Glutamine: A novel approach to chemotherapy-induced toxicity
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(01): 13-20
DOI: DOI: 10.4103/0971-5851.96962
Abstract
Treatment of cancer is associated with short- and long-term side-effects. Cancer produces a state of glutamine deficiency, which is further aggravated by toxic effects of chemotherapeutic agents leading to increased tolerance of tumor to chemotherapy as well as reduced tolerance of normal tissues to the side-effects of chemotherapy. This article reviews the possible role of glutamine supplementation in reducing the serious adverse events in patients treated with anticancer drugs. The literature related to the possible role of glutamine in humans with cancer and the supportive evidence from animal studies was reviewed. Searches were made and the literature was retrieved using PUBMED, MEDLINE, COCHRANE LIBRARY, CENAHL and EMBASE, with a greater emphasis on the recent advances and clinical trials. Glutamine supplementation was found to protect against radiation-induced mucositis, anthracycline-induced cardiotoxicity and paclitaxel-related myalgias/arthralgias. Glutamine may prevent neurotoxicity of paclitaxel, cisplatin, oxaplatin bortezomib and lenolidamide, and is beneficial in the reduction of the dose-limiting gastrointestinal toxic effects of irinotecan and 5-FU-induced mucositis and stomatitis. Dietary glutamine reduces the severity of the immunosuppressive effect induced by methotrexate and improves the immune status of rats recovering from chemotherapy. In patients with acute myeloid leukemia requiring parenteral nutrition, glycyl-glutamine supplementation could hasten neutrophil recovery after intensive myelosuppressive chemotherapy. Current data supports the usefulness of glutamine supplementation in reducing complications of chemotherapy; however, paucity of clinical trials weakens the clear interpretation of these findings.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Treatment of cancer is associated with short- and long-term side-effects. Cancer produces a state of glutamine deficiency, which is further aggravated by toxic effects of chemotherapeutic agents leading to increased tolerance of tumor to chemotherapy as well as reduced tolerance of normal tissues to the side-effects of chemotherapy. This article reviews the possible role of glutamine supplementation in reducing the serious adverse events in patients treated with anticancer drugs. The literature related to the possible role of glutamine in humans with cancer and the supportive evidence from animal studies was reviewed. Searches were made and the literature was retrieved using PUBMED, MEDLINE, COCHRANE LIBRARY, CENAHL and EMBASE, with a greater emphasis on the recent advances and clinical trials. Glutamine supplementation was found to protect against radiation-induced mucositis, anthracycline-induced cardiotoxicity and paclitaxel-related myalgias/arthralgias. Glutamine may prevent neurotoxicity of paclitaxel, cisplatin, oxaplatin bortezomib and lenolidamide, and is beneficial in the reduction of the dose-limiting gastrointestinal toxic effects of irinotecan and 5-FU-induced mucositis and stomatitis. Dietary glutamine reduces the severity of the immunosuppressive effect induced by methotrexate and improves the immune status of rats recovering from chemotherapy. In patients with acute myeloid leukemia requiring parenteral nutrition, glycyl-glutamine supplementation could hasten neutrophil recovery after intensive myelosuppressive chemotherapy. Current data supports the usefulness of glutamine supplementation in reducing complications of chemotherapy; however, paucity of clinical trials weakens the clear interpretation of these findings.
INTRODUCTION
Glutamine is a non-essential branched-chain amino acid. It is an important non-toxic nitrogen carrier in the body and an essential component of diet, especially dairy products, fish and green leafy vegetables. It participates in a variety of physiological functions, and is a major fuel source of enterocytes and is a substrate for gluconeogenesis in the kidney, lymphocytes and monocytes. It is also a nutrient in muscle protein metabolism in response to infection, inflammation and muscle trauma.[1] Because of glutamine's importance as a nitrogen carrier and respiratory fuel for enterocytes of the gut and other rapidly proliferating cells, including lymphocytes and fibroblasts, glutamine can be considered as a conditionally essential amino acid.[2] Although there are no known drug interactions with glutamine, physiological antagonism may occur with lactulose when given to treat high ammonia levels in liver failures. In some patients, glutamate may lead to brain excitation, and in patients with seizure, may make the drug less effective.
