Global Burden of Testicular Cancer and Its Risk Factors
CC BY 4.0 · Indian J Med Paediatr Oncol 2025; 46(02): 142-149
DOI: DOI: 10.1055/s-0044-1796675
Abstract
Testicular cancer (TC) is a rare cancer accounting for 5% of total urologic tumors. It occurs in distinct age groups of adolescents and young adults unlike other cancers peaking in the older age groups. About 95% of TC arises from germ cells. The histological classification of TC consists mainly of seminomas and nonseminomas. Based on GLOBOCAN 2022, the continent with the highest incidence rate was Europe (Age-adjusted rate-6.4), while Africa (0.59) had the lowest incidence. The highest mortality rates were estimated for Latin America and the Caribbean (0.58) followed by Europe (0.35) while the lowest was for the Asian continent (0.14). The highest prevalence of TC was in Europe followed by Oceania and Northern America, while Africa had the least prevalence of TC cases among all. A myriad of risk factors is associated with TC; Cryptorchidism is the strongest associated risk factor of TC increasing the risk by fivefold. Other risk factors identified include family history increasing the risk by four- to eightfold, increased adult height, infertility (1.6- to 2.8-fold), pesticide exposure (threefold), and gr/gr deletion (threefold). Clinically, TC generally presents as a painless scrotal swelling often mistaken as a hydrocele and the bulk of disease growing in the retroperitoneum can be asymptomatic even after growing to a huge size. This article aims to present the global burden of TC and also discusses its etiological risk factors.
Keywords
testicular cancer - epidemiology - GLOBOCAN - etiology - cryptorchidismPatient Consent
This a review article based on published literature, therefore patient consent was not required.
Authors' Contributions
S.M.: Writing original draft, data curation, and visualization.
S.B.: Writing - review and editing, data curation, and visualization.
S.S.: Writing - review and editing, data curation, and visualization.
P.K.: Writing - review and editing.
G.P.: Writing - review and editing.
A.B.: Conceptualization, writing - review and editing, and supervision.
Publication History
Article published online:
29 November 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Trend Analysis of Global Web Searches (2004–2022) on Oral Cancer and Its Major Risk FactorsKehinde Kazeem Kanmodi, Journal of Health and Allied Sciences NU
- Global Burden of StrokeMira Katan, Seminars in Neurology, 2018
- Global Burden of StrokeMira Katan, Revista Urología Colombiana / Colombian Urology Journal, 2018
- Venous thrombotic burden and the risk of subsequent overt cancerPaolo Prandoni, Thrombosis and Haemostasis, 2015
- Predictions Burden of Diabetes and Economics Cost: Contributing Risk Factors of Changing Disease Prevalence and its Pandemic Impact to QatarA. Bener, Experimental and Clinical Endocrinology & Diabetes, 2016
- IDDF2024-ABS-0356 The burden of early-onset pancreatic cancer and its risk factors: the India perspective from the global burden of disease study 2021<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global, regional and national burden of bladder cancer and its attributable risk factors in 204 countries and territories, 1990–2019: a systematic analysis for ...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global trends in incidence, death, burden and risk factors of early-onset cancer from 1990 to 2019<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- IDDF2024-ABS-0440 Global burden of colorectal cancer and its attributable risk factors in southeast asia, east asia, and oceania from 1990-2021: an insight from...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- IDDF2020-ABS-0181 Disease burden, risk factors, and recent trends of colorectal cancer: a global analysis of data from 186 countries<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
Abstract
Testicular cancer (TC) is a rare cancer accounting for 5% of total urologic tumors. It occurs in distinct age groups of adolescents and young adults unlike other cancers peaking in the older age groups. About 95% of TC arises from germ cells. The histological classification of TC consists mainly of seminomas and nonseminomas. Based on GLOBOCAN 2022, the continent with the highest incidence rate was Europe (Age-adjusted rate-6.4), while Africa (0.59) had the lowest incidence. The highest mortality rates were estimated for Latin America and the Caribbean (0.58) followed by Europe (0.35) while the lowest was for the Asian continent (0.14). The highest prevalence of TC was in Europe followed by Oceania and Northern America, while Africa had the least prevalence of TC cases among all. A myriad of risk factors is associated with TC; Cryptorchidism is the strongest associated risk factor of TC increasing the risk by fivefold. Other risk factors identified include family history increasing the risk by four- to eightfold, increased adult height, infertility (1.6- to 2.8-fold), pesticide exposure (threefold), and gr/gr deletion (threefold). Clinically, TC generally presents as a painless scrotal swelling often mistaken as a hydrocele and the bulk of disease growing in the retroperitoneum can be asymptomatic even after growing to a huge size. This article aims to present the global burden of TC and also discusses its etiological risk factors.
