Epidermal growth factor receptor mutation in adenocarcinoma lung in a North Indian population: Prevalence and relation with different clinical variables
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(03): 189-195
DOI: DOI: 10.4103/0971-5851.190356
Abstract
Introduction: Lung cancer is one of the most common causes of cancer deaths worldwide. Adenocarcinoma is taking over squamous cell lung cancer as the predominant histological subtype. Several cytotoxic drugs are available for the treatment of lung cancer, but side effects limit their use. Recently, targeted therapies for cancers have come into clinical practice. Aims and Objectives: To determine the prevalence of epidermal growth factor receptor (EGFR) mutation in adenocarcinoma lung in a North Indian population and its relation with different clinical variables. Materials and Methods: A total of 57 patients who met inclusion criteria were recruited into the study. Relevant history, clinical examination and investigations were done. EGFR mutation was done in all patients. Results: A total of twenty patients tested positive for EGFR mutation. EGFR was more frequently detected in female patients (53.8%), while as only 19.4% of the male patients expressed EGFR mutation, which was statistically very significant P = 0.007). EGFR mutation was more frequently detected in nonsmokers (52%) as compared to smokers (21.9%) which also was statistically significant P value of 0.018). EGFR mutation was more common in Stage III and IV adenocarcinomas (48%) as compared to Stage I and II (21.4%) which was statistically significant P value 0.034). Conclusion: EGFR mutation should be routinely done in all patients of adenocarcinoma lung particularly non-smoker females with Stage III and IV disease.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
Lung cancer is one of the most common causes of cancer deaths worldwide. Adenocarcinoma is taking over squamous cell lung cancer as the predominant histological subtype. Several cytotoxic drugs are available for the treatment of lung cancer, but side effects limit their use. Recently, targeted therapies for cancers have come into clinical practice.
Aims and Objectives:
To determine the prevalence of epidermal growth factor receptor (EGFR) mutation in adenocarcinoma lung in a North Indian population and its relation with different clinical variables.
Materials and Methods:
A total of 57 patients who met inclusion criteria were recruited into the study. Relevant history, clinical examination and investigations were done. EGFR mutation was done in all patients.
Results:
A total of twenty patients tested positive for EGFR mutation. EGFR was more frequently detected in female patients (53.8%), while as only 19.4% of the male patients expressed EGFR mutation, which was statistically very significant (P = 0.007). EGFR mutation was more frequently detected in nonsmokers (52%) as compared to smokers (21.9%) which also was statistically significant (P value of 0.018). EGFR mutation was more common in Stage III and IV adenocarcinomas (48%) as compared to Stage I and II (21.4%) which was statistically significant (P value 0.034).
Conclusion:
EGFR mutation should be routinely done in all patients of adenocarcinoma lung particularly non-smoker females with Stage III and IV disease.
INTRODUCTION
Lung cancer is the most common visceral malignancy, accounting for roughly one-third of all cancer deaths, and it is the most common cause of cancer-related death in both men and women 4.[1] The incidence of lung cancer peaks between age 55 and 65 years. Lung cancer accounts for 29% of all cancer deaths (31% in men and 26% in women).[2] It constitutes 6.8% deaths in India.[3] Lung cancer is major health problem in Kashmir valley and constitutes 9.9% of all cancers.[4] Smoking is the main risk factor for lung cancer, and it also potentiates the effects of many occupational/environmental carcinogenesis.[5,6,7]
