Gastric Teratoma: An Unusual Presentation and Location
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 563-565
DOI: DOI: 10.4103/ijmpo.ijmpo_182_16
Abstract
The gastric teratoma is a rare tumor that usually presents as an abdominal mass, with or without features of gastric outlet obstruction. We report two cases of gastric teratoma; one – mature in a male neonate and another – ruptured immature gastric teratoma in a female neonate.
Publication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The gastric teratoma is a rare tumor that usually presents as an abdominal mass, with or without features of gastric outlet obstruction. We report two cases of gastric teratoma; one – mature in a male neonate and another – ruptured immature gastric teratoma in a female neonate.
Introduction
Gastric teratomas are rare neoplasms, which account for <1 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759085/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659254237" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1,2] They mostly present as exogastric growths but can have exogastric as well as endogastric components.[1] They are classified into mature and immature teratomas based on the presence and degree of differentiation of neuroglial tissue. Mature gastric teratomas are benign and have a good prognosis after complete surgical excision.[1]
Case Reports
Case 1
A 5-day-old male neonate brought to us with nonbilious vomiting with progressively increasing abdominal distention since birth. The baby was found to have retroperitoneal mass at 32 weeks of gestation on ultrasonography (USG) scan and was born by full-term normal vaginal delivery with birth weight of 3.15 kg. Postnatal evaluation with USG abdomen and contrast-enhanced computed tomography (CECT) scan revealed heterogeneous mass of solid and cystic components with chunky calcifications, arising from the upper abdomen [Figure 1a]. Serum alfa fetoprotein (AFP) and Beta human chorionic gonadotrophin (HCG) were within the normal range. On laparotomy, we found well-encapsulated tumor having mixed solid and cystic lesion arising from posterior wall of stomach extending from greater curvature to lesser curvature and into the lesser sac having endogastric component of 2 cm × 3 cm with exogastric component of 6 cm × 7 cm. Mass was excised completely, keeping 1 cm margin, and the defect left extending from gastroesophageal junction (GEJ) to the antrum was closed in two layers with feeding jejunostomy tube placed. Postoperative period was uneventful. Histopathological examination (HPE) revealed various derivatives of ectoderm, endoderm, and mesoderm. Ectodermal derivatives included stratified squamous epithelium along with skin adnexal structures such as hair follicles. Among the endodermal derivatives, mucinous glands lined by cuboidal to columnar epithelium were found. Whereas mesodermal derivatives included mature cartilaginous tissue, hence overall histological picture was suggestive of mature gastric teratoma [Figure 2a and andb].b]. The patient is doing well at 3-year follow-up without any chemotherapy or radiotherapy.
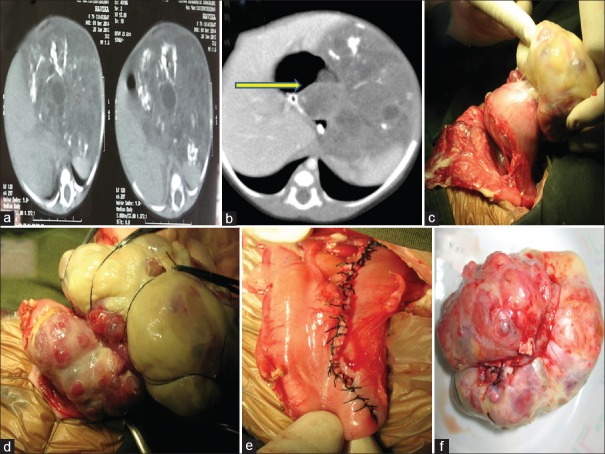
| Figure 1:(a) Computed tomography scan picture of mature gastric teratoma with areas of chunky calcifications. (b) Computed tomography scan picture of immature gastric teratoma showing heterogeneous tumor with specks of calcification; yellow-colored arrow pointing at intragastric component. (c) Ruptured immature gastric teratoma with slough over the surface, arising from anterior wall of stomach. (d) Intraoperative picture showing intragastric component. (e) Suture line after complete excision, extending from gastroesophageal junction to antrum. (f) Excised gastric teratoma

| Figure 2:Histopathological examination pictures in low power view. (a) Mature gastric teratoma showing skin and adnexal tissue. White-colored arrow pointing at cartilaginous tissue. (b) Mature gastric teratoma showing endodermal derivatives, i.e., glands and mesenchymal derivatives such as cartilaginous tissue. Yellow-colored arrow pointing at the multiple mucinous glands. (c) Immature gastric teratoma-low power view showing multiple tubular structures lined by primitive neuroepithelium. Blue-colored arrow pointing at a tubular structure lined by neuroepithelium. (d) Red-colored arrow pointing dentine epithelium suggestive of immature gastric teratoma
Case 2
A 1-month-old female baby born by full-term cesarean section, with birth weight of 2.8 kg, presented to us with progressive abdominal distention with breathing difficulty for 5 days. The routine antenatal USG scans were normal. Postnatal USG revealed large intraabdominal tumor of solid and cystic components, and CECT scan revealed large intraabdominal tumor with solid and cystic lesion having specks of calcification [Figure [Figure1b].1b]. At presentation, the baby had abdominal distention with respiratory difficulty. Serum beta HCG, AFP, and LDH were within normal range. On laparotomy, we found variegated mass of 10 cm × 8 cm arising from the anterior wall of stomach extending up to GE junction having endogastric as well exogastric component with tumor ruptured and gross peritoneal spillage [Figure [Figure1c,1c, ,dd and andf].f]. The tumor was resected with the margin of 1 cm and the resultant defect from GEJ to antrum was closed in two layers [Figure [Figure1e].1e]. The peritoneal cavity was washed thoroughly with normal saline. Postoperative period was uneventful. HPE revealed multiple tubules of varying sizes lined by primitive neuroepithelium which contributed to <10 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759085/figure/F2/" target="figure" class="fig-table-link figpopup" rid-figpopup="F2" rid-ob="ob-F2" co-legend-rid="lgnd_F2" xss=removed>[Figure2c2c and andd].d]. Baby is doing well at a year of follow-up.
