First report of upfront treatment with Gefitinib in comparison with chemotherapy in advanced non-small cell lung cancer patients from south India: Analysis of 120 patients
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(03): 146-154
DOI: DOI: 10.4103/0971-5851.103141
Abstract
Background: Lung cancer is the most common cause of cancer deaths in males and sixth among females in south India. Lung cancer is being increasingly recognized among non-smokers. Materials and Methods: Stage IIIB and IV advanced non-small cell lung cancer (NSCLC) patients (n=120) treated from January 2009 to December 2010 were retrospectively analyzed. Baseline clinical parameters, treatment protocol, response to therapy and survival were noted. Decision to use upfront Gefitinib was based on parameters like female sex, non-smoking status, adenocarcinoma histology and poor PS. Progression-free survival (PFS) and overall survival (OS) were analyzed by the Kaplan Meier method and prognosis by log rank test and Cox regression. Results: Baseline parameters: median age: 60 years (22-78 years); male sex: 83 (69.2%); Stage IV: 95(79.2%); adenocarcinoma: 109 (90.8%); smokers: 66 (55%); PS 2/3: 65(54.2%); first-line therapy: Gefitinib: 47 (39.2%), chemotherapy: 73 (60.8%). Among those progressing after chemotherapy, 17 (23%) received second-line Gefitinib. After a median follow-up of 7.5 months (1-26 months), median PFS and OS were 5 months (0-23 months) and 7.5 months (1-26 mo), respectively. On univariate analysis, PFS was significantly improved for non-smokers (7 months vs 4 months, P=0.010), females (7 months vs 5 months, P=0.024) and upfront treatment with Gefitinib (10 months vs 4 months, P=0.014). The only significant factor that affected OS was female sex (18 months vs 9 months, P=0.042). No factors were significant on multivariate analysis. Among PS 2/3 patients, PFS was significantly higher with Gefitinib (n=36) than with single-agent chemotherapy (n=29) [median PFS of 10 months vs 4 months (P=0.017)]. Conclusion: In the largest series on the use of first-line Gefitinib from India, we found it to be a useful agent in the treatment of NSCLC, especially in females patients with poor PS and non-smokers, even without Epidermal Growth Factor Receptor (EGFR) mutation testing. Second-line Gefitinib may have negated the OS differences. However, EGFR mutation studies may help in further individualization of therapy.
Publication History
Article published online:
02 August 2021
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background:
Lung cancer is the most common cause of cancer deaths in males and sixth among females in south India. Lung cancer is being increasingly recognized among non-smokers.
Materials and Methods:
Stage IIIB and IV advanced non-small cell lung cancer (NSCLC) patients (n=120) treated from January 2009 to December 2010 were retrospectively analyzed. Baseline clinical parameters, treatment protocol, response to therapy and survival were noted. Decision to use upfront Gefitinib was based on parameters like female sex, non-smoking status, adenocarcinoma histology and poor PS. Progression-free survival (PFS) and overall survival (OS) were analyzed by the Kaplan Meier method and prognosis by log rank test and Cox regression.
Results:
Baseline parameters: median age: 60 years (22–78 years); male sex: 83 (69.2%); Stage IV: 95(79.2%); adenocarcinoma: 109 (90.8%); smokers: 66 (55%); PS 2/3: 65(54.2%); first-line therapy: Gefitinib: 47 (39.2%), chemotherapy: 73 (60.8%). Among those progressing after chemotherapy, 17 (23%) received second-line Gefitinib. After a median follow-up of 7.5 months (1–26 months), median PFS and OS were 5 months (0–23 months) and 7.5 months (1–26 mo), respectively. On univariate analysis, PFS was significantly improved for non-smokers (7 months vs 4 months, P=0.010), females (7 months vs 5 months, P=0.024) and upfront treatment with Gefitinib (10 months vs 4 months, P=0.014). The only significant factor that affected OS was female sex (18 months vs 9 months, P=0.042). No factors were significant on multivariate analysis. Among PS 2/3 patients, PFS was significantly higher with Gefitinib (n=36) than with single-agent chemotherapy (n=29) [median PFS of 10 months vs 4 months (P=0.017)].
Conclusion:
In the largest series on the use of first-line Gefitinib from India, we found it to be a useful agent in the treatment of NSCLC, especially in females patients with poor PS and non-smokers, even without Epidermal Growth Factor Receptor (EGFR) mutation testing. Second-line Gefitinib may have negated the OS differences. However, EGFR mutation studies may help in further individualization of therapy.