Lung carcinoma is divided into small and non-small cell lung carcinoma (SCLC and NSCLC). Although NSCLC consists of squamous cell carcinoma, adenocarcinoma, and large cell carcinoma, it was considered as one group mainly because of lack of specific therapy for various histologic subtypes. Unfortunately, most patients with NSCLC are diagnosed at advanced stage and finding the best treatment for these patients is an important issue. The prognosis is poor for advanced stage NSCLC (Stage IIIB and IV). Chemotherapy for NSCLC remains marginally effective.[8] Platinum-based chemotherapy has been documented to improve overall survival and quality of life and is suggested for advanced stage NSCLC.[9,10] Nowadays the distinction between the adenocarcinoma and squamous cell carcinoma is extremely important due to certain reasons. One reason is adenocarcinoma of the lung is now increasing (now considered to be the most predominant histological subtype). Other reason is the potential uses of targeted therapy in cases showing epidermal growth factor receptor (EGFR) mutations.[11]
NSCLC frequently overexpresses receptors of the ErbB family, including the EGFR.[12,13] Since the 1980s, many studies showed EGFR overexpression in lung carcinoma particularly in squamous cell carcinoma using various techniques including immunohistochemistry. However, the significance of these over expressions continued to be controversial. The breakthrough took place in 2004 when Lynch et al.[14] reported that activating mutation of EGFR gene kinase domain resulted in responsiveness to tyrosine kinase inhibitors (TKI) in patient with lung adenocarcinoma. Simultaneously two independent group reported similar results.[15,16]
The EGFR (HER-1/ErbB1) is a member of the ErbB family of tyrosine kinase (TK) receptors, which includes HER-1/ErbB1, HER-2/neu/ErbB2, HER-3/ErbB3, and HER-4/ErbB4. It is composed of extracellular (ligand-binding), transmembrane and intracellular (TK) domain. On ligand binding and receptor homo or heterodimerization and activation, activated EGFR signals downstream to the P13K/AKT and RAS/RAF/MAPK pathways. These intracellular signaling pathways regulate key processes such as apoptosis and proliferation. In malignant cells, including NSCLC cells, the activity of the receptor may become dysregulated and no longer under the control of inherent inhibitory mechanism.[17] Spontaneous EGFR mutations often are oncogenic; that is, they activate the EGFR-signaling pathway in the absence of ligand and promote cell proliferation, survival, and anti-apoptotic signals. These signaling networks make EGFR-mutated cells dependent on a functional EGFR for their survival, rendering them addicted to receptor. Inhibition of EGFR leads to up-regulation of pro-apoptotic molecules and finally results in cell death through the activation of the intrinsic mitochondrial apoptotic pathway.
The data regarding the prevalence of EGFR mutation in adenocarcinoma lung from North India is not available. Given the grim prognosis of advanced NSCLC patients and a possible role of targeted therapies in advanced lung cancer, this study was carried out in one of the busiest tertiary care hospitals of North India.
Aims and objectives
- To determine the prevalence of EGFR mutation in Adenocarcinoma Lung in a North Indian population
- To study the association of EGFR mutation status with different clinical variables such as sex, residence, smoking history, and stage of disease.
MATERIALS AND METHODS
The present study was conducted in the Department of Medical Oncology (Regional Cancer Center) at Sher-i-Kashmir Institute of Medical Sciences, Jammu and Kashmir, one of the leading tertiary care institutions of North India catering to needs of around 13 million people. The study included all patients who met inclusion criteria from June 2011 to August 2013 and received treatment in our department. A written informed consent was received from each patient.
Inclusion criteria
- Diagnosis of adenocarcinoma lung
- Age less than 70 years
- Good performance status.
Exclusion criteria
- Age more than 70 years of age
- Patients not willing to give informed consent for EGFR mutation status
- Major medical comorbidities
- Poor performance status.
Sample collection/storage
The tissue specimen resected during surgery or obtained through computed tomography (CT) guided biopsy and bronchoscopy was collected from patients for the study. All tissue specimens were immediately stored at −70°C for nucleic acid extraction. Histopathologically confirmed adenocarcinoma tissues were used for mutational analysis of EGFR genes.
DNA extraction
High molecular weight DNA was isolated from the samples and confirmed by electrophoresis on 1% agarose gel. The concentration of the DNA obtained was measured in a microvolume UV-Vis Spectrophotometer (Thermo Scientific NanoDrop 2000) at 260 nm wavelength. The purity of DNA was checked using A260/A280 ratio.
