Gemcitabine and cisplatin-based combination chemotherapy in advanced hepatocellular carcinoma: An Indian experience
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(01): 42-47
DOI: DOI: 10.4103/0971-5851.96968
Abstract
Background: Gemcitabine, an anti-metabolite, has some activity in hepatocellular carcinoma (HCC) in terms of responses and median survival. Aims: To analyze our experience with the use of gemcitabine in combination with cisplatin in HCC with respect to response, toxicity and survival. Materials and Methods: We studied the records of patients of HCC treated from January 2000 to December 2005 with gemcitabine and cisplatin, and found 24 of them to be evaluable for response, toxicity and survival. Results: Of 24 patients receiving three or more cycles of chemotherapy, six (25%) had a partial response and an additional 12 (50%) had stable disease. The median overall survival (OS) was 7.5 months (95% confidence interval, 4.5-10.5 months) and 1-year survival was 18%. Grade 3 and 4 anemia, thrombocytopenia and neutropenia were observed in, respectively, 17, 17 and 33% patients. The most frequent non-hematologic toxicities were nausea and vomiting and peripheral neuropathy. Conclusion: We report a partial response rate of 25% with stable disease in an additional 50% to three or more cycles of chemotherapy with gemcitabine and cisplatin, with a median OS of 7.5 months (95% confidence interval, 4.5-10.5) and acceptable toxicity profile from our single-center retrospective study of 24 patients of HCC. We trust that, in HCC, gemcitabine is a good drug to be the foundation to build the chemotherapeutic or targeted agents′ combinations on.
Keywords
Chemotherapy - cisplatin - gemcitabine - hepatocellular carcinoma - median overall survival - response evaluation criteria in solid tumors - time to progressionPublication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Gemcitabine, an anti-metabolite, has some activity in hepatocellular carcinoma (HCC) in terms of responses and median survival.
Aims:
To analyze our experience with the use of gemcitabine in combination with cisplatin in HCC with respect to response, toxicity and survival.
Materials and Methods:
We studied the records of patients of HCC treated from January 2000 to December 2005 with gemcitabine and cisplatin, and found 24 of them to be evaluable for response, toxicity and survival.
Results:
Of 24 patients receiving three or more cycles of chemotherapy, six (25%) had a partial response and an additional 12 (50%) had stable disease. The median overall survival (OS) was 7.5 months (95% confidence interval, 4.5–10.5 months) and 1-year survival was 18%. Grade 3 and 4 anemia, thrombocytopenia and neutropenia were observed in, respectively, 17, 17 and 33% patients. The most frequent non-hematologic toxicities were nausea and vomiting and peripheral neuropathy.
Conclusion:
We report a partial response rate of 25% with stable disease in an additional 50% to three or more cycles of chemotherapy with gemcitabine and cisplatin, with a median OS of 7.5 months (95% confidence interval, 4.5–10.5) and acceptable toxicity profile from our single-center retrospective study of 24 patients of HCC. We trust that, in HCC, gemcitabine is a good drug to be the foundation to build the chemotherapeutic or targeted agents’ combinations on.
INTRODUCTION
Hepatocellular carcinoma (HCC) is, worldwide, one of the leading causes of death from malignancies.[1] More than 50% of the patients present with locally advanced or extra-hepatic tumor. Surgical resection is the most definitive and rewarding treatment, but very few patients are candidates for surgical resection. Only approximately 20–40% of patients are candidates for resection due to the burden of hepatic tumor, the presence of extrahepatic spread or the extent of underlying liver disease.[2] Sorafenib is the only systemic therapy proven to prolong overall survival (OS) in patients with advanced HCC.[3] For those patients who are not eligible for sorafenib treatment or for those who have failed on sorafenib, the only therapeutic option is systemic chemotherapy. Gemcitabine and its combinations with other agents have yielded variable results in HCC.[4] In a phase 2 trial from India, the gemcitabine–cisplatin combination yielded an overall response rate of 25%, with 58% showing stable disease.[5] Impelled by this protocol, we treated our patients of advanced HCC with the gemcitabine and cisplatin combination.
