Gestational Choriocarcinoma Manifesting as Spontaneous Hemothorax in Third Trimester of Pregnancy: A Case Report
CC BY 4.0 · Indian J Med Paediatr Oncol 2022; 43(06): 513-517
DOI: DOI: 10.1055/s-0042-1758525
Abstract
Gestational trophoblastic neoplasia (GTN) is an aggressive malignancy arising from the trophoblastic tissue. It is rarely seen in association with advanced intrauterine pregnancy. Most common manifestations are due to bleeding caused by the rich vascularity of trophoblastic tissue. We describe here a case of a 28-year-old female patient who presented to us at 32 weeks of pregnancy with sudden onset dyspnea and hemodynamic instability. On evaluation, imaging techniques revealed a gross left hemothorax requiring intercostal tube insertion for stabilization. Emergency thoracotomy and hemothorax drainage were performed wherein a tumor mass in the lower lobe of left lung was identified and resected. Histopathological examination confirmed the diagnosis of choriocarcinoma. Beta HCG levels were found to be elevated. Final diagnosis of a FIGO stage IV high-risk gestational choriocarcinoma was made. Following this, six cycles of multi-agent EMA-CO chemotherapy was administered to the patient. Patient had an excellent response to treatment with documented serial fall in β HCG levels and she continues to be in remission after 6 months of follow-up. In conclusion, in the circumstance of any pregnant women presenting with abnormal bleeding symptoms such as hemothorax, choriocarcinoma as a cause should be considered for early diagnosis and effective management.
Declaration of Patient Consent
Informed consent of the patient was acquired.
Publication History
Article published online:
29 November 2022
© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Gestational trophoblastic neoplasia (GTN) is an aggressive malignancy arising from the trophoblastic tissue. It is rarely seen in association with advanced intrauterine pregnancy. Most common manifestations are due to bleeding caused by the rich vascularity of trophoblastic tissue. We describe here a case of a 28-year-old female patient who presented to us at 32 weeks of pregnancy with sudden onset dyspnea and hemodynamic instability. On evaluation, imaging techniques revealed a gross left hemothorax requiring intercostal tube insertion for stabilization. Emergency thoracotomy and hemothorax drainage were performed wherein a tumor mass in the lower lobe of left lung was identified and resected. Histopathological examination confirmed the diagnosis of choriocarcinoma. Beta HCG levels were found to be elevated. Final diagnosis of a FIGO stage IV high-risk gestational choriocarcinoma was made. Following this, six cycles of multi-agent EMA-CO chemotherapy was administered to the patient. Patient had an excellent response to treatment with documented serial fall in β HCG levels and she continues to be in remission after 6 months of follow-up. In conclusion, in the circumstance of any pregnant women presenting with abnormal bleeding symptoms such as hemothorax, choriocarcinoma as a cause should be considered for early diagnosis and effective management.
Introduction
Gestational trophoblastic neoplasia (GTN) represents a spectrum of proliferative abnormalities of trophoblasts associated with pregnancy.[1] Choriocarcinoma is a highly aggressive form of gestational trophoblastic disease presenting with early distant metastasis due to high metastatic potential.[2] Although molar pregnancies are most commonly associated with choriocarcinoma, 25% of cases follow previous abortions, while 22.5% arise in normal pregnancy, and 2.5% are subsequent to ectopic pregnancy. All cases are associated with elevated β human chorionic gonadotrophin (HCG) levels.[3]
While choriocarcinoma generally presents with gynecological manifestations such as vaginal bleeding, as many as one-third of the patients can manifest with only non-gynecological symptoms due to metastases to various sites.[4] Favored sites of metastases are lungs (80%), followed by vagina (30%), brain (10%), and liver (10%).[2] Abnormal bleeding at these locations could be the primary presentation due to the rich vascularity of lesions at these sites.[5] Pulmonary metastatic choriocarcinoma mainly presents with hemoptysis, dyspnea, pleuritic pain, and cough[6]; pleural effusion or non-traumatic hemothorax being rare manifestations.[7] Metastatic gestational choriocarcinoma presenting in a normal intrauterine pregnancy is exceptionally rare and is assigned a higher World Health Organization (WHO) score with historically high maternal and fetal mortality.[8] Herein, we report a rare case of spontaneous hemothorax in the third trimester of pregnancy leading to a diagnosis of choriocarcinoma.
