Giant Sacrococcygeal Teratoma in Neonate: A Case Report and Review of Literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2019; 40(04): 573-575
DOI: DOI: 10.4103/ijmpo.ijmpo_16_18
Abstract
Sacrococcygeal teratoma is considered the most common tumor in the neonatal period with a female-to-male ratio of 3–4:1. They are believed to arise early in gestation from the totipotential cells of Hensen’s node and the derived ectoderm, mesoderm, and endoderm. A 2.25 kg cesarean-delivered male, term neonate presented with large nonpulsatile, globular erythematous mass with lobulated surface and variable consistency. Magnetic resonance imaging of the lumbosacral spine showed multilocular cystic and solid lesion with foci of calcification and subtle communication with spinal canal. Histopathological examination showed mature endodermal, ectodermal, and mesenchymal elements such as cartilage, glial tissues, keratin cyst, and glandular elements with focus of primary neuroepithelial and pancreatic elements. Entire tumor was excised. The baby expired secondary to wound infection. Early diagnosis by improving antenatal care with timely surgical intervention and good nursing care improves the outcome.
Publication History
Received: 17 June 2018
Accepted: 27 April 2018
Article published online:
03 June 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Sacrococcygeal teratoma is considered the most common tumor in the neonatal period with a female-to-male ratio of 3–4:1. They are believed to arise early in gestation from the totipotential cells of Hensen’s node and the derived ectoderm, mesoderm, and endoderm. A 2.25 kg cesarean-delivered male, term neonate presented with large nonpulsatile, globular erythematous mass with lobulated surface and variable consistency. Magnetic resonance imaging of the lumbosacral spine showed multilocular cystic and solid lesion with foci of calcification and subtle communication with spinal canal. Histopathological examination showed mature endodermal, ectodermal, and mesenchymal elements such as cartilage, glial tissues, keratin cyst, and glandular elements with focus of primary neuroepithelial and pancreatic elements. Entire tumor was excised. The baby expired secondary to wound infection. Early diagnosis by improving antenatal care with timely surgical intervention and good nursing care improves the outcome.
Introduction
Sacrococcygeal teratoma (SCT) is the most common congenital tumor in the neonatal period including still birth with reported incidence ranging from 1 in 35,000 to 1 in 40,000 live births, and approximately 80% of affected infants are female.[1] [2] Although their embryonic origin is still uncertain, they are believed to arise early in gestation from the totipotential cells of Hensen’s node, a remnant of the primitive streak in the coccygeal region. SCT has tissues derived from ectoderm, mesoderm, and endoderm. Mature (benign) teratomas are most common in neonates and older children. Immature teratomas are cystic, whereas malignant tumors are solid. Based on the American Academy of Pediatrics Surgical Section survey (1962–1972), Altman et al. proposed their famous classification for SCT in 1973. The classification included four types based on external component and intrapelvic/intra-abdominal extension of the tumor.[3] SCTs seen at birth are usually Altman Type I and II and rarely Type III, but Type IV is typically seen in later life.
Case Report
A 24 years old, non-consanguineously married mother and gravida 2, para 0, abortion 1 delivered a 2.25 kg male neonate at term by cesarean section. The baby was admitted to the Neonatal Intensive Care Unit with large mass in the sacrococcygeal region. Although the mother belonged to poor socioeconomic status, she attended regular antenatal clinic. Antenatal ultrasound in the second trimester had revealed polyhydramnino and mass over the lower back region. There was no history of any drug intake except iron, folic acid, and calcium. There was no irradiation or toxin exposure. There was a history of spontaneous abortion in the first trimester. On presentation, his activity was average and vitals were stable. A nonpulsatile, globular erythematous mass of size 30 cm × 20 cm was found in the sacrococcygeal region with lobulated surface and variable consistency. The anal opening was displaced anteriorly onto the mass. The mass gradually darkened in color, suggestive of internal hemorrhage [Figure 1]. Local ultrasound showed a large mass with cystic and solid components at the sacrococcygeal region. T1- and T2-weighted magnetic resonance imaging (MRI) of the lumbosacral spine showed a large, well-defined, multilocular predominantly cystic lesion of size 13 cm × 5.9 cm × 11.2 cm predominantly involving lower sacral vertebrae and the coccyx. There was a small solid component of size 2.7 cm × 1.2 cm which showed mild heterogeneous postcontrast enhancement. The lesion had tiny fatty component and foci of calcification. There was no obvious intraspinal extension of lesion; however, subtle communication with spinal canal through right S4 and S5 sacral foramen with partial involvement of S4 and S5 vertebrae and near-total involvement of the coccyx was noted which was suggestive of Altman Type II [Figure 2].