MATERIALS AND METHODS
Patient selection
We retrospectively analyzed the patients diagnosed with HCC at our institute who were treated with gemcitabine and cisplatin after being found to have unresectable disease by the surgical oncologist. The diagnosis of HCC was confirmed by histopathology or by triple-phase multi-detector computed tomography (MDCT) or magnetic resonance imaging (MRI) scans in addition to elevated values of alfa-fetoprotein (AFP) using the guidelines proposed by the American Association for the Study of Liver Diseases (AASLD).[6] Patients receiving at least three cycles of chemotherapy with gemcitabine and cisplatin between January 2000 and December 2005 were selected for this analysis. The American Joint Committee on Cancer (AJCC, 1998 edition) stages were used for tumor staging, and clinical stages were classified according to the Barcelona Clinic Liver Cancer (BCLC) staging system.[7] Patients treated with the standard schedule of administration of the agents (given below) were selected. Those treated with deviant schedules of administration were not included in this study. Those who were selected for chemotherapy belonged to BCLC stage C that entailed performance status of 0 to 2, and Child-Pugh class A or B. All patients were chemotherapy-naïve. None of the patients had locoregional therapy with ethanol, trans-arterial chemoembolization (TACE) or radiofrequency ablation (RFA) before or after chemotherapy.
Schedule of administration
The schedule of administration of chemotherapy was as follows: Gemcitabine 1.2 g/m2 on days 1 and 8 as 30-min infusion and Cisplatin 75 mg/m2 divided in two doses administered on days 1 and 2 with aggressive pre- and post-chemotherapy hydration. Appropriate anti-emetic pre- and post-medications were administered.
Disease measurement and response assessment
The disease status was assessed at baseline, after three cycles and after completion of the chemotherapy program at the end of six cycles with CT or MRI scans of abdomen. Although AFP and liver enzymes were performed, AFP levels were not used in the response assessment. Response evaluation criteria in solid tumors (RECIST) criteria were used for assessment of response to the combination chemotherapy with gemcitabine and cisplatin.[8]
Toxicity documentation
Data of toxicity documented in the records were included; toxicity with regard to the criteria not documented in the patient records was deemed to be absent or unreportable. The National Cancer Institute Common Toxicity Criteria for adverse events (CTC, version 2.0) were used for toxicity analysis.[9]
RESULTS
Patient characteristics
A total of 24 patients were included in the analysis. Majority of the patients (19) were males, and five were females. The median age was 57.5 years (range 22–71 years). Of all the patients, 14 were in AJCC stage III and another 10 were in stage IV disease. All patients (24) belonged to the BCLC clinical stage C. Serum aspartate aminotransferase (AST) ranged from 25 to 104 U/L, alanine aminotransferase (ALT) ranged from 22 to 132 U/L, total bilirubin ranged from 0.4 to 1.5 mg/dL, serum albumin ranged from 2.8 to 4.7 g/dL and AFP ranged from 12 to 96000 ng/mL. Characteristics of the patients (age, gender, distribution with respect to TNM stage, Okuda stage, BCLC stage, Child-Pugh class, performance status and biochemistry profile) have been tabulated [Table 1].
Table 1
Characteristics of the chemotherapytreated patients
Response
Twenty-four patients were evaluated at least mid-therapy (i.e., after three cycles) for disease status. Six of our patients (25%) had partial response by RECIST criteria, with an additional 12 patients (50%) having stable disease [Table 2]. One patient had near-complete response (more than 90% regression in the hepatic mass) [Figures [Figures11 and and22].