Case Presentation
A 28-year-old housewife presented at 32 weeks of gestation to the institution with 2-day history of progressive dyspnea and reduced fetal movements. She was triaged as G2P1L1 with a 3-year-old female child and present spontaneous conception. Her first and second trimesters were uneventful. She developed mild breathlessness and discomfort 2 days ago, which subsided on their own, however, were aggravated on the morning of day of admission. She had no significant medical or family history.
Patient was irritable and tachypneic with signs of tachycardia and hypoxia. Clinical examination was significant for reduced breath sounds in the left hemithorax. A chest X-ray showed a massive pleural effusion in the left lung. A subsequent CT scan of the thorax revealed gross left hemothorax with internal organized hyperdense content causing a contralateral shift of mediastinum and compression of the left lung. A few focal small hyperdense organized hematomas were also observed along the left diaphragmatic pleura. Another small hypodense lesion with peripheral eccentric nodular enhancement in collapsed left lower lobe was suspected of being a capillary hemangioma ([Fig. 1A]). An intercostal drainage tube was placed to decompress the left hemithorax. She had severe anemia requiring multiple blood transfusions. The obstetric ultrasonogram showed a single intrauterine fetal demise (IUD) of 33 weeks 4 days gestation with a normal placenta.
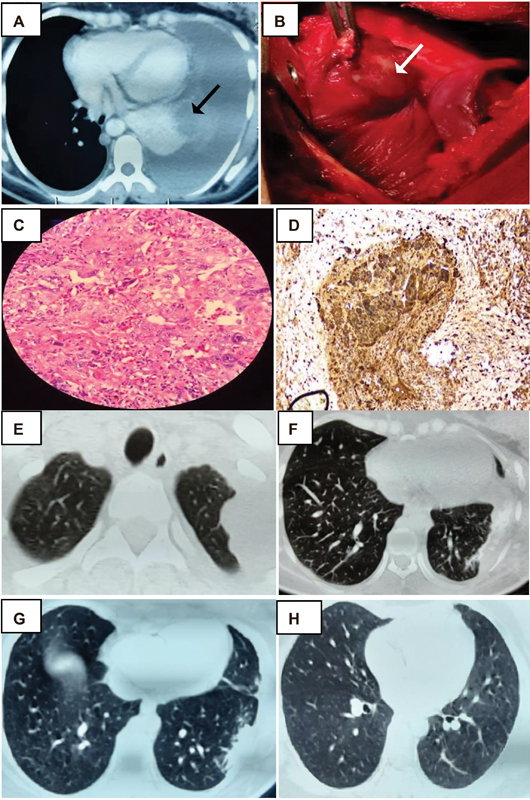
| Figure 1:CT thorax showing hemothorax, lesion in left lower lobe (black arrow). (A). Intraoperative lung mass (white arrow). (B) Syncitiotrophoblast surrounded by cytotrophoblast in biopsy and IHC positive for β HCG;. (C and D) Post-surgery CT thorax - residual lesions in the left upper and lower lobe. (E and F) CT thorax post treatment with soft calcified nodule over left upper lobe and normal lower lobe (G and H).
An emergency thoracotomy and hemothorax drainage with clot removal were performed. A mass in the lower lobe of the left lung ([Fig. 1B]) and another vascular mass in the apex of chest wall were identified. Wedge resection of the former site and sclerotherapy of the latter site were performed. The patient also underwent a lower segment cesarean section and a 2.2 kg preterm IUD fetus was delivered. The placental examination did not reveal any abnormality. The histopathological examination of the lung mass was suggestive of a poorly differentiated malignant tumor compatible with choriocarcinoma ([Fig. 1C]). These tumor cells showed positive immunohistochemical (IHC) staining for PANCK (pan cytokeratin) and β HCG suggestive of choriocarcinoma ([Fig. 1D]). Serum β HCG level was reported to be 25,600 mIU/mL and α fetoprotein level was found to be 5.3 ng/mL clinching the diagnosis of metastatic gestational choriocarcinoma.
Follow-up CT imaging of the thorax showed residual pleural-based lesions in apical segment of the left upper and lower lobes of the lungs ([Figs. 1E] and [1F]). An observation of left pleural minimal-organized hemothorax was also noted. CT scans of the abdomen and pelvis confirmed a bulky uterus with enhancing submucosal vasculature suggestive of post cesarean changes. MRI scan of the brain did not reveal any metastases.