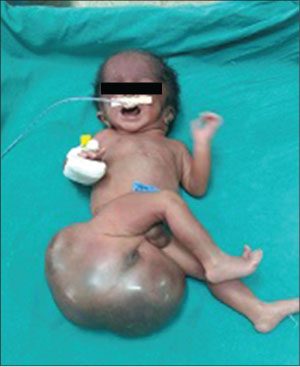
| Fig. 1 Neonate with huge mass on the sacrococcygeal region
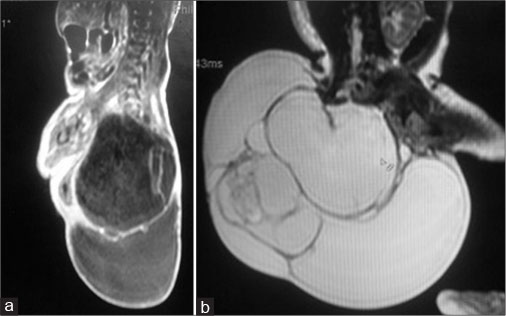
| Fig. 2 (a and b) Magnetic resonance imaging scan showed cystic solid lesion)
The baby was operated by Chevron incision extended by the side of rectum. Cystic component was drained to reduce the size, and the entire tumor was excised and division colostomy was done. The baby was managed with appropriate antibiotics and supportive care. Initially, the baby has passed stools from colostomy, but on the 3rd postoperative day, the baby had continuous bilious aspirate with nonpassage of stools suggestive of intestinal obstruction, so revision colostomy was done. On the 7th postoperative day, the baby developed gape in incision wound which subsequently became infected. In spite of appropriate antibiotic therapy and supportive care, the baby expired on the 24th day of life.
Histopathological examination of the excised mass showed mature endodermal, ectodermal, and mesenchymal elements in the form of cartilage, glial tissues, keratin cyst, and glandular elements with focus of primary neuroepithelial elements and pancreatic elements. These findings were suggestive of mature teratoma [Figure 3].
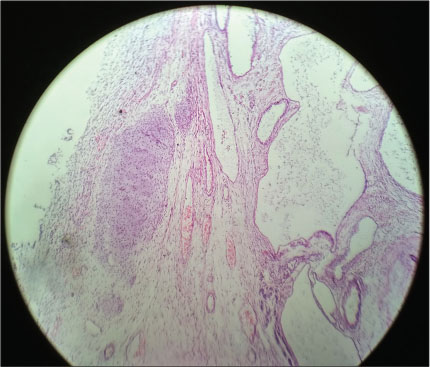
| Fig. 3 Histopathology of sacrococcygeal teratoma
Discussion
Although SCT is a rare tumor, it is considered the most common tumor in the neonatal period and may also present during infancy or childhood. Various studies report a female-to-male ratio of 3–4:1, but the exact reason for the female preponderance is not fully understood.[4] [5] The case presented here is a male neonate.