INTRODUCTION
Lung cancer is the most common cause of cancer deaths in males and ranks sixth among females in Chennai, South India. In The Madras Metropolitan Tumour Registry (MMTR), Chennai, lung cancer is the most common cancer among males (10.9% of all cancers in males) and ranked seventh among females (3.3% of all cancers among females).[1] Lung cancer is being increasingly recognized among non-smokers, although smoking remains the main etiology in the majority (80–85%) of patients.[2] The major causes for non-smoking-related lung cancer include, but are not limited to, exposure to environmental tobacco smoke (include passive smoking), radon, cooking fumes, asbestos, heavy metals, Human Papilloma Virus HPV, hormonal factors and inherited genetic susceptibility.[3] In a recent metaanalysis of studies assessing the risk of developing lung cancer in never-smoking women due to passive smoking from spouses, the pooled relative risk ranges from 1.15 to 1.31.[4] The relative contribution of passive smoking, exposure to cooking fumes, HPV and hormonal factors either as an independent factor or in combination in the causation of non-smoking-related lung cancer in India is largely unknown. Non-smoking-related lung cancer is being identified as a distinct entity with possible specific etiopathogenesis, both at the molecular and at the clinical level in terms of presentation, behavior, prognosis, response to specific targeted therapy and systemic chemotherapy.[5]
Earlier studies and a large recent publication from India regarding the treatment outcome of non-small cell lung cancer (NSCLC) patients has shown a median overall survival (OS) ranging from 27 to 38 weeks (6.2–8.7 months) and a median OS of 7 months for the cisplatin-based combination chemotherapy, respectively.[6–8]
The epidermal growth factor tyrosine kinase inhibitor Gefitinib and Erlotinib are now approved for first-line treatment of NSCLC tumors carrying the EGFR mutation.[9,10] Clinical experience with Gefitinib in Indian patients are available only as second-line therapy (for advanced or refractory NSCLC) in the form of subset analysis in the IRESSA Survival Evaluation in Lung (ISEL) study and Gefitinib expanded-access program in India.[11–13] However, clinical data on the use of Gefitinib as first-line treatment in advanced NSCLC in Indian patients is non-existent. Moreover, data comparing first-line chemotherapy versus first-line Gefitinib in advanced NSCLC patients from India is virtually not available.
Here, in this study, we present our first report on the upfront treatment of advanced unresectable stage III/stage IV NSCLC patients with Gefitinib and compare it with patients receiving first-line chemotherapy from South India. We found a significant PFS benefit for the female patients, non-smokers and upfront treatment with Gefitinib with significant OS benefit only for female NSCLC patients. Among PS 2/3 patients, PFS was significantly higher with Gefitinib than with single-agent chemotherapy.
MATERIALS AND METHODS
Stage IIIB and IV advanced NSCLC patients (n=120) treated from January 2009 to December 2010 were retrospectively analyzed by chart review. There were 186 registered cases during the above period, of which 120 patients were evaluable. The non-selected patients were either not treated, wished to take further treatment elsewhere, proven to be infectious or histology other than classical bronchial epithelial cancer, or had early-stage disease. The procedures followed were in accordance with the ethical standards of the Helsinki Declaration of 1975. Baseline clinical parameters including patient age, sex, ECOG performance status (PS), smoking status, stage, histology, treatment protocol including first-line therapy/agent used, response to therapy, time to progression, second-line agent used and survival were noted. Lifetime never smokers have been taken as non-smokers and the smokers′ category included those patients who have smoked cigarettes/beedies/used tobacco for more than at least 10 years. Former smokers and those who had quit smoking not more than 10 years ago have been included in the smoking category. Decision to use upfront Gefitinib (generic Gefitinib 250 mg, oral, once daily) was based on parameters like female sex, non-smoking status, adenocarcinoma histology and poor performance status as EGFR mutation data was not available in the majority. Poor PS patients included PS 2/3 patients thought not fit to receive either single-agent or doublet IV chemotherapy. Proper adenocarcinoma histology and pathologist report of “NSCLC not otherwise specified” were included in the adenocarcinoma histology for analysis purpose. IHC verification of NSCLC not otherwise specified type is done only for few. Only proper squamous cell carcinoma or carcinoma of squamous origin report is included in squamous histology. First-line chemotherapy options included the following schedules: platinum-based doublet like Cisplatin + Etoposide (EP schedule, Cisplatin 70 mg/ m2 D1 + Etoposide 100 mg/m2 D1-3 Q 3 weeks), Gemcitabine + Carboplatin (GC schedule, Gemcitabine 1250 mg/m2 D1,8 + Carboplatin AUC 5 Q 3 weeks), Pemetrexed + Carboplatin (Pem + Carb, Pemetrexed 500 mg/m2 D1 + Carboplatin AUC 5 Q 3 weeks), single-agent oral etoposide (Cap. Etoposide 50 mg/d 7-14/28 days per cycle). Patients who had completed at least one cycle of chemotherapy were included for intention to treat analysis. Standard response assessment was used to assign response category. Progression-free survival (PFS) and OS were analyzed by the Kaplan Meier method and prognosis by the log rank test and Cox regression.