A diagnosis of stage IV high-risk choriocarcinoma based on the International Federation of Gynecology and Obstetrics (FIGO) system was made. The WHO score was declared as 10 [antecedent pregnancy (2), HCG (2), site (4), number of metastases (1), size (1)]. The patient was started on chemotherapy that consisted of six cycles of intravenous EMA-CO protocol (etoposide 100 mg/m2, methotrexate 100 mg/m2 bolus, and 200 mg/m2 infusion over 12 hours and actinomycin D 0.5 mg on first day; etoposide 100 mg/m2, actinomycin D 0.5 mg on the second day with four doses of 15 mg folinic acid rescue every 12 hours from 24 hours after beginning of methotrexate; cyclophosphamide 600 mg/m2 and vincristine 1 mg/m2 on the eighth day and intrathecal methotrexate 12.5 mg) delivered every three weekly for all cycles.
There was remarkable response to chemotherapy in the form of shrinkage of metastatic foci and dramatic decline of serum β HCG to below-detectable levels. Post-therapy CT scan revealed a small nodule over the upper lobe of left lung with soft calcification ([Fig. 1G] and [1H]). The patient is currently on follow-up after 6 months and doing well with normal serum β HCG values ([Table 1]).
|
Date |
Beta HCG (mIU/mL) |
|
17/03/2021 |
25,600 |
|
31/03/2021 |
91,900 |
|
29/04/2021 |
1335 |
|
21/05/2021 |
23.66 |
|
25/06/2021 |
0.730 |
|
21/09/2021 |
0.187 |
|
19/10/2021 |
< 0.100 |
|
09/11/2021 |
< 0.100 |
|
16/02/2022 |
< 0.100 |
References
- Berkowitz RS, Goldstein DP. Chorionic tumors. N Engl J Med 1996; 335 (23) 1740-1748 DOI: 10.1056/NEJM199612053352306.
- Wreczycka-Cegielny P, Cegielny T, Oplawski M, Sawicki W, Kojs Z. Current treatment options for advanced choriocarcinoma on the basis of own case and review of the literature. Ginekol Pol 2018; 89 (12) 711-715 DOI: 10.5603/GP.a2018.0120.
- Braun-Parvez L, Charlin E, Caillard S. et al. Gestational choriocarcinoma transmission following multiorgan donation. Am J Transplant 2010; 10 (11) 2541-2546 DOI: 10.1111/j.1600-6143.2010.03275.x.
- Yu P, Diao W, Jiang X. A successfully treated metastatic choriocarcinoma coexistent with pregnancy: a case report of a 4-year follow-up. Medicine (Baltimore) 2016; 95 (21) e3505 DOI: 10.1097/MD.0000000000003505.
- Álvarez-Sarrado L, González-Ballano I, Herrero-Serrano R, Giménez-Molina C, Rodríguez-Solanilla B, Campillos-Maza JM. Hemoptysis as the first symptom in the diagnosis of metastatic choriocarcinoma in the third trimester of pregnancy: a case report. Case Rep Womens Health 2020; 27: e00211
- Mangla M, Singla D, Kaur H, Sharma S. Unusual clinical presentations of choriocarcinoma: a systematic review of case reports. Taiwan J Obstet Gynecol 2017; 56 (01) 1-8 DOI: 10.1016/j.tjog.2015.05.011.
- Saha K, Basuthakur S, Jash D, Bandyopadhyay A. Gestational choriocarcinoma presenting as hemothorax. Indian J Med Sci 2010; 64 (05) 237-240
- Steigrad SJ, Cheung AP, Osborn RA. Choriocarcinoma co-existent with an intact pregnancy: case report and review of the literature. J Obstet Gynaecol Res 1999; 25 (03) 197-203 DOI: 10.1111/j.1447-0756.1999.tb01147.x.
- Soper JT, Mutch DG, Schink JC. American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol 2004; 93 (03) 575-585 DOI: 10.1016/j.ygyno.2004.05.013.
- Buckley JD. The epidemiology of molar pregnancy and choriocarcinoma. Clin Obstet Gynecol 1984; 27 (01) 153-159 DOI: 10.1097/00003081-198403000-00022.