Discussion
Gastric teratomas (GTs) are rare tumors and constitute 1% of all the teratomas in the body. Most patients present in the neonatal period with male predilection. There are only nine reported female infants with GTs in the English literature till 2004, and in our series, one was a female neonate. Only about 112 cases of GTs are recorded till 2012, of which <15 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759085/#ref1" rid="ref1" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659254240" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>1] Typical presenting symptoms are abdominal lump, distension, and vomiting, but tumors with intramural extension presenting with gastrointestinal bleeding and gastric perforation have also been reported in literature.[3] GT presents as a palpable mass in 75% and/or abdominal distension in 56%.[2] Other rare presentations are peritonitis secondary to gastric perforation and tumor rupture.[4] In our series, case 1 presented with antenatal diagnosis of tumor and presented with abdominal distention. Case 2 presented with respiratory distress and early peritonitis which was unusual. Both of our patients had intragastric component along with major extragastric component and surprisingly had no bleeding or obstruction [Figure [Figure1b1b and andd].d]. Most of these tumors arise from the greater curvature and posterior wall of the stomach.[3] The growth of GTs has been exogastric in 65%, endogastric in 9%, and endogastric/exogastric in 26% of reported cases;[2,5,6]. both of our patients had the tumor arising from the lesser curvature with extension to GEJ which was not commonly reported. Case 2 had tumor which was arising from the anterior wall of the stomach in continuation from the lesser curvature which in itself is a rarity [Figure 1c].
Preoperative evaluation will require CECT abdomen and tumor markers apart from routine USG. In most cases, tumor markers are elevated, and they can be used as a prognostic marker in the follow-up. As a general approach, complete surgical excision is sufficient for mature teratoma and Grade 1 and 2 immature teratoma if serum AFP and beta HCG values are within normal range for the age and there is no malignant germ cell element. Elevated serum AFP levels may be the only alerting sign of the presence of malignant yolk sac component. However, the diagnostic utility of these oncofetal proteins is less in young infants because of the physiologically elevated levels.[5] In our series also, the tumor markers were within the age range. CECT abdomen did reveal a large heterogeneous mass arising from the retroperitoneum to the left of abdomen [Figure 1a and andb].b]. In both the patients, the gastric origin of the tumor was not identified preoperatively but was suspected in view of close proximity of the stomach to the tumor.
Complete surgical excision will result in good long-term outcome.[3] Mature teratoma and immature teratoma Grade 1 and 2 will only require total excision and no adjuvant therapy.[5] In both of our babies, we were able to achieve complete excision with rim of normal stomach. In case 2, the tumor was involving the GEJ, and the anterior esophageal defect was repaired with fundal flap covering [Figure 1e]. Both the patients had uneventful recovery.
Single most important prognostic factor is the histopathological grading of the tumor as the degree of immaturity correlates with the ultimate prognosis of children. Teratomas are neoplasms composed of elements representing all three germ layers and are subdivided into mature or immature subtypes depending on constituent element. Grade 0 is mature and regarded as benign, Grade 1 is immature with <10 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759085/#ref2" rid="ref2" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659254232" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>2,3,5,7,8] In our case 2, histology revealed tissue lined by dentine epithelium and primitive neuro epithelium comprising less than 10% of microscopic field, suggesting immature gastric teratoma grade 1.
