Syndrome: A localized cutaneous proliferation of macrophages frequently associated with chronic myeloproliferative disease. Eur J Dermatol 2015; 25: 335-41
- Apalla Z, Kanatli L, Sotiriou E, Manousari A, Papagarifallou I, Calonje E. Histiocytoid Sweet syndrome. Clin Exp Dermatol 2011; 36: 562-3
- Rehg JE, Bush D, Ward JM. The utility of immunohistochemistry for the identification of hematopoietic and lymphoid cells in normal tissues and interpretation of proliferative and inflammatory lesions of mice and rats. Toxicol Pathol 2012; 40: 345-74
- Peroni A, Colato C, Schena D, Rongioletti F, Girolomoni G. Histiocytoid Sweet syndrome is infiltrated predominantly by M2-like macrophages. J Am Acad Dermatol 2015; 72: 131-9
- Amanzada A, Malik IA, Blaschke M, Khan S, Rahman H, Ramadori G. et al. Identification of CD68(+) neutrophil granulocytes in in vitro model of acute inflammation and inflammatory bowel disease. Int J Clin Exp Pathol 2013; 6: 561-70
- Nakayama F, Nishihara S, Iwasaki H, Kudo T, Okubo R, Kaneko M. et al. CD15 expression in mature granulocytes is determined by α1,3-fucosyltransferase IX, but in promyelocytes and monocytes by α1,3-fucosyltransferase IV. J Biol Chem 2001; 276: 16100-06
- Tintle S, Patel V, Ruskin A, Halasz C. Azacitidine: A new medication associated with Sweet syndrome. J Am Acad Dermatol 2011; 64: e77-9
- Bonazza S, Dalton B, Hardin J, Metelitsa A. Histiocytoid variant of sweet syndrome associated with Azacitidine and recurrence upon rechallenge. Can J Hosp Pharm 2015; 68: 339-41
- Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA. et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981; 30: 239-45


 PDF
PDF  Views
Views  Share
Share