RESULTS
Baseline clinical parameters
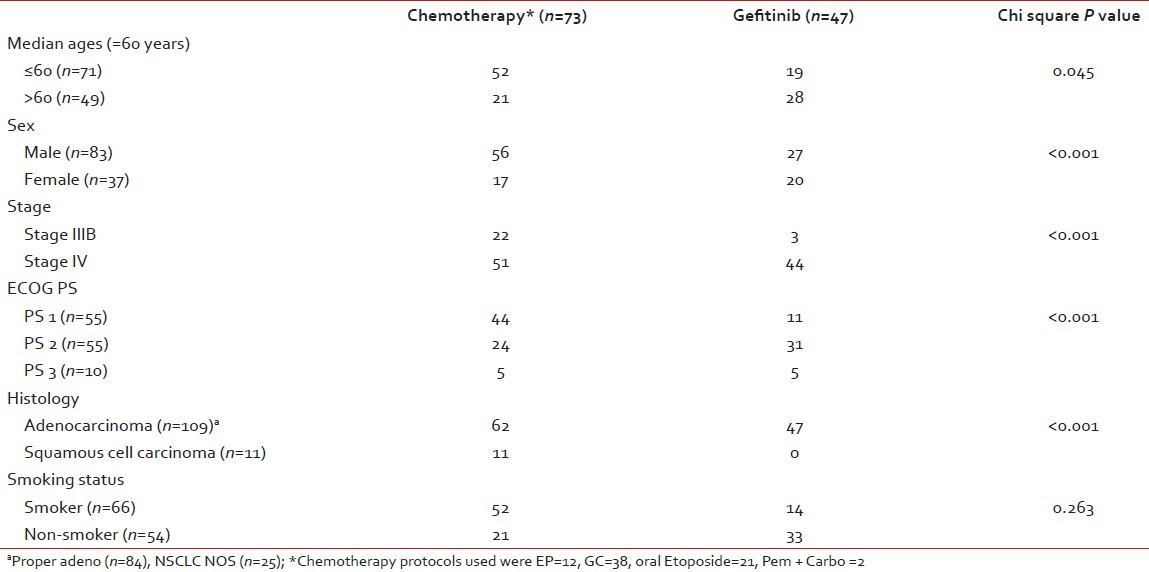
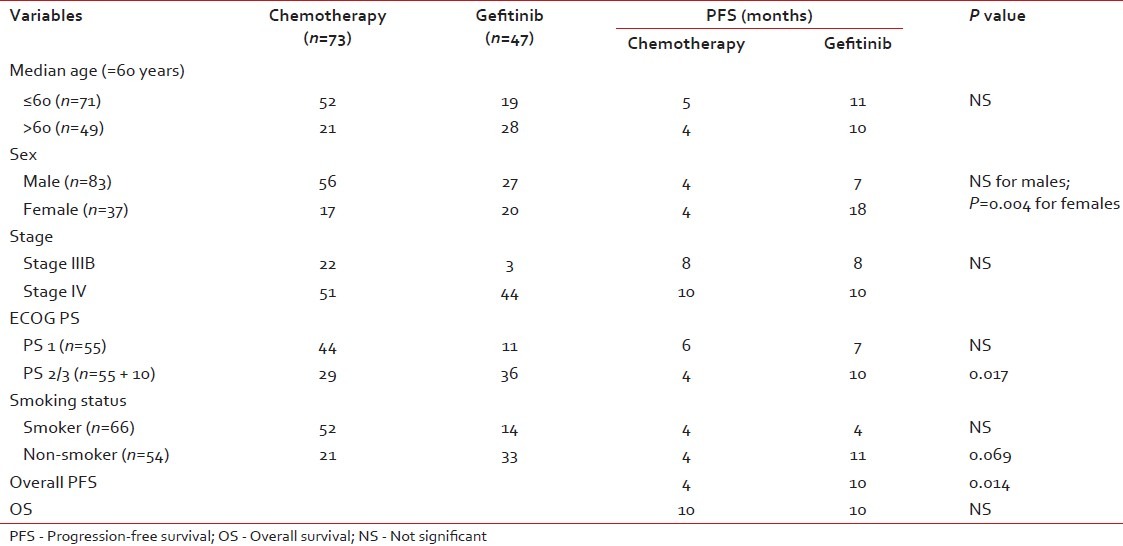
The median age of the study population was 60 years (22–78 years), with 83(69.2%) males and 37 (30.8%) females. Ninety-five patients (79.2%) were Stage IV and the remaining 25 (20.8%) had unresectable stage III disease [Table 1]. At the time of presentation, 55 patients (45.8%) were in PS 1/PS 2 and 10 patients (8.3%) were in PS 3. PS 2/3 constituted 65 (54.2%) of the total study population. Adenocarcinoma histology constituted 109 (90.8%) of the total and the remaining 11 (9.1%) constituted squamous cell carcinoma. Smoking and non-smoking patients constituted 55% and 45%, respectively. First-line chemotherapy was administered in 73 patients (60.8%) while 47 patients (39.2%) received first-line Gefitinib. Only 33 patients out of 120 patients (27.5%) received second-line therapy [Gefitinib (n=17) and chemotherapy (n=16)].
Table 1
Baseline clinical parameter distribution between upfront chemotherapy arm and upfront Gefitinib arm
Response assessment
Table 2 shows the response assessment for chemotherapy versus front-line Gefitinib. No CR was documented in either arm. However, there was a significant survival difference between the partial response (PR, n=16), stable disease (SD, n=49) and progressive disease (PD, n=55) categories (P=0.001).