Epidermal growth factor receptor amplification
Polymerase chain reaction (PCR) amplification using four set of primer pairs that span the mutational cluster region of the EGFR gene were used to amplify exons. The product sizes were 397, 306, 377, and 348 bps.[14,18,19,20]
All precautions were taken to ensure contamination free amplification of DNA. Prevention of carry-over by separation of pre- and post-PCR steps, and use of aerosol-free tips were strictly followed. Results were considered valid only on the reproduction of same in two independent experiments.
Molecular analysis [Table 1]
Table 1
Primer sequences and annealing temperatures of epidermal growth factor receptor genes for direct sequencing

DNA isolated from samples was subjected to PCR for amplification of different exons of EGFR gene. Data from DNA sequencing was analyzed for the alterations in the respective amplicons of EGFR gene. Lung tumor tissue samples were sequenced with the primers used for amplification of the exons of EGFR gene. The sequencing data were analyzed and interpreted manually or using Clustal X software.
DNA sequencing was carried out by automated DNA sequencing method on ABI Prism 310 (Perkin Elmer). The automated DNA sequencing is based on the chain termination reaction.
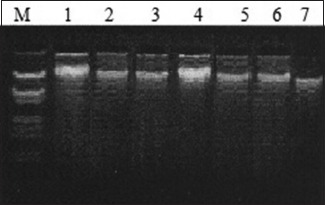
A: Representative gel picture of genomic DNA isolated, tumor tissue, lung cancer patients. Lane M – Lambda DNA EcoRI and Hind III digest; Lanes 1–7 – DNA extracted from different tissues/blood samples.
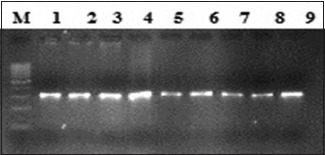
B: Lane M, 100 bp ladder; lanes 1–9 amplified product 306 bp (exon19).
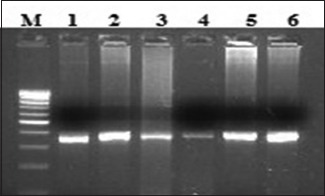
C: Lane M, 100 bp ladder; lanes 1–6 amplified product 377 bp (exon 20).
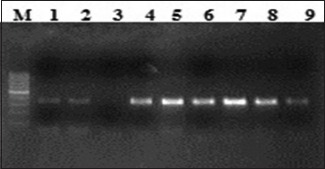
D: Lane M, 100 bp ladder; lanes 1–9 amplified product 348 bp (exon 21).
Statistical methods
The statistical analyses were done by standard statistical methods applicable to the study, including Chi-square/Student's t-test. Statistical Package for Social Sciences 16.0 (IBM in 2009) and Microsoft Excel Software was used for data analysis. P < 0.05 was taken as significant.
RESULTS
Baseline characteristics
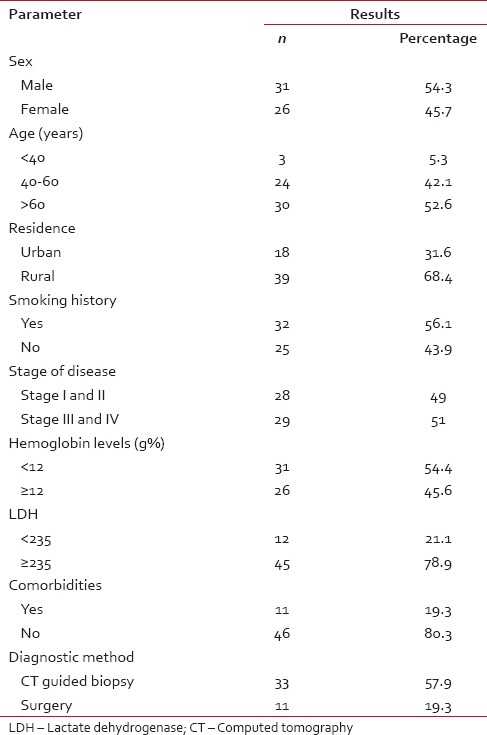