Antenatal diagnosis of SCT Altman Type I and II and even large Type III and IV are usually done by prenatal sonogram in the 24th–34th weeks of gestation.[6] The presence of a heterogeneous, well-circumscribed exophytic mass at the caudal end of the fetus is pathognomonic. The presence of placentomegaly, cardiomegaly, or nonimmune hydrops fetalis is indicative of poor outcome. Fetal MRI may provide additional anatomical information. Alpha-fetoprotein level and close antenatal monitoring are of paramount importance to look for complication. More recently, there has been an increasing incidence in the antenatal diagnosis of SCT, and there are reports on the feasibility of fetal intervention in selected cases.[2] [7] Cesarean delivery is advised to a mother whose fetuses harbor large SCTs (>10 cm diameter) with high vascularity and in tumors larger than 5 cm in order to prevent the risk of rupture and bleeding.[1]
Altman et al. defined the size as small (2–5 cm), moderate (5–10 cm), and large (>10 cm) diameter and also classified on the basis of the extent of tumor. Size of a tumor does not predict its biological behavior, but a hypervascular lesion often leads to congestive heart failure, coagulopathy from intramural bleeding, and consumption of clotting factors. The incidence of other congenital malformations associated with SCTs ranges from 5% to 26%, and the most common are anorectal and genital, but spinal dysraphism, sacral agenesis, and cardiac anomalies have been rarely noted.[6] Our case had Altman Type II large tumor mass without other congenital anomalies.
The recurrence rate without removal of the coccyx is as high as 37%.[8] Hence, complete surgical excision of the tumor with coccygectomy is of paramount importance in the prevention of recurrence. Wound infection, being the most common complication, is due to proximity of the surgical site to the anus. Other complications include neuropathic bladder, bowel incontinence, constipation, and diarrhea. Septicemia due to wound infection is the most common cause of death in the early postoperative period. Poor cosmetic results in the buttock region are the most common long-term complication and can lead to distortion of body image, particularly in teenage, and can even cause psychological disturbances and depression. Providing good cosmesis may be challenging in the neonatal period due to lack of local tissue, large tumor, and thinned/stretched out muscles.
The incidence of malignancy is more in large size SCTs, and Altman Type III/IV due to delay in diagnosis and presence of solid areas. Excellent survival rate has been reported in the literature; however, mortality rate for the mass larger than 10 cm is 18%.[3] [9] Close follow-up every 3–6 months, with physical examination including rectal examination, serum alpha-fetoprotein, and diagnostic imaging, is advisable for at least 3 years as chances of recurrence range from 11% to 22% even in completely excised mature neonatal SCTs.[9] [10]
Conclusion
SCTs in the neonatal period are mostly of mature type and benign in nature. Most of the cases have a favorable outcome with timely intervention and appropriate surgical intervention. Hence, it is our prime responsibility to improve antenatal care and at least one ultrasound of the mother should be performed to facilitate early diagnosis. Good nursing and supportive care is more important to improve early postoperative mortality because wound infection and septicemia are the most common complications and cause of death in early postoperative period.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Conflict of Interest
There are no conflicts of interest.