Table 2
Response assessment for chemotherapy vs front-line Gefitinib
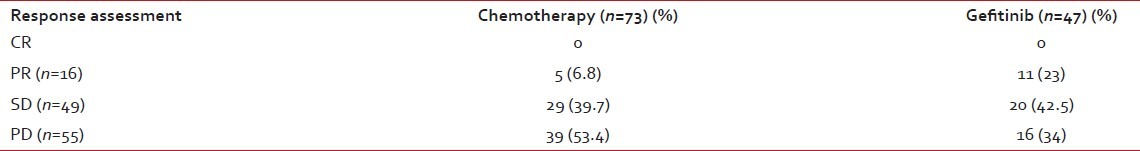
Survival results for the entire study population
Table 3 shows the results of the univariate analysis of the prognostic factors discussed below.
Table 3
Univariate analysis of prognostic factors
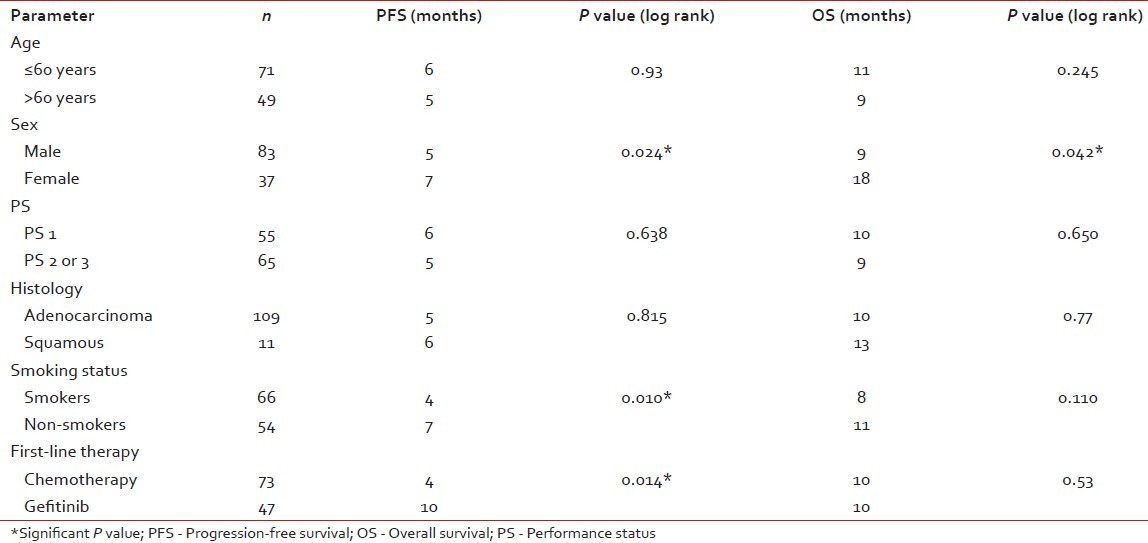
The PFS and OS for age ≤60 years vs age >60 years was not significant. The median PFS and median OS were significantly in favor of females when compared with males (7 months and 18 months vs 5 months and 9 months, respectively) [Figures [Figures11 and and2].2]. While there is a significant PFS benefit for non-smokers when compared with smokers (7 months vs 4 months; P=0.010), there is no significant OS difference noted between them [Figures [Figures33 and and4].4]. There is no significant OS or PFS difference between the different PS categories (PS1 vs PS2/3 or PS1/2 vs PS 3) and specific histology type, even though numbers were small in squamous histology. The median PFS and OS were not significant between stage III and stage IV.
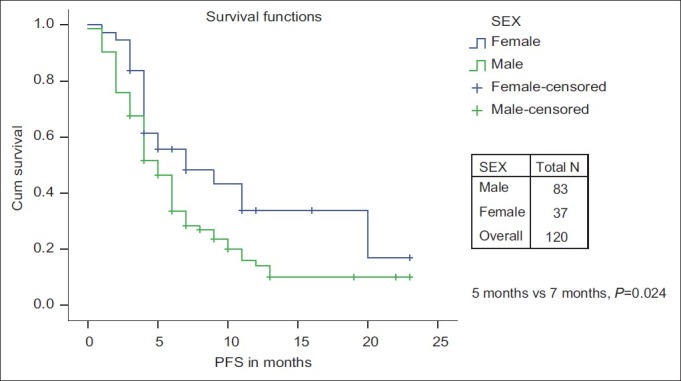
| Fig. 1 Progression-free survival: Male vs female
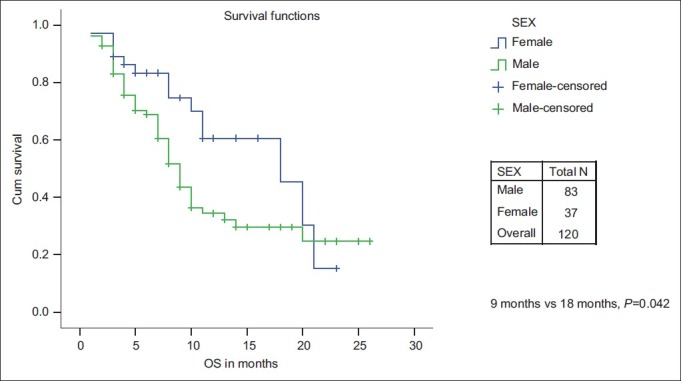
| Fig. 2 Overall survival: Male vs female
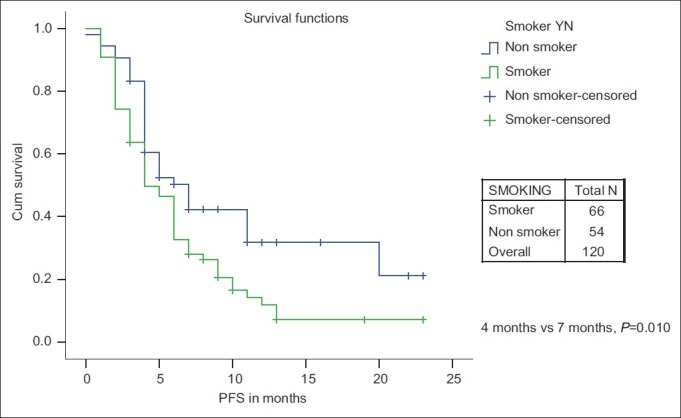