A total of 57 patients were included in the study, all of whom had a diagnosis of Adenocarcinoma lung [Table 2]. Among these 31 (54%) were male and 26 (46%) female. The mean age of our study patients was 56.97, with the youngest diagnosed at the age of 28 and the oldest aged 70 years. At the time of diagnosis, 56.1% had been active smokers. Smoking was more prevalent in males (77.4%), in comparison, only 30% females had a history of smoking. Only 11 (19.3%) patients had significant comorbidities such as hypertension or diabetes. However, anemia defined as hemoglobin levels <12 g% was seen in 31 (54.4%) patients, more common in males. Serum lactate dehydrogenase (normal ≤235) was increased in 45 (78.9%) patients. The majority of our patients presented with a cough and breathlessness 70.2% as compared to other presentation 29.8%. Most common finding on examination was decreased air entry and signs of effusion (66.7%)
Table 2
Baseline parameters
Diagnosis of cancer
Diagnostic methods used in procuring samples for histopathology and EGFR status were open surgery; CT-guided biopsy and bronchoscopic biopsy. Out of 57 patients, 11 (19.3%) underwent open surgery, while as CT-guided biopsy was used in 33 (57.9%) and bronchoscopy in 13 (22.8%) patients for diagnosis of cancer. The standard TNM score was used for staging of lung cancer. Stage III and IV disease was found in 29 (51%) patients while as 28 (49%) patients had Stage I and II disease.
Epidermal growth factor receptor mutation and its relation to different clinical variables
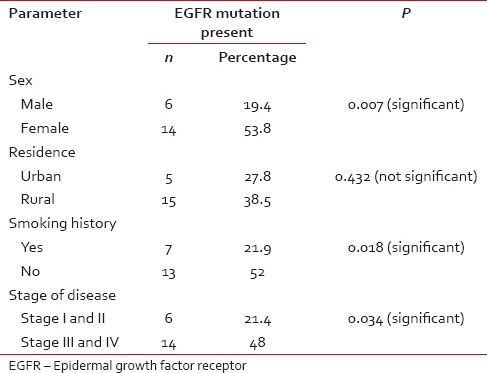
A total of 20 (35.1%) patients tested positive for EGFR in this study population [Table 3]. Of these 14 were female and 6 male. EGFR was more frequently detected in female patients (53.8%), while as only 19.4% of the male patients expressed EGFR mutation, which was statistically very significant (P = 0.007) [Figure 1]. There was no significant difference between the expression of EGFR mutation among patients from rural and urban areas (P = 0.43). There was no significant difference between the expression of EGFR mutation among patients from rural and urban areas (P = 0.43). Seven (35.1%) patients who tested positive for EGFR mutation were smokers while as 13 (64.1%) were nonsmokers [Figure 2]. EGFR mutation was more frequently detected in nonsmokers (52%) as compared to smokers (21.9%), which was also statistically significant with a P value of 0.018. Six (35.1%) patients who tested positive for EGFR mutation had Stage I and II disease while as 14 (64.9%) patients had Stage III and IV disease [Figure 3]. EGFR mutation was more frequent in Stage III and IV adenocarcinomas (48%) as compared to Stage I and II (21.4%) which was statistically significant (P value 0.034).