Keywords
testicular cancer - epidemiology - GLOBOCAN - etiology - cryptorchidism
Introduction
The burden of testicular cancer (TC) has doubled in the past 40 years. Coded as C62 as per the International Classification of Disease-Oncology–3rd Edition, it accounts for 5%-of urologic tumors, globally.[1] [2] [3] Despite being rare, it is an important public health issue due to its impact on the quality of life in men.[4] Due to data scarcity, the epidemiology of TC is not explored to its full potential, unlike other cancer sites.[5] However, increased attention is required due to its grim consequences affecting the quality of life in men due to treatment of TC such as cytotoxicity and cardiometabolic issues affecting the most productive years of adolescents and young adults.[6]
Depending on the cell type from which the cancer has originated, TC is divided into two types; those from the germ cells and the other arising from the nongerm cells of the testis.[7] Around 95%-of TC arises from germ cells, while the remaining 5%-arises from sex cord or stromal cells and miscellaneous nonspecific stromal cells.[8] Of these, 95%-of testicular germ cell tumors (TGCTs) are further divided based on the histologic features into seminomas, nonseminomas, and spermatocyte seminomas.[4]
The burden of TC is observed to peak in the age group 15 to 40, thus it is predominantly regarded as the cancer of adolescents and young adults.[5] [6] [9] There is a lack of clear appearance of signs and symptoms of TC with the exception of a unilateral lump or painless swelling; detecting TC cases in the early stage is a challenge. This calls for a clear understanding of the etiology as well as the current epidemiology of TC. This article aims to add to the literature on TC emphasizing its epidemiology and etiology.
Burden of Testicular Cancer
The current epidemiology of TC across continents is described in terms of incidence, mortality, prevalence, and survival. [Table 1] presents the burden of TC in different continents as per GLOBOCAN 2022.[10]
|
Continents |
Incidence |
Mortality |
Prevalence |
|||
|---|---|---|---|---|---|---|
|
Cases |
AAR per 100,000 |
Deaths |
AAR per 100,000 |
Previous cases |
Proportion per 100,000 |
|
|
Africa |
3,139 |
0.59 |
1,080 |
0.23 |
9,026 |
1.3 |
|
Latin America and Caribbean |
13,650 |
3.8 |
2,103 |
0.58 |
53,322 |
16.3 |
|
North America |
10,546 |
5.5 |
565 |
0.26 |
49,417 |
26.7 |
|
Asia |
19,388 |
6.4 |
3,660 |
0.35 |
70,947 |
30.2 |
|
Europe |
24,070 |
5.5 |
1,611 |
0.20 |
109,109 |
25.7 |
|
Oceania |
1,247 |
0.76 |
49 |
0.14 |
5,633 |
3.0 |
|
Total |
72,040 |
1.7 |
9,068 |
0.21 |
297,454 |
7.5 |
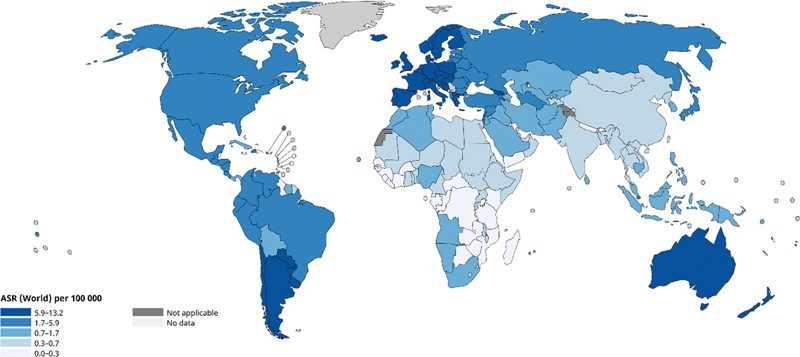
| Fig 1 :Age-standardized rate (world) per 100,000, incidence of testicular cancer as per GLOBOCAN 2022.