BIOCHEMISTRY
Glutamine is a major component of tissue of the skeletal muscle, which is the main site for the synthesis and storage of L-glutamine. When the supply of glutamine in plasma is inadequate to meet the demand, glutamine synthesis occurs in skeletal muscle and liver. Glutamine is transported to the neurons and, by the enzyme glutaminase (GA), is converted to glutamate – the potential excitotoxin. L-glutamine accounts for 30–35% of the amino acid nitrogen in the plasma. It contains two ammonia groups, one from its precursor, glutamate, and the other from free ammonia in the bloodstream. Glutamine plays an important role to prevent fluctuations in the levels of ammonia in blood by acting as a “nitrogen shuttle.” It does so by acting as a buffer, accepting and then releasing excess ammonia when needed to form other amino acids, amino sugars, nucleotides and urea. This capacity to accept and donate nitrogen makes glutamine the major vehicle for nitrogen transfer among tissues. Glutamine is one of the three amino acids involved in glutathione (GSH) synthesis. GSH, an important intracellular antioxidant and hepatic detoxifier, is comprised of glutamic acid, cysteine and glycine.[3,4] Glutamine is one of the most important substrates for ammoniagenesis in the gut and the kidney due to its important role in the regulation of acid–base homeostasis.[5] It decomposes readily to yield ammonia and glutamate or via intramolecular catalysis to pyroglutamate. Deamidation of glutamine via GA produces glutamate, a precursor of gamma-amino butyric acid. The transfer of the amide nitrogen from glutamine via the amido transferase reaction is involved in the biosynthesis of purines and pyrimidines and in the production of hexosamines. Glutamine via glutamate is converted to α-ketoglutarate, an integral component of the citric acid cycle. It is a component of the antioxidant GSH and of the polyglutamated folic acid. The cyclization of glutamate produces proline, an amino acid important for the synthesis of collagen and connective tissue [Figure 1]. However, excess glutamine in a protein is of pathological importance, and a number of neurodegenerative diseases have been found to be due to a CAG expansion that causes expansion of glutamine repeats in affected proteins (CAA and CAG codons are responsible for the insertion of glutamine from its transfer RNA with its anti-codon triplet into the genetically determined position of the coded polypeptide chain). This leads to abnormal protein folding[6] and neuronal diseases.[7]
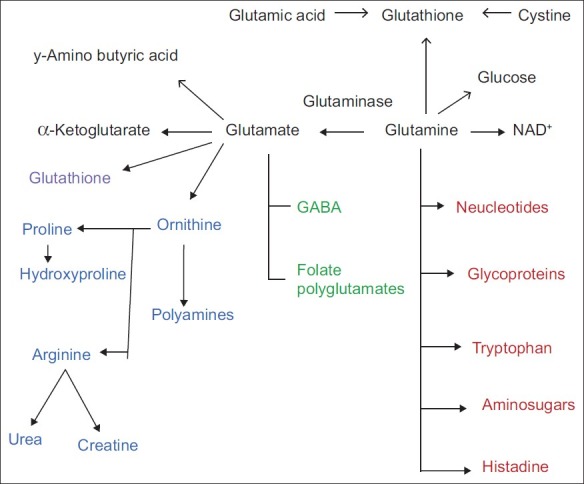
| Fig. 1 Metabolism of glutamine
Glutamine is involved in many metabolic processes in the body. Glutamine is converted to glucose when more glucose is required by the body as an energy source. Glutamine also plays a part in maintaining proper blood glucose levels and the right pH range. It is also used by white blood cells and is important for immune function. Glutamine assists in maintaining the proper acid/alkaline balance in the body, and is the basis of the building blocks for the synthesis of RNA and deoxyribonucleic acid (DNA). Glutamine regulates the expression of certain genes, including those that govern certain protective enzymes, and helps regulate the biosynthesis of DNA and RNA. Construction of DNA is dependent on adequate amounts of glutamine. It also increases the body's ability to secrete human growth hormone, which assists in metabolizing body fat and helps to support new muscle tissue growth. The glutamic acid–glutamine interconversion is of central importance to the regulation of the levels of toxic ammonia in the body, and thus among all the amino acids of blood plasma, glutamine has the highest concentration.