Table 3
Epidermal growth factor receptor status in relation to different clinical variables
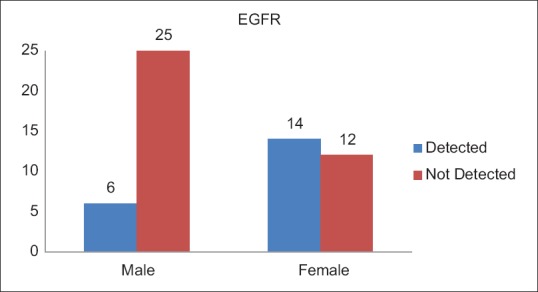
| Fig. 1 Epidermal growth factor receptor status with relation to gender
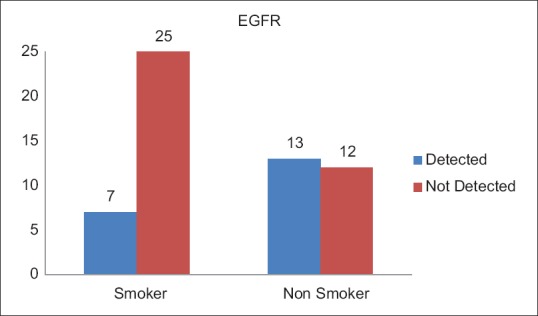
| Fig. 2 Epidermal growth factor receptor status with relation to smoking history
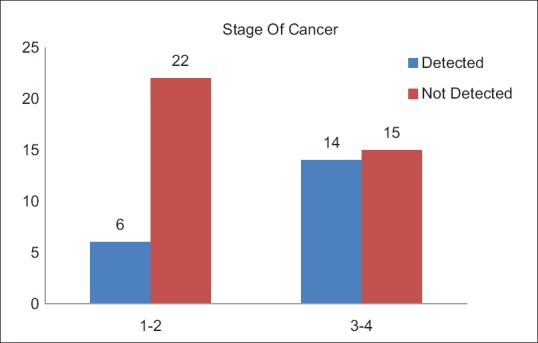
| Fig. 3 Epidermal growth factor receptor status in relation to stage of cancer
Type of mutation
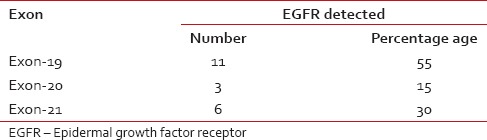
EGFR mutation was further qualified with the exon on which deletion had occurred. Exon 19 deletion was seen more frequently (55%) as compared to Exon 21 (30%) and Exon 20 which expressed EGFR mutation (15%) [Table 4].
Table 4
Epidermal growth factor receptor mutation with respect to different exons
DISCUSSION
Lung cancer remains one of the most common causes of cancer-related morbidity and mortality worldwide. The distinction between SCLC and NSCLC is an initial step in the management of lung cancer patients, as former are treated with chemoradiation and later usually subjected to surgery. However, advanced NSCLC remains a therapeutic challenge. Chemotherapy, particularly platinum-based compounds, provides a marginal benefit in advanced NSCLC. However cytotoxic chemotherapy is not without side effects and majority of advanced NSCLC usually have a low-performance score and usually opt out of treatment protocols. In the past decade, a dramatic shift in cancer therapy has occurred. Although traditional cytotoxic chemotherapy remains the treatment of choice for many malignancies, targeted therapies are increasingly taking over as an important component of cancer treatment regimens. Traditional cytotoxic chemotherapy works primarily through the inhibition of cell division. In addition to cancer cells, other normal rapidly dividing cells of the body are affected by these drugs and hence an unfavorable side effect profile. In contrast, targeted therapy blocks the proliferation of cancer cells by interfering with specific molecules required for tumor development and growth.
A number of mutations have been reported in lung cancer. These mutations, collectively called as oncogenic driver mutations, are responsible for initiation and maintenance of cancer. These mutations are often found in genes that are critical for maintaining normal cellular proliferation and survival, and the presence of such mutations confers a growth advantage on cancer cells.[18] The EGFR is a member of the ErbB family of TK receptors that regulate key processes such as apoptosis and proliferation. In recent years, attention has been paid to the role of driver mutations in EGFR in the tumorigenesis of adenocarcinomas and their potential use as targets for therapy.[14,18,20]
Different studies report the different prevalence of EGFR mutation in NSCLC, ranging from 43% to 89%. Paez et al. observed EGFR mutation in 32% of adenocarcinoma lung patients while as Kosaka et al. found EGFR mutation in 49% of patient with adenocarcinoma lung.[15,21] Mitsudomi et al. observed EGFR mutation in 56% of lung tumor whereas Sahoo et al. found EGFR mutation in 51.8% patients of lung cancer.[22,23] Marchetti et al. found EGFR mutation in 42% of adenocarcinoma patients. In this study, EGFR mutation was found in 35.1% patients.[24] This variation in different studies could be attributed to various factors such as environmental, geographical, and ethnic difference. In addition, this variation can result from technical errors which could occur during storage, transportation, and processing of the sample. It can also result from various methodologies used to detect EGFR mutation in different studies.