- Balagopal P, Pandey M, Chandramohan K, Somanathan T, Kumar A. Unusual presentation of choriocarcinoma. World J Surg Oncol 2003; 1 (01) 4
- Huang CY, Chen CA, Hsieh CY, Cheng WF. Intracerebral hemorrhage as initial presentation of gestational choriocarcinoma: a case report and literature review. Int J Gynecol Cancer 2007; 17 (05) 1166-1171 DOI: 10.1111/j.1525-1438.2007.00934.x.
- Kulkarni R, Lister UG. Metastatic choriocarcinoma coexisting with full term viable pregnancy. Postgrad Med J 1985; 61 (721) 1013-1014 DOI: 10.1136/pgmj.61.721.1013.
- Sudduth CD, Strange C, Campbell BA, Sahn SA. Metastatic choriocarcinoma of the lung presenting as hemothorax. Chest 1991; 99 (02) 527-528 DOI: 10.1378/chest.99.2.527b.
- DeFrance JH, Blewett Jr JH, Ricci JA, Patterson LT. Massive hemothorax: two unusual cases. Chest 1974; 66 (01) 82-84 DOI: 10.1378/chest.66.1.82.
- Wolkove N, Gervais A, Kreisman H, Frank H, Lachance RC. Choriocarcinoma presenting as hemothorax. Can Med Assoc J 1981; 124 (12) 1599-1600
- Lazovic B, Milenkovic V. Gestational choriocarcinoma with hemorrhagic pleural effusion. Arch Oncol 2010; 18 (03) 86-87 DOI: 10.2298/aoo1003086l.
- Yangki S. Pulmonary metastatic choriocarcinoma presenting as hemodynamically unstable patient with hemothorax. The Turkish Journal of Thoracic and Cardiovascular Surgery. 2016; 24 (03) 582-584 DOI: 10.5606/tgkdc.dergisi.2016.11699.
- Seetharaman ML, Arora R, Arora VK. Gestational choriocarcinoma with haemorrhagic pleural effusion. Indian J Chest Dis Allied Sci 1991; 33 (01) 39-42
- Buza N, Baine I, Hui P. Precision genotyping diagnosis of lung tumors with trophoblastic morphology in young women. Mod Pathol 2019; 32 (09) 1271-1280 DOI: 10.1038/s41379- 019–0275-z.
- Ngan HYS, Seckl MJ, Berkowitz RS. et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet 2018; 143 (Suppl. 02) 79-85 DOI: 10.1002/ijgo.12615.
- Dhanda S, Ramani S, Thakur M. Gestational trophoblastic disease: a multimodality imaging approach with impact on diagnosis and management. Radiol Res Pract 2014; 2014: 842751 DOI: 10.1155/2014/842751.
- Cagayan MS. High-risk metastatic gestational trophoblastic neoplasia. Primary management with EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine) chemotherapy. J Reprod Med 2012; 57 (5-6): 231-236
- May T, Goldstein DP, Berkowitz RS. Current chemotherapeutic management of patients with gestational trophoblastic neoplasia. Chemother Res Pract 2011; 2011: 806256 DOI: 10.1155/2011/806256.
- Frijstein MM, Lok CAR, Short D. et al. The results of treatment with high-dose chemotherapy and peripheral blood stem cell support for gestational trophoblastic neoplasia. Eur J Cancer 2019; 109: 162-171 DOI: 10.1016/j.ejca.2018.12.033.
- Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol 2017; 36 (02) 146-153 DOI: 10.1097/PGP.0000000000000305.
- Ghorani E, Kaur B, Fisher RA. et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet 2017; 390 (10110): 2343-2345 DOI: 10.1016/S0140-6736(17)32894-5.
- Clark JJ, Slater S, Seckl MJ. Treatment of gestational trophoblastic disease in the 2020s. Curr Opin Obstet Gynecol 2021; 33 (01) 7-12 DOI: 10.1097/GCO.0000000000000674.
- You B, Bolze PA, Lotz JP. et al. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort A of the TROPHIMMUN phase II trial. J Clin Oncol 2020; 38 (27) 3129-3137 DOI: 10.1200/JCO.20.00803.
- Soper JT. Gestational trophoblastic disease: current evaluation and management. [published correction appears in Obstet Gynecol. 2022 Jan 1;139(1):149] Obstet Gynecol 2021; 137 (02) 355-370 DOI: 10.1097/AOG.0000000000004240.