Table 2
Toxicity
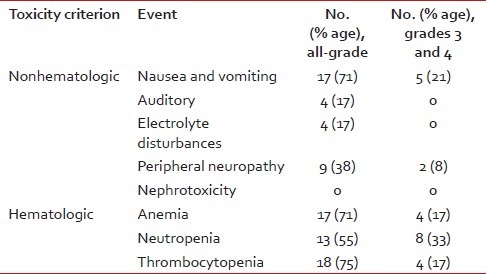
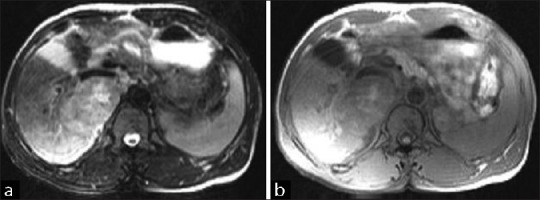
| Fig. 1 (a) Baseline scan (T2FS image) of a patient of hepatocellular carcinoma (HCC) before commencement of chemotherapy with gemcitabine and cisplatin. (b) Baseline scan (T1FS image) of a patient of HCC before commencement of chemotherapy with gemcitabine and cisplatin
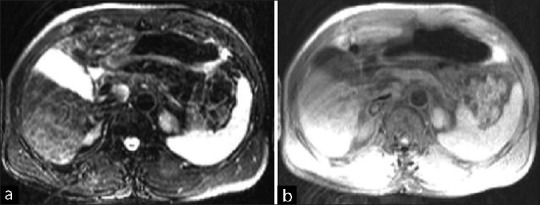
| Fig. 2 (a) T2FS image of very good partial response after six cycles of chemotherapy. (b) T1FS image of very good partial response after six cycles of chemotherapy
Toxicity
All 24 patients were eligible for toxicity analysis. A median number of four cycles of chemotherapy was delivered. Grade 3 and 4 anemia was observed in 17% patients. Grade 3 and 4 thrombocytopenia of 17% was observed. Grade 3 and 4 neutropenia was observed in 33% patients. Grade 3 and 4 non-hematological toxicities – nausea and vomiting – and peripheral neuropathy were seen in 21 and 8% patients, respectively. Electrolyte disturbances were encountered less commonly, as electrolyte supplementation was/is a part of the cisplatin administration protocol at our institute. None of the patients had delays, dose modifications or skipping of the dose.
Survival
For those patients of HCC who were given three or more cycles of chemotherapy with gemcitabine and cisplatin, the time to progression and median OS were, respectively, 12 weeks and 7.5 months (95% confidence interval, 4.5–10.5 months) for 23 patients, one patient being censored. The one-year survival was 18%. The Kaplan-Meier survival curve has been plotted in Figure 3.
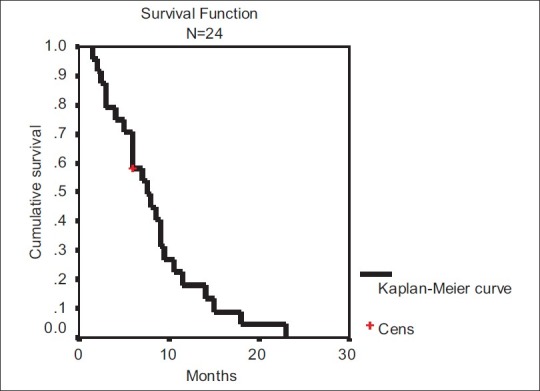
| Fig. 3 Kaplan-Meier survival curve of the patients treated with three or more cycles of chemotherapy based on gemcitabine and cisplatin
DISCUSSION
The age-adjusted incidence rate of HCC in India ranges in regional cancer registries from 1.9 to 3.4 cases per 100,000 population. The mean incidence rates of HCC in four population-based registries in India for males and females are, respectively, 2.77 and 1.38%.[10] The age-adjusted incidence of HCC in the United States has risen over the last three decades from 1.6 to 4.6 per 100,000 individuals.[11]
HCC is a disease with very abysmal prognosis. The survival rate in untreated HCC varies greatly: A median survival of less than 1 month from diagnosis in sub-Saharan African patients is contrasted by an average survival of 4 months in virus-induced HCC associated with cirrhosis.[12] Surgical resection is the only curative treatment available at this point in time, and only BCLC stage A1 patients are the candidates for it. Patients in BCLC stages A2-4 may be candidates for local therapies like percutaneous ethanol injection (PEI), RFA or cryosurgery. BCLC stage B patients may be treated with TACE.[7] A small minority of patients – BCLC stages A2-4 – meet Milan Criteria, University of California San Francisco criteria or United Network for Organ Sharing criteria for liver transplantation; many of those who meet the criteria do not have an access to liver transplantation for the lack of logistics in developing countries.[13,14]