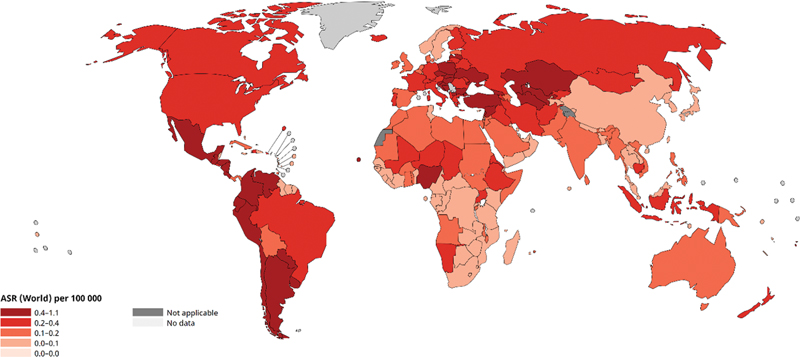
| Fig 2: Age-standardized rate (World) per 100,000, mortality of testicular cancer as per GLOBOCAN 2022.
Survival
The data on the survival of TC is scarce. According to the Surveillance, Epidemiology and End Results organization, a very high 5-year overall survival rate of 95%- was observed for all-stage TC and 99.2%-for localized TC in the United States.[2] The increase in survival was attributed to the introduction of platinum-based chemotherapy regimens and guidelines to help standardize tumor management, thus increasing the 5-year survival rates from 63%-to more than 90%-during the last three decades.[10] [13] Improved survival can also be attributed to increased awareness, wider use of ultrasonography at the primary level, and centralization of care and guidelines.[14]
In the Context of Cancer Registries Represented in CI5 XII
Based on the data from Cancer Incidence in Five Continents Volume XII, the range of incidence rates for TC in cancer registries from different continents is presented in [Table 2]. Of the total 589 cancer registries represented in CI5 XII, the cancer registry with the highest AAR for TC was the Chile, Valdivia Cancer Registry with AAR of 15.5 per 100,000 population. This registry belongs to the South, Central America and the Caribbean continent. The lowest incidence rate for TC was recorded in the Eldoret, Kenya registry in the African continent and the Nebraska Cancer Registry in the North American continent.[15]
|
Continent |
Number of registries represented in CI5 |
Range of testicular cancer incidence rate |
|||
|---|---|---|---|---|---|
|
High |
Low |
||||
|
Name of registry |
AAR |
Name of registry |
AAR |
||
|
Africa |
14 |
France, La Réunion |
2.8 |
Kenya, Eldoret |
0 |
|
America, Central and South, and Caribbean |
27 |
Chile, Valdivia |
15.5 |
Brazil, Recife |
0.7 |
|
America, North |
175 |
Canada, Yukon |
13.2 |
USA, Nebraska: Black |
0.3 |
|
Asia |
230 |
Türkiye, Trabzon |
6.3 |
China, Yiyuan County China, Yongkang City China, Yunmeng County |
0.1 |
|
Europe |
123 |
Switzerland, Graubünden Glarus |
14.4 |
Russian Federation, Arkhangelsk |
1.7 |
|
Oceania |
20 |
Australia, Tasmania |
9.9 |
USA, Hawaii: Filipino |
1.9 |
|
Risk factors identified |
References |
|---|---|
|
Cryptorchidism[a] |
|
|
Age |
|
|
Family history • TC in father and brother |
|
|
Perinatal or maternal factors • Low birth weight, maternal exposure to estrogen, maternal smoking, gestational weight gain, inguinal hernia, birth defects, serum cholesterol levels |
|
|
Physical features • Increased height, increased BMI |
|
|
Hormonal or reproductive factors • Infertility, vasectomy (-), increasing sibship size (-) |
|
|
Diet • Dairy products, cheese, cocoa, fruits, and vegetable (-) |
|
|
Physical activity |
[14] |
|
Occupational factors • Pesticides, textile dust, aliphatic, alicyclic hydrocarbons, organic solvents, endocrine disrupting factors such as polychlorinated biphenyls, organochlorines, nonionizing radiation, radiofrequency emitters, electrical machines, and high voltage lines |
|
|
Socioeconomic factors • Lower levels of education and socioeconomic position |
[25] |
|
Genetic factors |
[42] |
|
Environmental exposure • Extreme heat exposure, γ-radiation and electromagnetic fields, organochlorines, and polychlorinated biphenyls • Testicular dysgenesis |
References
- Tian YQ, Yang JC, Hu JJ, Ding R, Ye DW, Shang JW. Trends and risk factors of global incidence, mortality, and disability of genitourinary cancers from 1990 to 2019: systematic analysis for the Global Burden of Disease Study 2019. Front Public Health 2023; 11: 1119374
- Giona S. The Epidemiology of Testicular Cancer. In: Barber N, Ali A. eds. Brisbane, AU: Exon Publications Urologic Cancers; 2022
- Tongaonkar H. Testicular cancer: current management and controversial issues. Indian J Urol 2010; 26 (01) 63
- Yazici S, Del Biondo D, Napodano G. et al. Risk factors for testicular cancer: environment, genes and infections-is it all?. Medicina (Kaunas) 2023; 59 (04) 724