Address for correspondence
Suma Devaraj, MDDepartment of Medical Oncology, Institute of Medical Sciences & SUM HospitalBhubaneswar 751003, OdishaIndiaEmail: sumadevaraj26@gmail.comPublication History
Article published online:
29 November 2022© 2022. The Author(s). This is an open access article published by Thieme under the terms of the Creative Commons Attribution License, permitting unrestricted use, distribution, and reproduction so long as the original work is properly cited. (https://creativecommons.org/licenses/by/4.0/)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India

| Figure 1:CT thorax showing hemothorax, lesion in left lower lobe (black arrow). (A). Intraoperative lung mass (white arrow). (B) Syncitiotrophoblast surrounded by cytotrophoblast in biopsy and IHC positive for β HCG;. (C and D) Post-surgery CT thorax - residual lesions in the left upper and lower lobe. (E and F) CT thorax post treatment with soft calcified nodule over left upper lobe and normal lower lobe (G and H).
References
- Berkowitz RS, Goldstein DP. Chorionic tumors. N Engl J Med 1996; 335 (23) 1740-1748 DOI: 10.1056/NEJM199612053352306.
- Wreczycka-Cegielny P, Cegielny T, Oplawski M, Sawicki W, Kojs Z. Current treatment options for advanced choriocarcinoma on the basis of own case and review of the literature. Ginekol Pol 2018; 89 (12) 711-715 DOI: 10.5603/GP.a2018.0120.
- Braun-Parvez L, Charlin E, Caillard S. et al. Gestational choriocarcinoma transmission following multiorgan donation. Am J Transplant 2010; 10 (11) 2541-2546 DOI: 10.1111/j.1600-6143.2010.03275.x.
- Yu P, Diao W, Jiang X. A successfully treated metastatic choriocarcinoma coexistent with pregnancy: a case report of a 4-year follow-up. Medicine (Baltimore) 2016; 95 (21) e3505 DOI: 10.1097/MD.0000000000003505.
- Álvarez-Sarrado L, González-Ballano I, Herrero-Serrano R, Giménez-Molina C, Rodríguez-Solanilla B, Campillos-Maza JM. Hemoptysis as the first symptom in the diagnosis of metastatic choriocarcinoma in the third trimester of pregnancy: a case report. Case Rep Womens Health 2020; 27: e00211
- Mangla M, Singla D, Kaur H, Sharma S. Unusual clinical presentations of choriocarcinoma: a systematic review of case reports. Taiwan J Obstet Gynecol 2017; 56 (01) 1-8 DOI: 10.1016/j.tjog.2015.05.011.
- Saha K, Basuthakur S, Jash D, Bandyopadhyay A. Gestational choriocarcinoma presenting as hemothorax. Indian J Med Sci 2010; 64 (05) 237-240
- Steigrad SJ, Cheung AP, Osborn RA. Choriocarcinoma co-existent with an intact pregnancy: case report and review of the literature. J Obstet Gynaecol Res 1999; 25 (03) 197-203 DOI: 10.1111/j.1447-0756.1999.tb01147.x.
- Soper JT, Mutch DG, Schink JC. American College of Obstetricians and Gynecologists. Diagnosis and treatment of gestational trophoblastic disease: ACOG Practice Bulletin No. 53. Gynecol Oncol 2004; 93 (03) 575-585 DOI: 10.1016/j.ygyno.2004.05.013.
- Buckley JD. The epidemiology of molar pregnancy and choriocarcinoma. Clin Obstet Gynecol 1984; 27 (01) 153-159 DOI: 10.1097/00003081-198403000-00022.
- Balagopal P, Pandey M, Chandramohan K, Somanathan T, Kumar A. Unusual presentation of choriocarcinoma. World J Surg Oncol 2003; 1 (01) 4
- Huang CY, Chen CA, Hsieh CY, Cheng WF. Intracerebral hemorrhage as initial presentation of gestational choriocarcinoma: a case report and literature review. Int J Gynecol Cancer 2007; 17 (05) 1166-1171 DOI: 10.1111/j.1525-1438.2007.00934.x.
- Kulkarni R, Lister UG. Metastatic choriocarcinoma coexisting with full term viable pregnancy. Postgrad Med J 1985; 61 (721) 1013-1014 DOI: 10.1136/pgmj.61.721.1013.