HCC is a highly chemoresistant disease.[15] Systemic chemotherapy yields response rates of the order of 0–20%. Agents with some potency in HCC are doxorubicin, 5-FU, interferon and cisplatin. Gemcitabine is a pyrimidine anti-metabolite that exhibits a broad range of activity against a variety of tumors. It has been shown to have a minimal activity in HCC in phase II studies.[16–19] High-dose gemcitabine was shown to be of no great promise.[19] Gemcitabine was shown to have a modest activity in combination with doxorubicin.[20] With cisplatin, it showed a partial response rate of 20% and stable disease in 43% patients, and with oxaliplatin it was shown to yield a response rate of 19%.[21,22] In another phase II study, a triplet of gemcitabine, oxaliplatin and bevacizumab was found to have an objective response rate of 20%, along with the proportion of stable disease of additional 27%.[23]
Although the phase 1/2 study of gemcitabine in combination with doxorubicin by Yang et al. yielded a partial response rate of 11.8%, with a median survival of 4.6 months, in stark contrast, the most recent phase 2 study of gemcitabine in combination with pegylated liposomal doxorubicin (PLD) in advanced HCC by Lombardi et al. has shown a complete response rate of 7% and a partial response rate of 17% along with a median OS of 22.5 months.[20,24]
In a multicenter, double-blind, placebo-controlled randomized phase III trial (SHARP trial) for advanced HCC, sorafenib, a multi-tyrosine kinase inhibitor, was found to give a median OS of 10.7 months as against 7.9 months in the placebo group.[3] After the demonstration of this increase of nearly 3 months in OS, sorafenib became the drug of choice for advanced HCC.
In an anecdotal instance, pulmonary metastases from HCC were reported to have disappeared with docetaxel-based systemic chemotherapy.[25] Other agents demonstrated to have an activity in HCC in small retrospective and prospective studies are thalidomide and arsenic trioxide.[26,27]
As there is no standard treatment, our center was participating in a phase II multicentric study of gemcitabine and cisplatin in advanced HCC. Ethical committee waiver was obtained to give the same medications to patients not enrolled in the trial. Based on the promising results and favorable toxicity profile seen in that trial, those who were not eligible and who were willing to take this form of chemo were offered this combination. We evaluated our patients treated with gemcitabine and cisplatin. The magnitude of hematologic toxicities was the same as in any other study.[21] In our cohort, patients were assessed at the end of three cycles, and those who completed six cycles were assessed at the end of this period. Subsequent scans or investigations were performed only on the clinical or ultrasound evidence of progression.