- Singh D, Singh P, Mandal A. Epidemiology and treatment outcomes of testicular germ cell tumor at tertiary care center in Patna, India: a retrospective analysis. Asian Pac J Cance Care 2020; 5: 45-50
- Cai Q, Chen Y, Zhang D. et al. Estimates of over-time trends in incidence and mortality of testicular cancer from 1990 to 2030. Transl Androl Urol 2020; 9 (02) 182-195
- Cheng L, Albers P, Berney DM. et al. Testicular cancer. Nat Rev Dis Primers 2018; 4 (01) 29
- Heidenreich A, Paffenholz P, Nestler T, Pfister D. European Association of Urology guidelines on testis cancer: important take home messages. Eur Urol Focus 2019; 5 (05) 742-744
- Shanmugalingam T, Soultati A, Chowdhury S, Rudman S, Van Hemelrijck M. Global incidence and outcome of testicular cancer. Clin Epidemiol 2013; 5: 417-427
- Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74 (03) 229-263
- Kusler KA, Poynter JN. International testicular cancer incidence rates in children, adolescents and young adults. Cancer Epidemiol 2018; 56: 106-111
-
United Nations. Human Development Index. Human Development Reports. Accessed Jun 10, 2024 at: https://hdr.undp.org/data-center/human-development-index
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer 2006; 118 (12) 3099-3111
- Huang J, Chan SC, Tin MS. et al. Worldwide distribution, risk factors, and temporal trends of testicular cancer incidence and mortality: a global analysis. Eur Urol Oncol 2022; 5 (05) 566-576
- Bray F, Colombet M, Aitken JF. et al. Cancer Incidence in Five Continents (IARC CancerBase No. 19). Vol. XII. International Agency Research Cancer; 2023. . Accessed November 15, 2024 at: https://ci5.iarc.who.int
- Nicolás PP, José RC, Carolina BM. et al. Testicular cancer mortality in Chile: is it as high as we think? 41 years of experience in a single center. Arch Urol Res 2019; 3: 9-11
- Braga LH, Lorenzo AJ. Cryptorchidism: a practical review for all community healthcare providers. Can Urol Assoc J 2017; 11 (1-2 suppl1): S26-S32
- Sheldon CA. Undescended testis and testicular torsion. Surg Clin North Am 1985; 65 (05) 1303-1329
- Garner MJ, Turner MC, Ghadirian P, Krewski D. Epidemiology of testicular cancer: an overview. Int J Cancer 2005; 116 (03) 331-339
- Herrinton LJ, Zhao W, Husson G. Management of cryptorchism and risk of testicular cancer. Am J Epidemiol 2003; 157 (07) 602-605
- Florou M, Tsilidis KK, Siomou E. et al. Orchidopexy for congenital cryptorchidism in childhood and adolescence and testicular cancer in adults: an updated systematic review and meta-analysis of observational studies. Eur J Pediatr 2023; 182 (06) 2499-2507
- Moirano G, Zugna D, Grasso C. et al. Postnatal risk factors for testicular cancer: the EPSAM case-control study. Int J Cancer 2017; 141 (09) 1803-1810
- Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer 2001; 92 (01) 144-150
- Baird DC, Meyers GJ, Hu JS. Testicular cancer: diagnosis and treatment. Am Fam Physician 2018; 97 (04) 261-268
- Richardson LC, Neri AJ, Tai E, Glenn JD. Testicular cancer: a narrative review of the role of socioeconomic position from risk to survivorship. Urol Oncol 2012; 30 (01) 95-101
- McGlynn KA, Petrick JL, Gamborg M, Aarestrup J, Baker JL. Childhood height and risk of testicular germ cell tumors in adulthood. Int J Cancer 2018; 143 (04) 767-772
- McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol 2009; 5 (09) 1389-1402
- Levy M, Hall D, Sud A. et al. Mendelian randomisation analysis provides no evidence for a relationship between adult height and testicular cancer risk. Andrology 2017; 5 (05) 914-922
- Duan H, Deng T, Chen Y. et al. Association between vasectomy and risk of testicular cancer: A systematic review and meta-analysis. PLoS One 2018; 13 (03) e0194606
- Grasso C, Zugna D, Fiano V. et al. Subfertility and risk of testicular cancer in the EPSAM case-control study. PLoS One 2016; 11 (12) e0169174
- McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol 2012; 9 (06) 339-349