GLUTAMINE METABOLISM
In healthy animals, the small intestine is the principal site of dietary glutamine uptake, whereas skeletal muscle and lung can be major export sites. In sepsis, the liver becomes the major organ of glutamine uptake. Following endotoxin administration[8] or starvation,[9] uptake can increase by up to 10-fold. In septic states, increased glutamine in the liver is mediated by tumor necrosis factor-alfa, glucocorticoids and prostaglandins.[10] In skeletal muscle, glutamine synthetase (GS) expression and activity increases greatly whereas no change in GA activity is observed during sepsis. Despite an increase in GS expression and activity, the rate of release exceeds that of synthesis, resulting in intracellular glutamine pool depletion.[11] If skeletal muscle is the major glutamine exporter, the high flow of blood through the pulmonary circulation and the presence of GS make the lungs a site for glutamine metabolism.[12] The GS gene expression is induced during sepsis.[13] Lymphocytes and macrophages are also great glutamine consumers, while in an inflammatory state, glutamine utilization is 10-fold greater in proliferating lymphocytes than in resting cells.[14] In the bowel, decreased glutamine utilization during sepsis occurs in the mucosal cells rather than in lymphatic tissue,[15] and reduced intestinal uptake of circulating glutamine has been observed during interleukin-1 (IL-1) treatment.[16] The maintenance of blood acid–base balance is essential for survival. Thus, increased renal ammoniagenesis and gluconeogenesis from plasma glutamine constitute an adaptative response that restores in part the acid–base balance during metabolic acidosis.[17] Renal catabolism of glutamine is acutely activated in response to the onset of metabolic acidosis and is primarily due to increased glutamine plasma and cellular decrease of glutamate and alfa-ketoglutarate (α-KG). During normal acid–base balance, approximately two-thirds of the ammonium ions produced from glutamine are trapped in the tubular lumen and excreted in acidified urine. Acute activation of the Na+ / H+ exchanger acidifies the fluid in the tubular lumen and facilitates the trapping and excretion of ammonium ions. Changes in renal glutamine metabolism after endotoxin administration suggest that, during sepsis, early renal failure and altered glutamine metabolism may impair the kidney's ability to maintain acid/base homeostasis.[18] In humans, it has been shown that insulin, glucagons and epinephrine affect glutamine metabolism.[19] The gluconeogenesis from glutamine occurs principally in the kidney, whereas alanine conversion is essentially limited to the liver.[20] Renal gluconeogenesis contributes to 20–25% of whole body glucose production.[21] Overall, glutamine gluconeogenesis is responsible for about 5% of systemic glucose, and renal production of glucose from glutamine accounts for nearly 75% of all glucose derived from glutamine. Thus, it seems that glutamine is a significant contributor to whole body glucose homeostasis, while glutamate is not.
THERAPEUTIC ROLES
Glutamine is one of the most common plasma amino acids, and its concentration often decreases post-operatively,[22,23] during sepsis[24] and after multiple trauma[25] or major burns,[26] similar to a fall in the concentrations of many other amino acids, electrolytes, minerals and trace elements; therefore, it seems prudent to give glutamine supplementation in all these conditions.[27]
BONE MARROW TRANSPLANT
Bone marrow transplant (BMT) is a sophisticated procedure consisting of the administration of high-dose chemoradiotherapy followed by intravenous infusion of hemopoietic stem cells to re-establish marrow function when the bone marrow is damaged or defective. BMT is used in the treatment of solid tumors, hematological diseases and autoimmune disorders. Glutamine has protein-anabolic effects and has shown a clear reduction of complications in patients undergoing BMT who exhibit post-transplant body protein wasting, gut mucosal injury leading to mucositis of gastrointestinal tract, acute graft versus host disease and immunodeficiency. Studies indicate that enteral and parenteral glutamine supplementation is well tolerated and potentially efficacious after high-dose chemotherapy or BMT for cancer treatment. Although not all studies demonstrate benefits, sufficient data has been published to suggest that this nutrient should be considered as adjunctive metabolic support of some individuals undergoing marrow transplant.[28–39] However, BMT is a rapidly evolving clinical procedure with regard to the conditioning and supportive protocols used. Thus, additional randomized, double-blind, controlled clinical trials are indicated to define the efficacy of glutamine with current BMT regimens.[28]
GLUTAMINE SUPPLEMENTATION IN CHEMOTHERAPY