- Sudduth CD, Strange C, Campbell BA, Sahn SA. Metastatic choriocarcinoma of the lung presenting as hemothorax. Chest 1991; 99 (02) 527-528 DOI: 10.1378/chest.99.2.527b.
- DeFrance JH, Blewett Jr JH, Ricci JA, Patterson LT. Massive hemothorax: two unusual cases. Chest 1974; 66 (01) 82-84 DOI: 10.1378/chest.66.1.82.
- Wolkove N, Gervais A, Kreisman H, Frank H, Lachance RC. Choriocarcinoma presenting as hemothorax. Can Med Assoc J 1981; 124 (12) 1599-1600
- Lazovic B, Milenkovic V. Gestational choriocarcinoma with hemorrhagic pleural effusion. Arch Oncol 2010; 18 (03) 86-87 DOI: 10.2298/aoo1003086l.
- Yangki S. Pulmonary metastatic choriocarcinoma presenting as hemodynamically unstable patient with hemothorax. The Turkish Journal of Thoracic and Cardiovascular Surgery. 2016; 24 (03) 582-584 DOI: 10.5606/tgkdc.dergisi.2016.11699.
- Seetharaman ML, Arora R, Arora VK. Gestational choriocarcinoma with haemorrhagic pleural effusion. Indian J Chest Dis Allied Sci 1991; 33 (01) 39-42
- Buza N, Baine I, Hui P. Precision genotyping diagnosis of lung tumors with trophoblastic morphology in young women. Mod Pathol 2019; 32 (09) 1271-1280 DOI: 10.1038/s41379- 019–0275-z.
- Ngan HYS, Seckl MJ, Berkowitz RS. et al. Update on the diagnosis and management of gestational trophoblastic disease. Int J Gynaecol Obstet 2018; 143 (Suppl. 02) 79-85 DOI: 10.1002/ijgo.12615.
- Dhanda S, Ramani S, Thakur M. Gestational trophoblastic disease: a multimodality imaging approach with impact on diagnosis and management. Radiol Res Pract 2014; 2014: 842751 DOI: 10.1155/2014/842751.
- Cagayan MS. High-risk metastatic gestational trophoblastic neoplasia. Primary management with EMA-CO (etoposide, methotrexate, actinomycin D, cyclophosphamide and vincristine) chemotherapy. J Reprod Med 2012; 57 (5-6): 231-236
- May T, Goldstein DP, Berkowitz RS. Current chemotherapeutic management of patients with gestational trophoblastic neoplasia. Chemother Res Pract 2011; 2011: 806256 DOI: 10.1155/2011/806256.
- Frijstein MM, Lok CAR, Short D. et al. The results of treatment with high-dose chemotherapy and peripheral blood stem cell support for gestational trophoblastic neoplasia. Eur J Cancer 2019; 109: 162-171 DOI: 10.1016/j.ejca.2018.12.033.
- Veras E, Kurman RJ, Wang TL, Shih IM. PD-L1 expression in human placentas and gestational trophoblastic diseases. Int J Gynecol Pathol 2017; 36 (02) 146-153 DOI: 10.1097/PGP.0000000000000305.
- Ghorani E, Kaur B, Fisher RA. et al. Pembrolizumab is effective for drug-resistant gestational trophoblastic neoplasia. Lancet 2017; 390 (10110): 2343-2345 DOI: 10.1016/S0140-6736(17)32894-5.
- Clark JJ, Slater S, Seckl MJ. Treatment of gestational trophoblastic disease in the 2020s. Curr Opin Obstet Gynecol 2021; 33 (01) 7-12 DOI: 10.1097/GCO.0000000000000674.
- You B, Bolze PA, Lotz JP. et al. Avelumab in patients with gestational trophoblastic tumors with resistance to single-agent chemotherapy: cohort A of the TROPHIMMUN phase II trial. J Clin Oncol 2020; 38 (27) 3129-3137 DOI: 10.1200/JCO.20.00803.
- Soper JT. Gestational trophoblastic disease: current evaluation and management. [published correction appears in Obstet Gynecol. 2022 Jan 1;139(1):149] Obstet Gynecol 2021; 137 (02) 355-370 DOI: 10.1097/AOG.0000000000004240.


 PDF
PDF  Views
Views  Share
Share