- Bonner MR, McCann SE, Moysich KB. Dietary factors and the risk of testicular cancer. Nutr Cancer 2002; 44 (01) 35-43
- Giannandrea F. Correlation analysis of cocoa consumption data with worldwide incidence rates of testicular cancer and hypospadias. Int J Environ Res Public Health 2009; 6 (02) 568-578
- Walschaerts M, Muller A, Auger J. et al. Environmental, occupational and familial risks for testicular cancer: a hospital-based case-control study. Int J Androl 2007; 30 (04) 222-229
- Yousif L, Hammer GP, Emrich K, Blettner M, Zeeb H. Occupational risk factors for testicular cancer: a registry-based case-control study in Rhineland Palatinate-Germany. Ger Med Sci 2013; 11: Doc16
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008; 100 (09) 663-671
- Guo J, Pukkala E, Kyyrönen P, Lindbohm ML, Heikkilä P, Kauppinen T. Testicular cancer, occupation and exposure to chemical agents among Finnish men in 1971-1995. Cancer Causes Control 2005; 16 (02) 97-103
- Pollán M, Gustavsson P, Cano MI. Incidence of testicular cancer and occupation among Swedish men gainfully employed in 1970. Ann Epidemiol 2001; 11 (08) 554-562
- Hardell L, Carlberg M, Ohlson CG, Westberg H, Eriksson M, Hansson Mild K. Use of cellular and cordless telephones and risk of testicular cancer. Int J Androl 2007; 30 (02) 115-122
- Saju SV, Radhakrishnan V, Ganesan TS. et al. Factors that impact the outcomes in testicular germ cell tumors in low-middle-income countries. Med Oncol 2019; 36 (03) 28
- Gurney JK, Florio AA, Znaor A. et al. International trends in the incidence of testicular cancer: lessons from 35 years and 41 countries. Eur Urol 2019; 76 (05) 615-623
- Nathanson KL, Kanetsky PA, Hawes R. et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet 2005; 77 (06) 1034-1043
- Guo F, Kuo YF, Shih YCT, Giordano SH, Berenson AB. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer 2018; 124 (17) 3500-3509
- Meeks JJ, Sheinfeld J, Eggener SE. Environmental toxicology of testicular cancer. Urol Oncol 2012; 30 (02) 212-215
- Auriemma RS, Davide M, Cristina de A. et al. The role of the environment in testicular dysgenesis syndrome. Endocrinology 2023; •••: 1-38
- Selvi I, Ozturk E, Yikilmaz TN, Sarikaya S, Basar H. Effects of testicular dysgenesis syndrome components on testicular germ cell tumor prognosis and oncological outcomes. Int Braz J Urol 2020; 46 (05) 725-740
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16 (05) 972-978
- Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder?. Life Sci 2018; 194: 120-129
- van den Driesche S, Kilcoyne KR, Wagner I. et al. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2017; 2 (06) e91204
- Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl 2010; 33 (02) 298-303
- Zhang ZF, Vena JE, Zielezny M. et al. Occupational exposure to extreme temperature and risk of testicular cancer. Arch Environ Health 1995; 50 (01) 13-18
- Verhovsky G, Giladi M, Tzur D. et al. Varicocoele in adolescence and testicular cancer in young adulthood. Andrology 2022; 10 (08) 1575-1580
- Stewart BW, Kleihues P. World Cancer Report. International Agency For Research On Cancer Press, Lyon; 2003. (ISBN 9283204115). Accessed November 15, 2024 at:: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2003
- Capocaccia R, Gatta G, Dal Maso L. Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol 2015; 26 (06) 1263-1268
- Budukh AM, Thakur JS, Dora TK. et al. Overall survival of prostate cancer from Sangrur and Mansa cancer registries of Punjab state, India. Indian J Urol 2023; 39 (02) 148-155
- Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods part II. Completeness. Eur J Cancer 2009; 45 (05) 756-764
-
Cancer Samiksha. National Cancer Registry Programme (NCRP), National Centre for Disease Informatics and Research, Bangalore, India. Accessed January 4, 2024 at: https://ncdirindia.org/cancersamiksha/