| Fig. 3 Progression-free survival: Smoker vs non-smoker
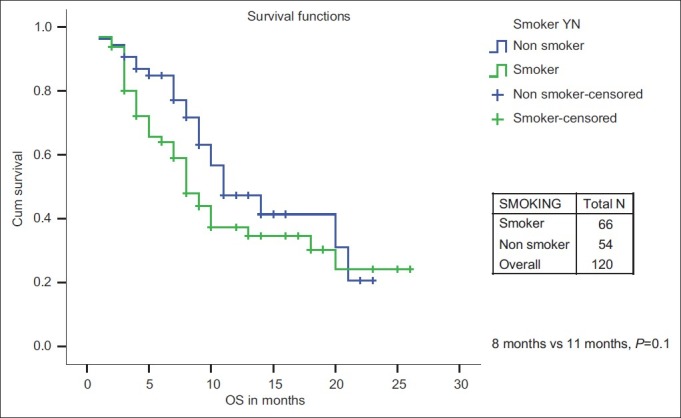
| Fig. 4 Overall survival: Smoker vs non-smoker
Survival results for upfront chemotherapy vs upfront Gefitinib arm
There is no PFS or OS difference for age, stage and PS (PS 1 vs PS 2/3) with either chemotherapy or Gefitinib as first-line therapy [Table 4]. However, when the PS 2/3 patients were analyzed separately, only PFS and not OS was significantly higher with Gefitinib (n=36) than with single-agent chemotherapy (n=29) [median PFS of 10 months (95% CI 6.4–13.5 months) vs 4 months (95%CI 3.4–4.8 months) (P=0.017)] [Figures [Figures55 and and66].
Table 4
Analysis of prognostic factors – Chemotherapy vs Gefitinib
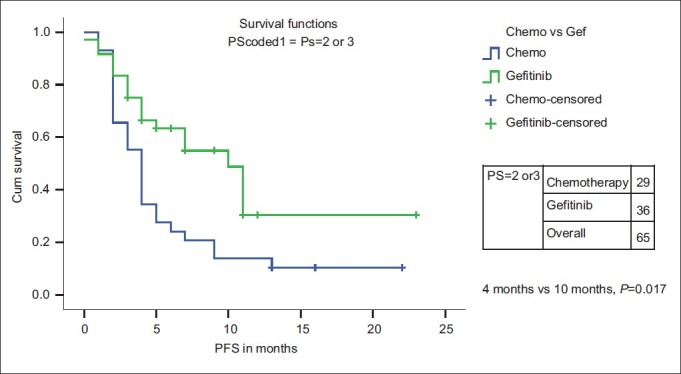
| Fig. 5 Progression-free survival for PS 2/3 patients: Chemotherapy vs first-line Gefitinib
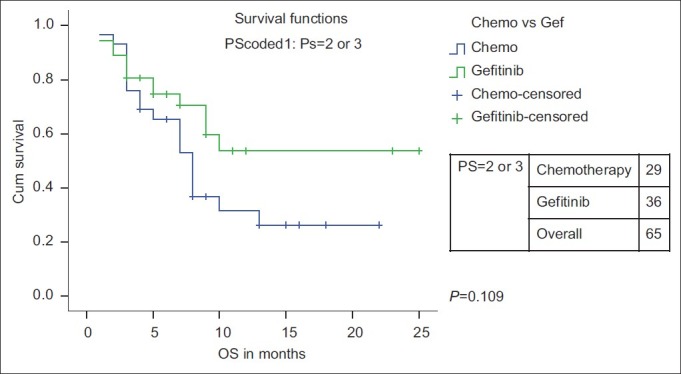
| Fig. 6 Overall survival for PS 2/3 patients: Chemotherapy vs firstline Gefitinib
Although there is no significant gender difference for PFS in the chemotherapy arm (4 months vs 4 months, P=0.9), significant PFS difference in favor of the Gefitinib arm for females (7 months vs 18 months, P=0.004) was noted. Similarly non-smokers receiving upfront Gefitinib had better PFS than smokers receiving upfront chemotherapy or Gefitinib.