Marina et al. suggested that it seems safe to treat all patients with extragonadal immature teratomas by surgical excision followed by close observation, withholding chemotherapy until there is evidence of disease recurrence. Although adjuvant therapies (i.e., chemotherapy or radiotherapy) are not recommended with completely resected Grades 2 and 3 immature teratomas, optimal treatment in these groups is still controversial.[5]
Conclusion
GTs are rare and more so in female neonates. Surgery is the primary treatment modality and has good outcome. We are reporting these cases for their rarity of presentations, difficulty in preoperative diagnosis, and their unusual locations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Sugandhi N, Gupta AK, Bhatnagar V. Gastric teratoma: Unusual location and difficulties in diagnosis. Trop Gastroenterol 2012;33:75-7.
- Yoon SE, Goo HW, Jun S, Lee IC, Yoon CH. Immature gastric teratoma in an infant: A case report. Korean J Radiol 2000;1:226-8.
- Kumar V, Godara R, Bharadwaj R, Arora M. Gastric teratoma-unusual cause of neonatal obstructive jaundice: A case report. Indian J Surg 2013;75:421-4.
- Ahmed H, Al-Salem A. Immature gastric teratoma in a newborn. Med Case Stud 2012;3:12-6.
- Marina NM, Cushing B, Giller R, Cohen L, Lauer SJ, Ablin A, et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children's Cancer Group Intergroup Study. J Clin Oncol 1999;17:2137-43.
- Ijaz L, Aslam I, Sheikh A, Mirza B. Mature gastric teratoma: The mixed exogastric and endogastric variety. APSP J Case Rep 2011;2:17.
- Ramani M, Husain KW, Geetha K. Histopathological spectrum of teratomas in paediatric age group. J Evol Med Sci Dent Sci 2013;322:6001-10.
- Cushing B, Perlman EJ, Marina NM, Castleberry RP. Germ cell tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2002. p. 1091-113.

| Figure 1:(a) Computed tomography scan picture of mature gastric teratoma with areas of chunky calcifications. (b) Computed tomography scan picture of immature gastric teratoma showing heterogeneous tumor with specks of calcification; yellow-colored arrow pointing at intragastric component. (c) Ruptured immature gastric teratoma with slough over the surface, arising from anterior wall of stomach. (d) Intraoperative picture showing intragastric component. (e) Suture line after complete excision, extending from gastroesophageal junction to antrum. (f) Excised gastric teratoma

| Figure 2:Histopathological examination pictures in low power view. (a) Mature gastric teratoma showing skin and adnexal tissue. White-colored arrow pointing at cartilaginous tissue. (b) Mature gastric teratoma showing endodermal derivatives, i.e., glands and mesenchymal derivatives such as cartilaginous tissue. Yellow-colored arrow pointing at the multiple mucinous glands. (c) Immature gastric teratoma-low power view showing multiple tubular structures lined by primitive neuroepithelium. Blue-colored arrow pointing at a tubular structure lined by neuroepithelium. (d) Red-colored arrow pointing dentine epithelium suggestive of immature gastric teratoma
References
- Sugandhi N, Gupta AK, Bhatnagar V. Gastric teratoma: Unusual location and difficulties in diagnosis. Trop Gastroenterol 2012;33:75-7.
- Yoon SE, Goo HW, Jun S, Lee IC, Yoon CH. Immature gastric teratoma in an infant: A case report. Korean J Radiol 2000;1:226-8.
- Kumar V, Godara R, Bharadwaj R, Arora M. Gastric teratoma-unusual cause of neonatal obstructive jaundice: A case report. Indian J Surg 2013;75:421-4.
- Ahmed H, Al-Salem A. Immature gastric teratoma in a newborn. Med Case Stud 2012;3:12-6.
- Marina NM, Cushing B, Giller R, Cohen L, Lauer SJ, Ablin A, et al. Complete surgical excision is effective treatment for children with immature teratomas with or without malignant elements: A Pediatric Oncology Group/Children's Cancer Group Intergroup Study. J Clin Oncol 1999;17:2137-43.
- Ijaz L, Aslam I, Sheikh A, Mirza B. Mature gastric teratoma: The mixed exogastric and endogastric variety. APSP J Case Rep 2011;2:17.
- Ramani M, Husain KW, Geetha K. Histopathological spectrum of teratomas in paediatric age group. J Evol Med Sci Dent Sci 2013;322:6001-10.
- Cushing B, Perlman EJ, Marina NM, Castleberry RP. Germ cell tumors. In: Pizzo PA, Poplack DG, editors. Principles and Practice of Pediatric Oncology. Philadelphia: Lippincott Williams and Wilkins; 2002. p. 1091-113.


 PDF
PDF  Views
Views  Share
Share