The results of glutamine supplementation and oncology in animals and humans are conflicting,[40] In vitro studies reveal an increase in cellular growth with glutamine supplementation.[41] While in vivo studies show the opposite effect, i.e. reduction in tumor growth.[42,43] Glutamine uptake in patients with colon cancer, regardless of tumor size and cell type, is comparable to uptake in patients with healthy intestinal tissue,[44,45] also enteral diet containing glutamine increase muscle glutamine in rats by 60% without increasing tumor growth or tumor glutamine use.[46] Glutamine supplementation in rats receiving methotrexate chemotherapy causes reduction in methotrexate-induced side-effects, including mucositis, and improved survival is observed.[47] Mucosal ulceration in rats subjected to abdominal radiation is also prevented.[48]
Growth of tumor leads to reactive increases in nucleotide and protein synthesis. High rates of protein synthesis in rapidly dividing tumors increase the demand for both essential and non-essential amino acids.[49] This demand is met by assimilating not only the nitrogen from diet but also the nitrogen from host proteins, raising the concept of tumors as “nitrogen traps,” actively competing with the host for nitrogen compounds.[50] Tumors use the incorporated amino acids for both oxidation and protein synthesis.[51] Because glutamine is the main vehicle for circulation of ammonia in a non-toxic form,[52] some authors consider that tumors indeed behave as “glutamine traps.”[53,54] The highly tumorigenic human breast cancer TSE cell line exhibits up-regulation of glutamine synthetase protein and mRNA levels and a decline in intracellular glutamine content on chronic glutamine deprivation.[55]
Tumors elicit a specific response in the host nitrogen metabolism, i.e. to mobilize and augment circulating glutamine.[56–59] There is a net flux of glutamine from host to tumor, which is possibly due to a net production of glutamine by host tissues as a result of an increase in the GS/GA ratio. As a rule, malignant cells transport glutamine across their plasma membranes at a faster rate than their non-malignant counterparts.[54,60,61]
There is increasing evidence supporting a protective role for glutamine supplementation in enteral or total parenteral nutrition.[62] In relation to cancer, it seems that a supplementation of glutamine in the diet may be beneficial for several reasons. Tumor progression has been found to be associated with an avid consumption of host glutamine by tumor cells and a depression in the activity of natural killer cells due to a decrease in GSH concentrations in these cells. Therefore, dietary supplementation of glutamine could have the beneficial effect of restoring the levels of GSH inside natural killer cells. At the same time, it could have the deleterious effect of feeding the tumor. However, because glutamine consumption by tumors is almost absolutely dissipative, an increase in the growth rate of the tumor due to this process should not be expected.[63,64] The experimental data indicate that a dietary supplement diminishes tumor growth by restoring the function of natural killer cells and improves protein metabolism of the host or patient.[65,66] Additionally, an oral supplement of glutamine can increase the selectivity of antitumor drugs[67–69] by protecting the patient from oxidative damage through an increase in GSH contents.[70] Several groups have shown that glutamine can also protect against oxidative damage induced by radiotherapy.[66,69,70]
GLUTAMINE AND CANCER
Numerous studies on glutamine metabolism in cancer indicate that many tumors are avid glutamine consumers in vivo and in vitro. As a consequence of progressive tumor growth, host glutamine depletion develops and becomes a hallmark. This glutamine depletion occurs in part because the tumor behaves as a “glutamine trap” and also because of cytokine-mediated alterations in glutamine metabolism in host tissues. Animal and human studies that have investigated the use of glutamine-supplemented nutrition in the host with cancer suggest that pharmacologic doses of dietary glutamine may be beneficial. Understanding the control of glutamine metabolism in the tumor-bearing host not only improves the knowledge of metabolic regulation in the patient with cancer but also leads to improved nutritional support regimens targeted to benefit the host.
GLUTAMINE: ROLE IN INCREASING SELECTIVITY OF CHEMOTHERAPEUTIC AGENTS
Chemotherapy doses are limited by toxicity to normal tissues. Intravenous glutamine protects liver cells from oxidant injury by increasing intracellular GSH content.[71,72] The effects of oral glutamine on tumor and host GSH metabolism and response to methotrexate (MTX) have been studied in rat models of sarcoma as well as in human patients with inflammatory breast cancer. Feeding the glutamine-enriched diets to rats receiving MTX decreases tumor GSH while increasing or maintaining host GSH stores.[73–75] Diminished GSH levels in tumor cells increases susceptibility to chemotherapy. Significantly decreased GSH content in tumor cells in the glutamine-supplemented group correlates with enhanced tumor volume loss.[76] These data suggest that oral glutamine supplementation will enhance the selectivity of antitumor drugs by protecting normal tissues from and possibly sensitizing tumor cells to radiation-induced and chemotherapy treatment-related injury.[77,76]