While PFS was significantly improved for upfront treatment with Gefitinib when compared with chemotherapy (10 months vs 4 months, P=0.014), there is no significant OS difference (10 months vs 10 months, P=0.53) [Figures [Figures77 and and88].
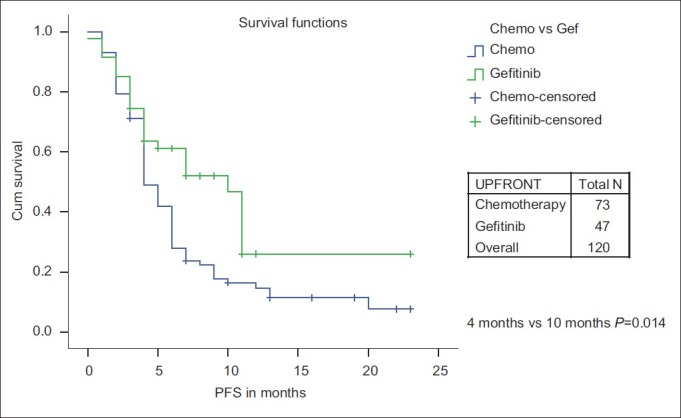
| Fig. 7 Progression-free survival: Chemotherapy vs first-line Gefitinib
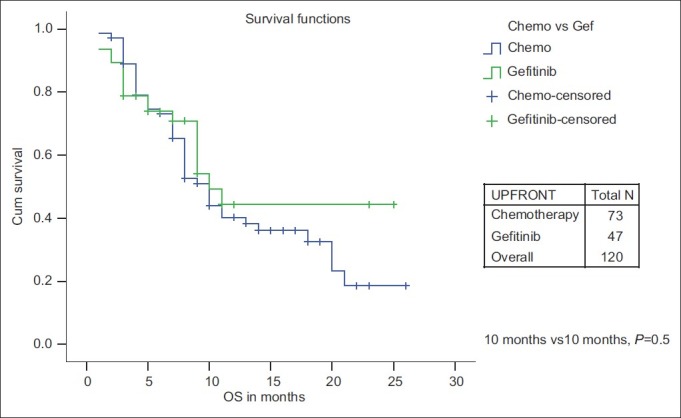
| Fig. 8 Overall survival: Chemotherapy vs first-line Gefitinib
Survival rate
The 12-month PFS rate is 16.3% for the chemotherapy vs 26% for the Gefitinib group. The 12-month OS rate is 40.3% for the chemotherapy and 44.3% for Gefitinib group, respectively. For PS 2/3 patients, the 12-month PFS rate is 13% vs 40% for chemotherapy and Gefitinib, respectively.
Toxicity (Data not shown)
First-line Gefitinib therapy is well tolerated when compared with upfront chemotherapy. The toxicity issues with Gefitinib are mainly rash and diarrhea, both limited to grade 1and 2 of Common Terminology Criteria Version 4 in more than 90% of the patients, and are manageable with either oral Doxycycline, local Clindamycin gel or temporary stoppage of drug for 2 or 3 days in case of severe toxicity. And, such patients are able to restart Gefitinib without much symptoms and continued therapy as outpatients. Higher toxicity with Gefitinib was seen in the first 2 months, and this subsequently decreased in intensity. None of the patients discontinued Gefitinib because of toxicity. On the other hand, chemotherapy adverse effects such as nausea, vomiting, fatigue, alopecia and myelosupression were commonly seen and frequent admissions for supportive care were noticed while on chemotherapy, although formal quality of life (QOL) assessment was not available between both arms.
DISCUSSION
In this study, we show for the first time from India upfront treatment with Gefitinib in advanced NSCLC patients.
Overall study result
After a median follow-up of 7.5 months, median PFS and OS were 5 months and 7.5 months, respectively. Our median PFS and OS reported is consistent with previous publications from India. Older studies from India quoted a median OS of 6.2–8.7 months for chemotherapy.[6,7] In a recent large publication of chemotherapy for advanced NSCLC from India (194 evaluable patients), a median PFS of 6 months and a median OS of 7 months was quoted using both first-generation and second-generation chemotherapy regimens.[8]
Our 1-year survival rate for the chemotherapy group is 40.3%, which is comparable to 29.8% in the recent published series from India.[8]
On univariate analysis, PFS was significantly improved for non-smokers (7 months vs 4 months; P=0.010), female sex (7 months vs 5 months; P=0.024) and upfront treatment with Gefitinib (10 months vs 4 months; P=0.014). Our results were consistent with the above-mentioned recent Indian publication in which female sex and non-smoking status were significant on univariate analysis.[8]
First-line Gefitinib therapy
Forty-seven of 120 patients (39.2%) received first-line Gefitinib therapy. Upfront treatment with Gefitinib has significant PFS benefit when compared with upfront chemotherapy. There is no study published from India with which we can compare our results of upfront Gefitinib with chemotherapy.