- Shivshankar S, Mhamane S, Budukh AM. Representation of Indian and Chinese cancer registries from 1964 to 2017 in cancer incidence in five continents: growing burden but low coverage. Int J Cancer 2024; 154 (12) 2200-2203
- Suzuki S, Nagumo Y, Kandori S. et al. The prognostic impact of treatment centralization in patients with testicular germ cell tumors: analysis of hospital-based cancer registry data in Japan. Int J Clin Oncol 2024; 29 (03) 318-324
Address for correspondence
Publication History
Article published online:
29 November 2024
© 2024. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
- Trend Analysis of Global Web Searches (2004–2022) on Oral Cancer and Its Major Risk FactorsKehinde Kazeem Kanmodi, Journal of Health and Allied Sciences NU
- Global Burden of StrokeMira Katan, Seminars in Neurology, 2018
- Venous thrombotic burden and the risk of subsequent overt cancerPaolo Prandoni, Thrombosis and Haemostasis, 2015
- Global Burden of StrokeMira Katan, Revista Urología Colombiana / Colombian Urology Journal, 2018
- Predictions Burden of Diabetes and Economics Cost: Contributing Risk Factors of Changing Disease Prevalence and its Pandemic Impact to QatarA. Bener, Experimental and Clinical Endocrinology & Diabetes, 2016
- The burden of cardiovascular risk factors: a global perspective<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2019<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global burden of atrial fibrillation/atrial flutter and its attributable risk factors from 1990 to 2021<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global, regional, and national burden of ischaemic heart disease and its attributable risk factors, 1990–2017: results from the Global Burden of Disease Study 2...<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">
- Global, regional, and national burden and attributable risk factors of transport injuries: Global Burden of Disease Study 1990–2019<svg viewBox="0 0 24 24" fill="none" xmlns="http://www.w3.org/2000/svg">

| Fig 1 :Age-standardized rate (world) per 100,000, incidence of testicular cancer as per GLOBOCAN 2022.

| Fig 2: Age-standardized rate (World) per 100,000, mortality of testicular cancer as per GLOBOCAN 2022.
References
- Tian YQ, Yang JC, Hu JJ, Ding R, Ye DW, Shang JW. Trends and risk factors of global incidence, mortality, and disability of genitourinary cancers from 1990 to 2019: systematic analysis for the Global Burden of Disease Study 2019. Front Public Health 2023; 11: 1119374
- Giona S. The Epidemiology of Testicular Cancer. In: Barber N, Ali A. eds. Brisbane, AU: Exon Publications Urologic Cancers; 2022
- Tongaonkar H. Testicular cancer: current management and controversial issues. Indian J Urol 2010; 26 (01) 63
- Yazici S, Del Biondo D, Napodano G. et al. Risk factors for testicular cancer: environment, genes and infections-is it all?. Medicina (Kaunas) 2023; 59 (04) 724
- Singh D, Singh P, Mandal A. Epidemiology and treatment outcomes of testicular germ cell tumor at tertiary care center in Patna, India: a retrospective analysis. Asian Pac J Cance Care 2020; 5: 45-50
- Cai Q, Chen Y, Zhang D. et al. Estimates of over-time trends in incidence and mortality of testicular cancer from 1990 to 2030. Transl Androl Urol 2020; 9 (02) 182-195
- Cheng L, Albers P, Berney DM. et al. Testicular cancer. Nat Rev Dis Primers 2018; 4 (01) 29
- Heidenreich A, Paffenholz P, Nestler T, Pfister D. European Association of Urology guidelines on testis cancer: important take home messages. Eur Urol Focus 2019; 5 (05) 742-744
- Shanmugalingam T, Soultati A, Chowdhury S, Rudman S, Van Hemelrijck M. Global incidence and outcome of testicular cancer. Clin Epidemiol 2013; 5: 417-427
- Bray F, Laversanne M, Sung H. et al. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2024; 74 (03) 229-263
- Kusler KA, Poynter JN. International testicular cancer incidence rates in children, adolescents and young adults. Cancer Epidemiol 2018; 56: 106-111
-
United Nations. Human Development Index. Human Development Reports. Accessed Jun 10, 2024 at: https://hdr.undp.org/data-center/human-development-index