EFFECT OF GLUTAMINE ON METHOTREXATE EFFICACY AND TOXICITY
Oral glutamine has been shown to decrease the gut toxicity seen with MTX treatment while enhancing its tumoricidal effect. Studies in both laboratory rats and in breast cancer indicate a three-fold increase in total MTX in the tumor in the glutamine receiving group as compared with the control group, and this increase was in both the diglutamated and in the pentaglutamated MTX. There was also a significant decrease in the total polyglutamated MTX in the gut in the glutamine group. In a phase I trial, patients diagnosed with inflammatory breast cancer received glutamine during MTX neoadjuvant therapy in escalating doses for 3 weeks, followed by a doxorubicin-based regimen. No toxicity of oral glutamine was detected. No patient showed any sign of chemotherapy-related toxicity and all patients responded to the chemotherapy regimen, suggesting that glutamine supplementation is safe and may augment the response of MTX-based regimens. Thus, glutamine by selectively increasing tumor retention of MTX compared with normal host tissue increases the therapeutic window of chemotherapeutic drugs.[75] Experimental evidence indicates a very good correlation between the activity of mitochondrial GA and the rate of respiration of tumor mitochondria in the presence of glutamine.[78]
ROLE OF GLUTAMINE IN METABOLISM-DEPENDENT TOXICITY OF CYCLOPHOSPHAMIDE
Studies have shown that acrolein produced during the metabolism of cyclophosphamide (CP) binds to proteins and, by doing so, may denature these proteins and acrolein in vivo, preferentially reacting with GSH and sulfhydryl-containing compounds, and may protect against acrolein toxicity and at the same time not interfere with the chemotherapeutic activity of CP.[79] The main source of this GSH is glutamine. Besides CP, GSH is also extremely effective in trapping metabolites of acetaminophen, phenacetin and bromobenzene and in preventing their covalent binding to protein both in vivo and in vitro.
ORAL GLUTAMINE IN THE PREVENTION OF FLUOROURACIL-INDUCED INTESTINAL TOXICITY
5-Fluorouracil (FU) in association with folinic acid (FA) is the most frequently used chemotherapeutic agent in colorectal cancer, but it often causes diarrhea. Animal and human studies suggest that glutamine stimulates intestinal mucosal growth. A double-blind randomized trial with placebo and oral glutamine has shown that glutamine reduces changes in intestinal absorption and permeability (assessed by d-xylose urinary excretion and cellobiose-mannitol test, respectively) induced by FU and may have a protective effect on the FU-induced diarrhea.[80] Although there is no evidence of a beneficial effect of glutamine during pelvic radiotherapy-induced diarrhea for colonic carcinoma[81] a beneficial effect of oral glutamine for FU-induced diarrhea in colonic carcinoma is supported.[82] Even mild diarrhea must not be discounted when evaluating the effect of chemotherapy-induced diarrhea. Thus, careful monitoring, early identification and aggressive management must be applied to all grades of diarrhea.[83] There is also some evidence showing that glutamine and alanine-glutamine have trophic effects not only in the ileum but also in the proximal and distal colon. This could be important during parenteral nutrition, when mucosal atrophy may weaken the gut barrier.[84]
ORAL GLUTAMINE TO PREVENT CHEMOTHERAPY-INDUCED STOMATITIS
Mucositis is a common toxicity of cancer chemotherapy. Glutamine appears to be the major energy source for intestinal epithelium, and animal studies have suggested that dietary supplementation with glutamine may protect the gut from both radiation and chemotherapy. Experimental studies have shown that oral supplementation with glutamine can significantly decrease the severity of chemotherapy-induced stomatitis, an important cause of morbidity in the treatment of patients with cancer,[85,86] although the dose given and duration of therapy varies widely upon pathological conditions.[87] Other modalities of prevention of chemotherapy-induced mucositis, like oral cooling, granulocyte-macrophage colony-stimulating factor or granulocyte colony-stimulating factor and laser, are available, none of them have uniform efficacy.[88] Studies in mice suggest that alanyl-glutamine, not glutamine, speeds intestinal recovery when compared in 5-FU-treated mice, predominantly by enhancing mitotic activity and crypt length.[89] To evaluate the effect of therapeutic interventions in patients with chemotherapy-induced mucositis, testing intestinal permeability via the measurement of[51] Cr-EDTA urinary excretion after oral challenge may be useful as shown in some experiments.[90,91]
GLUTAMINE AND PROTECTION AGAINST ANTHRACYCLINE-INDUCED CARDIOTOXICITY
Glutamine supplementation maintained cardiac GSH levels in animal models given methotrexate or CP. In rat models given lethal and sublethal doses of CP, glutamine-supplemented rats were observed to have much less cardiotoxicity and had improved survival rates compared with those who were not supplemented, possibly through the upregulation of cardiac GSH.[92,93]