However, in a pivotal study, Iressa Pan-Asia Study (IPASS) comparing Gefitinib or Carboplatin–Paclitaxel in Pulmonary Adenocarcinoma by Tony S. Mok et al. in a mutation-unselected population, the median PFS was 5.7 months in the Gefitinib group and 5.8 months in the carboplatin–paclitaxel group, demonstrating the non-inferiority of upfront Gefitinib therapy and superior outcome in mutation-positive patients when compared with chemotherapy.[14]
Another trial from the North-East Japan Study Group comparing Gefitinib or chemotherapy for NSCLC with mutated EGFR,[15] the Gefitinib group had a significantly longer median PFS (10.8 months vs 5.4 months) than the chemotherapy group, which is very similar to our result even though we did not select our patients for EGFR mutation.
A similar trial from the West Japan Oncology Group (WJOG) comparing Gefitinib vs cisplatin plus docetaxel in EGFR-mutation positive NSCLC reported a significant median PFS of 9·2 months versus 6·3 months in favor of Gefitinib.[16]
Two prospective phase III studies, OPTIMAL and EURTAC, evaluating the efficacy of first-line erlotinib versus chemotherapy in EGFR activating mutation-positive patients has been reported. The preliminary and updated data of the OPTIMAL trial has shown a significant median PFS of 13.1 months vs 4.6 months for erlotinib and Gemcitabine/carboplatin, respectively (P=0.0001). The efficacy results of the EURTAC trial are similar (median PFS 9.4 months vs 5.2 months in favor of erlotinib with a non-significant OS difference).[17,18]
Clinical experience with Gefitinib in Indian patients who had participated in the IRESSA Survival Evaluation in Lung (ISEL) study and Gefitinib expanded-access program in India for advanced or refractory NSCLC as second-line therapy reported a median survival of 6.4 months with Gefitinib and 5.1 months with placebo (P-value not calculated). However, in the overall ISEL population, 5.6 months for Gefitinib versus 5.1 months in placebo, P = ns was reported.[11–13]
Table 5 shows and summarizes the comparative efficacy report from studies comparing first-line Gefitinib/erlotinib vs chemotherapy.
Table 5
Comparative efficacy report from studies comparing first-line Gefitinib/erlotinib versus chemotherapy
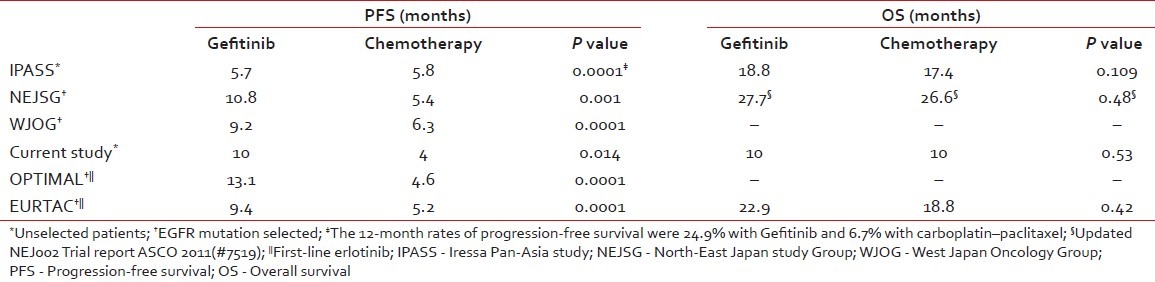
The 12-month PFS rate for upfront Gefitinib vs chemotherapy (26% vs 16%) in our study is similar to the largest East Asian study of first-line Gefitinib vs chemotherapy (IPASS study – rate for Gefitinib is 24.9% and 6.7% with carboplatin–paclitaxel).[14]
Also, first-line Gefitinib therapy is well tolerated when compared with upfront chemotherapy. The side-effect profile of Gefitinib therapy is manageable when compared with the obvious adverse effects associated with chemotherapy. Contrary to chemotherapy, patients on Gefitinib were managed in the outpatient department and rarely required discontinuation of therapy or admissions for supportive care.
No overall survival difference between upfront Gefitinib vs chemotherapy
However, there is no difference in the 1-year OS rate for chemotherapy vs Gefitinib group (40.3% vs 44.3%) and no significant median OS difference between chemotherapy and upfront Gefitinib therapy (10 months; P=0.53) as seen in the IPASS study and the North-East Japan Study Group study comparing first-line Gefitinib vs chemotherapy.[14,15] The only significant factor that affected OS was female sex (18 months vs 9 months; P=0.042). No factors were significant on multivariate analysis.
In the updated final analysis of the pivotal IPASS trial, OS was similar for Gefitinib and carboplatin/paclitaxel (median OS was 18.8 months with Gefitinib and 17.4 months with standard chemotherapy), which was likely to be due to subsequent lines of therapy as 60–70% of the patients received additional treatment post study completion. Also, there is no significant OS difference in the mutation-positive subgroup.[19]
In the final OS results of the NEJ002 study, there is no significant difference in OS between the Gefitinib group (27.7 months) and the chemotherapy group (26.6 months) as 96% of the chemotherapy patients received second-line Gefitinib and 90% of the Gefitinib patients received subsequent chemotherapy after progression.[20]
In contrast, only 27.5% of our study patients received second-line therapy after progression. About 51% (17/33 patients) received second-line Gefitinib therapy.