- Bray F, Richiardi L, Ekbom A, Pukkala E, Cuninkova M, Møller H. Trends in testicular cancer incidence and mortality in 22 European countries: continuing increases in incidence and declines in mortality. Int J Cancer 2006; 118 (12) 3099-3111
- Huang J, Chan SC, Tin MS. et al. Worldwide distribution, risk factors, and temporal trends of testicular cancer incidence and mortality: a global analysis. Eur Urol Oncol 2022; 5 (05) 566-576
- Bray F, Colombet M, Aitken JF. et al. Cancer Incidence in Five Continents (IARC CancerBase No. 19). Vol. XII. International Agency Research Cancer; 2023. . Accessed November 15, 2024 at: https://ci5.iarc.who.int
- Nicolás PP, José RC, Carolina BM. et al. Testicular cancer mortality in Chile: is it as high as we think? 41 years of experience in a single center. Arch Urol Res 2019; 3: 9-11
- Braga LH, Lorenzo AJ. Cryptorchidism: a practical review for all community healthcare providers. Can Urol Assoc J 2017; 11 (1-2 suppl1): S26-S32
- Sheldon CA. Undescended testis and testicular torsion. Surg Clin North Am 1985; 65 (05) 1303-1329
- Garner MJ, Turner MC, Ghadirian P, Krewski D. Epidemiology of testicular cancer: an overview. Int J Cancer 2005; 116 (03) 331-339
- Herrinton LJ, Zhao W, Husson G. Management of cryptorchism and risk of testicular cancer. Am J Epidemiol 2003; 157 (07) 602-605
- Florou M, Tsilidis KK, Siomou E. et al. Orchidopexy for congenital cryptorchidism in childhood and adolescence and testicular cancer in adults: an updated systematic review and meta-analysis of observational studies. Eur J Pediatr 2023; 182 (06) 2499-2507
- Moirano G, Zugna D, Grasso C. et al. Postnatal risk factors for testicular cancer: the EPSAM case-control study. Int J Cancer 2017; 141 (09) 1803-1810
- Dong C, Hemminki K. Modification of cancer risks in offspring by sibling and parental cancers from 2,112,616 nuclear families. Int J Cancer 2001; 92 (01) 144-150
- Baird DC, Meyers GJ, Hu JS. Testicular cancer: diagnosis and treatment. Am Fam Physician 2018; 97 (04) 261-268
- Richardson LC, Neri AJ, Tai E, Glenn JD. Testicular cancer: a narrative review of the role of socioeconomic position from risk to survivorship. Urol Oncol 2012; 30 (01) 95-101
- McGlynn KA, Petrick JL, Gamborg M, Aarestrup J, Baker JL. Childhood height and risk of testicular germ cell tumors in adulthood. Int J Cancer 2018; 143 (04) 767-772
- McGlynn KA, Cook MB. Etiologic factors in testicular germ-cell tumors. Future Oncol 2009; 5 (09) 1389-1402
- Levy M, Hall D, Sud A. et al. Mendelian randomisation analysis provides no evidence for a relationship between adult height and testicular cancer risk. Andrology 2017; 5 (05) 914-922
- Duan H, Deng T, Chen Y. et al. Association between vasectomy and risk of testicular cancer: A systematic review and meta-analysis. PLoS One 2018; 13 (03) e0194606
- Grasso C, Zugna D, Fiano V. et al. Subfertility and risk of testicular cancer in the EPSAM case-control study. PLoS One 2016; 11 (12) e0169174
- McGlynn KA, Trabert B. Adolescent and adult risk factors for testicular cancer. Nat Rev Urol 2012; 9 (06) 339-349
- Bonner MR, McCann SE, Moysich KB. Dietary factors and the risk of testicular cancer. Nutr Cancer 2002; 44 (01) 35-43
- Giannandrea F. Correlation analysis of cocoa consumption data with worldwide incidence rates of testicular cancer and hypospadias. Int J Environ Res Public Health 2009; 6 (02) 568-578
- Walschaerts M, Muller A, Auger J. et al. Environmental, occupational and familial risks for testicular cancer: a hospital-based case-control study. Int J Androl 2007; 30 (04) 222-229
- Yousif L, Hammer GP, Emrich K, Blettner M, Zeeb H. Occupational risk factors for testicular cancer: a registry-based case-control study in Rhineland Palatinate-Germany. Ger Med Sci 2013; 11: Doc16
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008; 100 (09) 663-671
- Guo J, Pukkala E, Kyyrönen P, Lindbohm ML, Heikkilä P, Kauppinen T. Testicular cancer, occupation and exposure to chemical agents among Finnish men in 1971-1995. Cancer Causes Control 2005; 16 (02) 97-103