References
- 1 Sinha S, Sarin YK, Deshpande VP. Neonatal sacrococcygeal teratoma: Our experiences with 10 cases. J Neonat Surg 2013; 2: 4
- 2 Abouzeid AA, Mohammad SA, Elsherbeny M, Radwan NA, El-Naggar O. Preoperative grading of sacrococcygeal teratoma: A roadmap to successful resection. J Neonatal Surg 2017; 6: 75
- 3 Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 1974; 9: 389-98
- 4 Aly KA, Shoier M, Badrawy T. Sacrococcygeal teratoma: A neonatal surgical problem. Ann Pediatr Surg 2006; 2: 106-11
- 5 Pathak M, Kotabagi A, Narula D, Barkesia B. Sacrococcygeal teratoma: A retrospective study of 23 surgically treated cases at a tertiary care centre. IOSR J Dent Med Sci 2016; 15: 11-6
- 6 Tuladhar R, Patole SK, Whitehall JS. Sacrococcygeal teratoma in the perinatal period. Postgrad Med J 2000; 76: 754-9
- 7 Usui N, Kitano Y, Sago H, Kanamori Y, Yoneda A, Nakamura T. et al. Outcomes of prenatally diagnosed sacrococcygeal teratomas: The results of a Japanese nationwide survey. J Pediatr Surg 2012; 47: 441-7
- 8 Gonzalez-Crussi F, Winkler RF, Mirkin DL. Sacrococcygeal teratomas in infants and children: Relationship of histology and prognosis in 40 cases. Arch Pathol Lab Med 1978; 102: 420-5
- 9 Bilik R, Shandling B, Pope M, Thorner P, Weitzman S, Ein SH. et al. Malignant benign neonatal sacrococcygeal teratoma. J Pediatr Surg 1993; 28: 1158-60
- 10 Rescorla FJ, Sawin RS, Coran AG, Dillon PW, Azizkhan RG. Long-term outcome for infants and children with sacrococcygeal teratoma: A report from the children’s cancer group. J Pediatr Surg 1998; 33: 171-6
Address for correspondence
Dr. Rajkumar Motiram MeshramDepartment of Paediatrics, Govt. Medical College and HospitalNagpur, MaharashtraIndiaEmail: dr_rajmeshram@rediffmail.comPublication History
Received: 17 June 2018
Accepted: 27 April 2018
Article published online:
03 June 2021© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/).
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Fig. 1 Neonate with huge mass on the sacrococcygeal region

| Fig. 2 (a and b) Magnetic resonance imaging scan showed cystic solid lesion)

| Fig. 3Histopathology of sacrococcygeal teratoma
References
- 1 Sinha S, Sarin YK, Deshpande VP. Neonatal sacrococcygeal teratoma: Our experiences with 10 cases. J Neonat Surg 2013; 2: 4
- 2 Abouzeid AA, Mohammad SA, Elsherbeny M, Radwan NA, El-Naggar O. Preoperative grading of sacrococcygeal teratoma: A roadmap to successful resection. J Neonatal Surg 2017; 6: 75
- 3 Altman RP, Randolph JG, Lilly JR. Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section Survey-1973. J Pediatr Surg 1974; 9: 389-98
- 4 Aly KA, Shoier M, Badrawy T. Sacrococcygeal teratoma: A neonatal surgical problem. Ann Pediatr Surg 2006; 2: 106-11
- 5 Pathak M, Kotabagi A, Narula D, Barkesia B. Sacrococcygeal teratoma: A retrospective study of 23 surgically treated cases at a tertiary care centre. IOSR J Dent Med Sci 2016; 15: 11-6
- 6 Tuladhar R, Patole SK, Whitehall JS. Sacrococcygeal teratoma in the perinatal period. Postgrad Med J 2000; 76: 754-9
- 7 Usui N, Kitano Y, Sago H, Kanamori Y, Yoneda A, Nakamura T. et al. Outcomes of prenatally diagnosed sacrococcygeal teratomas: The results of a Japanese nationwide survey. J Pediatr Surg 2012; 47: 441-7
- 8 Gonzalez-Crussi F, Winkler RF, Mirkin DL. Sacrococcygeal teratomas in infants and children: Relationship of histology and prognosis in 40 cases. Arch Pathol Lab Med 1978; 102: 420-5
- 9 Bilik R, Shandling B, Pope M, Thorner P, Weitzman S, Ein SH. et al. Malignant benign neonatal sacrococcygeal teratoma. J Pediatr Surg 1993; 28: 1158-60
- 10 Rescorla FJ, Sawin RS, Coran AG, Dillon PW, Azizkhan RG. Long-term outcome for infants and children with sacrococcygeal teratoma: A report from the children’s cancer group. J Pediatr Surg 1998; 33: 171-6


 PDF
PDF  Views
Views  Share
Share