Of the patients responding to the gemcitabine–cisplatin regimen, one patient with advanced, high-volume, bulky unresectable disease with adrenal involvement had very good partial response (PR) [Figures [Figures11 and and2]2] with more than 90% regression of the mass. He had a documented progression-free interval of 7 months after the completion of the chemotherapy program. At the end of the 7-month progression-free interval, he was operated (extended hepatectomy) at another center and had an OS of 26 months from the onset of symptoms.[28]
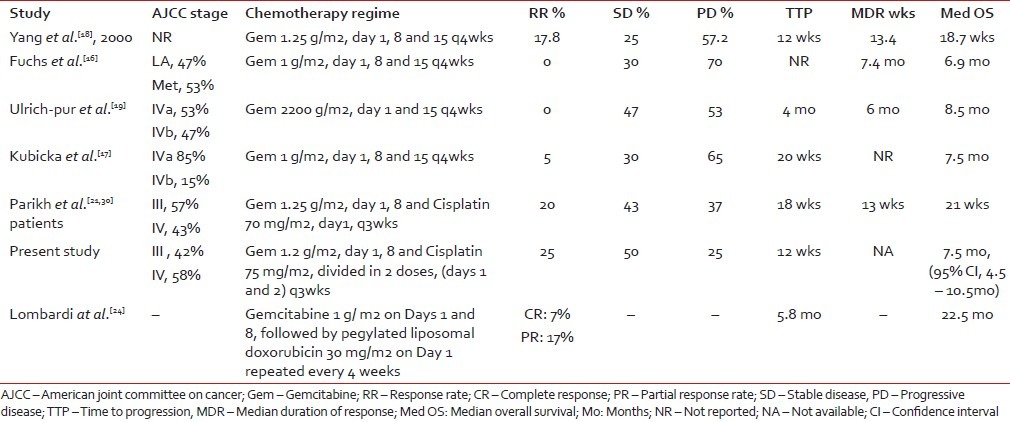
Table 3 shows the cross-trial comparison of the present study of the authors with the gemcitabine-based arms of studies by other authors. Except for the most recent phase 2 study of gemcitabine and PLD that yielded a 7% complete response and median OS of 22.5 months, compared with all other phase 2 trials of gemcitabine, alone or in combination with cisplatin, our retrospective analysis gives almost similar information with respect to response rate and median OS. Yang et al. reported a response rate of 17.8% with gemcitabine alone.[18] Other investigators, namely Fuchs et al., Kubicka et al. and Ulrich-Pur et al., have reported irreproducibility of similar response rates.[16,17,19] Parikh et al. reported a response of 20% in a cohort of 30 patients to the gemcitabine–cisplatin combination; our 25% response is comparable.[21]
Table 3
Cross-study comparison of the studies of gemcitabine with or without cisplatin
 With the advent of sorafenib, chemotherapy for advanced HCC had waned. But, if the sharply increased median survival of 22.5 months demonstrated by Lombardi et al. with gemcitabine and PLD can be replicated in further studies, there will likely be a regeneration of interest in chemotherapy in advanced HCC, and sorafenib or other targeted agents with chemotherapy combinations may be tested in the future. As the best response obtained with sorafenib was only stable disease, to improve the results further, we need to explore either combination with other targeted agents or chemotherapeutic agents. That could be the answer to the question: “where next after sorafenib?”[29]
With the advent of sorafenib, chemotherapy for advanced HCC had waned. But, if the sharply increased median survival of 22.5 months demonstrated by Lombardi et al. with gemcitabine and PLD can be replicated in further studies, there will likely be a regeneration of interest in chemotherapy in advanced HCC, and sorafenib or other targeted agents with chemotherapy combinations may be tested in the future. As the best response obtained with sorafenib was only stable disease, to improve the results further, we need to explore either combination with other targeted agents or chemotherapeutic agents. That could be the answer to the question: “where next after sorafenib?”[29]CONCLUSION
We report a response rate of 25% with stable disease in an additional 50% to three or more cycles of chemotherapy with gemcitabine and cisplatin with a median OS of 7.5 months (95% confidence interval, 4.5–10.5) and acceptable toxicity profile from our single-center retrospective study of selected patients with HCC.
Footnotes
Source of Support: Nil
REFERENCES

| Fig. 1 (a) Baseline scan (T2FS image) of a patient of hepatocellular carcinoma (HCC) before commencement of chemotherapy with gemcitabine and cisplatin. (b) Baseline scan (T1FS image) of a patient of HCC before commencement of chemotherapy with gemcitabine and cisplatin

| Fig. 2 (a) T2FS image of very good partial response after six cycles of chemotherapy. (b) T1FS image of very good partial response after six cycles of chemotherapy

| Fig. 3 Kaplan-Meier survival curve of the patients treated with three or more cycles of chemotherapy based on gemcitabine and cisplatin


 PDF
PDF  Views
Views  Share
Share