- Pollán M, Gustavsson P, Cano MI. Incidence of testicular cancer and occupation among Swedish men gainfully employed in 1970. Ann Epidemiol 2001; 11 (08) 554-562
- Hardell L, Carlberg M, Ohlson CG, Westberg H, Eriksson M, Hansson Mild K. Use of cellular and cordless telephones and risk of testicular cancer. Int J Androl 2007; 30 (02) 115-122
- Saju SV, Radhakrishnan V, Ganesan TS. et al. Factors that impact the outcomes in testicular germ cell tumors in low-middle-income countries. Med Oncol 2019; 36 (03) 28
- Gurney JK, Florio AA, Znaor A. et al. International trends in the incidence of testicular cancer: lessons from 35 years and 41 countries. Eur Urol 2019; 76 (05) 615-623
- Nathanson KL, Kanetsky PA, Hawes R. et al. The Y deletion gr/gr and susceptibility to testicular germ cell tumor. Am J Hum Genet 2005; 77 (06) 1034-1043
- Guo F, Kuo YF, Shih YCT, Giordano SH, Berenson AB. Trends in breast cancer mortality by stage at diagnosis among young women in the United States. Cancer 2018; 124 (17) 3500-3509
- Meeks JJ, Sheinfeld J, Eggener SE. Environmental toxicology of testicular cancer. Urol Oncol 2012; 30 (02) 212-215
- Auriemma RS, Davide M, Cristina de A. et al. The role of the environment in testicular dysgenesis syndrome. Endocrinology 2023; •••: 1-38
- Selvi I, Ozturk E, Yikilmaz TN, Sarikaya S, Basar H. Effects of testicular dysgenesis syndrome components on testicular germ cell tumor prognosis and oncological outcomes. Int Braz J Urol 2020; 46 (05) 725-740
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001; 16 (05) 972-978
- Xing JS, Bai ZM. Is testicular dysgenesis syndrome a genetic, endocrine, or environmental disease, or an unexplained reproductive disorder?. Life Sci 2018; 194: 120-129
- van den Driesche S, Kilcoyne KR, Wagner I. et al. Experimentally induced testicular dysgenesis syndrome originates in the masculinization programming window. JCI Insight 2017; 2 (06) e91204
- Jørgensen N, Rajpert-De Meyts E, Main KM, Skakkebaek NE. Testicular dysgenesis syndrome comprises some but not all cases of hypospadias and impaired spermatogenesis. Int J Androl 2010; 33 (02) 298-303
- Zhang ZF, Vena JE, Zielezny M. et al. Occupational exposure to extreme temperature and risk of testicular cancer. Arch Environ Health 1995; 50 (01) 13-18
- Verhovsky G, Giladi M, Tzur D. et al. Varicocoele in adolescence and testicular cancer in young adulthood. Andrology 2022; 10 (08) 1575-1580
- Stewart BW, Kleihues P. World Cancer Report. International Agency For Research On Cancer Press, Lyon; 2003. (ISBN 9283204115). Accessed November 15, 2024 at:: http://publications.iarc.fr/Non-Series-Publications/World-Cancer-Reports/World-Cancer-Report-2003
- Capocaccia R, Gatta G, Dal Maso L. Life expectancy of colon, breast, and testicular cancer patients: an analysis of US-SEER population-based data. Ann Oncol 2015; 26 (06) 1263-1268
- Budukh AM, Thakur JS, Dora TK. et al. Overall survival of prostate cancer from Sangrur and Mansa cancer registries of Punjab state, India. Indian J Urol 2023; 39 (02) 148-155
- Parkin DM, Bray F. Evaluation of data quality in the cancer registry: principles and methods part II. Completeness. Eur J Cancer 2009; 45 (05) 756-764
-
Cancer Samiksha. National Cancer Registry Programme (NCRP), National Centre for Disease Informatics and Research, Bangalore, India. Accessed January 4, 2024 at: https://ncdirindia.org/cancersamiksha/
- Shivshankar S, Mhamane S, Budukh AM. Representation of Indian and Chinese cancer registries from 1964 to 2017 in cancer incidence in five continents: growing burden but low coverage. Int J Cancer 2024; 154 (12) 2200-2203
- Suzuki S, Nagumo Y, Kandori S. et al. The prognostic impact of treatment centralization in patients with testicular germ cell tumors: analysis of hospital-based cancer registry data in Japan. Int J Clin Oncol 2024; 29 (03) 318-324


 PDF
PDF  Views
Views  Share
Share