High-dose doxorubicin therapy causes both acute and, more commonly, chronic cardiac toxicity, which manifests as dose-related cardiomyopathy due to doxorubicin-induced free radicals. Oxidative damage due to doxorubicin-induced free radical results in decreased GSH and depletes superoxide dysmutase in cardiac muscle.[94–96] These molecules alter DNA, causing mitochondrial dysfunction, eliciting a cellular calcium overload, causing acute depression of GSH levels and inducing the release of catecholamine.[97,98]
The incidence of cardiac toxicity can be reduced by using a weekly schedule of doxorubicin rather than every 21 days (lower peak drug levels), or by the use of dexrazoxane, a derivative of EDTA.[98] This agent may enhance mucositis and myelosuppression during treatment, and also has been reported in one study to alter antitumor efficacy.[99] Mechanisms by which glutamine plays a protective role in the myocardial cell is through upregulation of GSH and, secondly, induction of heat shock protein (HSP). HSP 72 is known to be protective to the myocardium against hypoxic/ischemic injury. Induction of HSP 27 (another protective heat shock protein) has been shown to be protective against doxorubicin-induced cardiac injury.[100] Glutamine also appears to be a potent inducer of myocardial HSP 72 in an in vivo rat model.[101] Several trials are being conducted presently to prove the possible role of glutamine protection via HSP 72/27 induction in the heart. In addition, recent evidence indicate that glutamine can preserve myocardial high-energy phosphate levels and prevent accumulation of lactate following a variety of stress, including infection and ischemia/reperfusion injury.[102,103]
GLUTAMINE AS A NEUROPROTECTIVE AGENT IN TAXANE AND OTHER NEUROTOXIC THERAPY
Glutamine has been proposed as a potentially neuroprotective agent in patients receiving paclitaxel. In one non-randomized study, neurological signs and symptoms and changes in nerve-conduction studied in 46 consecutive patients receiving high-dose paclitaxel either with or without glutamine was assessed. Patients who received glutamine developed significantly less weakness, less loss of vibratory sensation and less toe numbness than controls. The percent change in the compound motor action potential and sensory nerve action potential amplitudes after paclitaxel treatment was lower in the glutamine group, but this finding was not statistically significant in these small groups.[104]
Another clinical trial evaluating the role of glutamine taken orally in the prevention of neurotoxicity among 86 patients with colorectal cancer who were being treated with Eloxatin, a common agent causing neurotoxicity, showed a significantly lower incidence of peripheral neuropathy compared with controls.[105]
GLYCYL-GLUTAMINE-DIPEPTIDE IN THE PARENTERAL NUTRITION OF PATIENTS WITH ACUTE LEUKEMIA UNDERGOING INTENSIVE CHEMOTHERAPY
The effects of parenteral glycyl-glutamine supplementation in patients with acute leukemia receiving intensive conventional chemotherapy was evaluated in a randomized, double-blind, controlled study that compared a standard glutamine-free parenteral nutrition with a glycyl-glutamine-supplemented parenteral nutrition containing 20 g of glutamine. There was significant faster neutrophil recovery in the group that received glutamine supplementation along with high-dose cytarabine chemotherapy as compared with those patients receiving cytarabine regimen alone. There was no significant difference in the recovery of CD4+ or CD8+ lymphocytes or monocyte activation between the two groups. The authors concluded that there is a possible role of glutamine in the stimulation of lymphocyte proliferation.[106]
CONCLUSIONS
The influence of glutamine on body homeostasis is protean. States of physiologic stress, including those resulting from the treatment of malignant disease, are characterized by a relative deficiency of glutamine. Supplementation with this inexpensive dietary supplement may have an important role in the prevention of gastrointestinal, neurologic and, possibly, cardiac complications of cancer therapy. These complications often negatively affect the quality of life and may also lead to changes in therapy, which potentially alter efficacy. Glutamine may also improve the therapeutic index of both chemotherapy and radiation, increasing cytotoxicity while concurrently protecting against toxicity. However, the current evidence is not sufficient to recommend its regular use. Further studies of glutamine supplementation in these areas is warranted and multicentric, placebo-controlled phase III studies are needed to evaluate the role of glutamine for the prevention of mucositis, neurotoxicity and cardiotoxicity, and for the prevention of hepatic venoocclusive disease in patients undergoing hematopoietic cell transplantation before any definitive recommendation can be made.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Metabolism of glutamine


 PDF
PDF  Views
Views  Share
Share