Patients with PS 2/3 category
PS 2/3 patients with lung cancer were included in our analysis. They constituted 54.1% of our study population as compared with only 10% and less than 2% in the IPASS study and the NEJ002 study, respectively.[14,15] Among PS 2/3 patients, PFS was significantly higher with Gefitinib (n=36) than with single-agent chemotherapy (n=29) [median PFS of 10 months (95%CI 6.4–13.5 months) vs 4 months (95%CI 3.4–4.8 months) (P=0.017)].
Equivalent survival of stage IIIB and stage IV
In our study, there is no significant PFS or OS difference between stage IIIB vs stage IV patients, which is consistent with the recent previous publication from India.[8] Both stage IIIB and stage IV cases were included in advanced NSCLC in large published trials comparing Gefitinib vs chemotherapy. Around 13–27.3% of the patients were stage IIIB in the study quoted.[14,15] However, only three stage IIIB patients (three of a total of 25) were included in our Gefitinib arm. Two of them are alive at 4 months and 8 months at the time of analysis.
Similar results between unresectable stage III and stage IV patients may be multifactorial, like poor PS, occult stage IV disease, heterogenous use of radiation therapy, tolerance issues and completion rate of first-line therapy, employment of second-line therapy, coexistent co- morbidity and economic and social support available.
EGFR mutation data of Indian patients
EGFR mutation data is available for only three patients (two male smokers – mutation negative – and one female non-smoker – mutation positive). Low rate of EGFR mutation testing may be because of lack of adequate biopsy material available, technical issues and lack of expertise in testing EGFR mutation on fine needle aspiration cytology (FNAC), bronchoalveolar lavage (BAL) fluid, bronchial brushing, pleural fluid tissue and cost consideration. However, despite all the practical difficulties, routine testing of EGFR mutation is now recommended by ASCO and NCCN for personalized lung cancer therapy (use of oral tyrosine kinase inhibitors like Gefitinib or erlotinib) for NSCLC.[9,10]
There may be a higher incidence of mutated EGFR in our population, especially in females and non-smoking individuals, as reflected in our study result of improved outcome for those patients receiving upfront Gefitinib therapy even though mutation status is not available for the majority of the patients. In a recent report of screening for EGFR mutations in lung cancer from India,[21] the overall prevalence of EGFR mutation is 51.8%. The mutation is more often significantly found in females (58/97 (59.9%)) than in males (56/123 (45.5%); P=0.04), but non-significant in non-smokers (55%) than in smokers (48%) (P=0.29). However, there was a significant increase in mutation status in the non-smoking group compared with the smoking group in exons 19 and 21 together (z = −2.55; P=0.01).
Limitations of the study
Some of the limitations of our study include the retrospective nature of chart review, inclusion of NOS NSCLC in adenocarcinoma histology, female patients assumed to be non-smokers until strong clinical history is elicited otherwise [even though elements like passive smoking and in-home cooking fumes (including coal/wood smoke during cooking in rural areas) were not qualified], absence of EGFR mutation status in the majority of first-line Gefitinib patients, second-line Gefitinib therapy in the majority of chemotherapy-refractory patients, recent employment of maintenance chemotherapy in some patients completing first-line chemotherapy regimens and use of oral etoposide in PS 2/3 patients although no standard chemotherapy recommendations can be made in such a patient population.
In summary, we have shown improved outcome (PFS benefit and not OS) for stage IV NSCLC patients when treated with upfront Gefitinib therapy, especially female sex, non-smoking status and poor PS patients, even when not selected by EGFR mutation status in our population when compared with upfront chemotherapy.
CONCLUSION
In the largest series on the use of first-line Gefitinib from India, we found it to be a useful agent in the treatment of NSCLC, especially in females, patients with poor PS and non-smokers, even without EGFR mutation testing. Second-line Gefitinib may have negated OS differences. However, EGFR mutation studies may help in further individualization of therapy.
Footnotes
Source of Support: Nil,
Conflict of Interest: None declared
REFERENCES

| Fig. 1 Progression-free survival: Male vs female

| Fig. 2 Overall survival: Male vs female

| Fig. 3 Progression-free survival: Smoker vs non-smoker

| Fig. 4 Overall survival: Smoker vs non-smoker

| Fig. 5 Progression-free survival for PS 2/3 patients: Chemotherapy vs first-line Gefitinib

| Fig. 6 Overall survival for PS 2/3 patients: Chemotherapy vs firstline Gefitinib

| Fig. 7 Progression-free survival: Chemotherapy vs first-line Gefitinib

| Fig. 8 Overall survival: Chemotherapy vs first-line Gefitinib


 PDF
PDF  Views
Views  Share
Share