Several studies report higher prevalence of EGFR mutations in female lung cancer patients. The reason for this difference is largely unknown. In this study, 53.8% of females tested positive for EGFR mutation as compared to only 19.4% males that were statistically significant (P = 0.007). Such results have been found in other studies also. Qin et al. found in their study that EGFR mutation was present in 36.4% females as compared to 20% in males.[25] Tokumo et al. showed the prevalence of EGFR mutation was more in female (67%) compared to male (33%).[26] Tomizawa showed the prevalence of EGFR mutation 16% in female as compared to male 0.075%.[27] Sonobe et al. found that female with adenocarcinoma has a frequency of EGFR mutation of (76.3%) as compared to male (34%).[28] Adenocarcinoma is the most common histologic type of lung cancer in nonsmokers. EGFR mutation follows a similar pattern. Shigematsu et al. observed EGFR mutation in (47%) of nonsmoker patient as compared to (24%) in a patient with smoking history.[29] Kosaka et al. (2004) showed the prevalence of EGFR mutation in (66%) in nonsmoker as compared to (22%) in smoker.[21] The present study also showed that the percentage of EGFR mutation was more in nonsmokers (52%) as compared to smokers (21.9%). Stage of the disease has also been found to be a predictor of EGFR status in certain studies. The prevalence of EGFR mutation has been found to be more in Stage III–IV of lung cancer as compared to Stage I–II. Such an issue, however, remains controversial and whether such findings are just incidental or carry some prognostic significance remains debateable. Although Tomizawa et al. showed a higher prevalence of EGFR mutation in advanced lung cancer; such findings were not seen in studies done by Jang et al.[27,30] This study, however, supports the claims of former, with EGFR mutation being more common in Stage II–IV disease as compared to Stage I–II disease (48% vs. 21.4% respectively) that was statistically significant. The reasons behind such differences remain to be unknown. However, given the fact that such findings have been found in more than few studies does make it more than incidental.
In this study, we also found that EGFR mutation was most commonly seen in exon-19 (50%) followed by exon-21 (30%) which was followed by exon-20 (15%). This result was in consistent with various studies done previously. Hofman et al. found mutation in exon-19 in 58% cases and exon-21 in 34% of patients.[31] Boch et al. found in their study that exon-19 was mutant in (56%) and exon-21 was mutant in (30%).[32] Unal et al. also found that patients with EGFR mutation on exon-20 are less responsive to TKIs than those with mutation on exon 19 and exon-21.[33] This study revealed the higher percentage of EGFR mutation on exon-20 as compared to other studies. It could because many of our patients were already started on chemotherapy before going for EGFR mutation analysis. Sequist et al. found that expression of Exon-20 mutation is associated with acquired resistance to TKIs and can be due to previous therapy.[34] Furthermore, there may be genetic or racial preponderance which needs further studies for confirmation. Furthermore, it explains the response rate in such patients who are receiving TKIs based chemotherapy.
Different studies have found higher prevalence of EGFR mutations in East Asian population as compared to Caucasians (47-64% vs. 10-15%).[35,36,37,38] Only few studies are available from India. Mehtashowed 35% of lung cancer patients’ harbored EGFR mutation; the majority of whom were Adenocarcinoma with no significant gender difference.[39] Shankar et al. found very high prevalence of EGFR mutation (89%) in adenocarcinoma lung in South Indian population.[40] Pungliya et al. found that South Indians had a higher mutation rate (68% ± 17%) than North Indians (41% ± 21%, P = 0.06).[41] In this study, 35.1% patients of adenocarcinoma lung tested positive for EGFR mutation with statistically significant differences with respect to gender, smoking history, and stage of lung cancer.
CONCLUSION
Lung cancer is a very common malignancy with significant morbidity and mortality. Few treatment options exist for advanced NSCLC. Due to significant treatment-related toxicity patients usually opt out of the treatment protocols which compound the problem. Targeted therapies have come a long way in the management of a variety of diseases including cancers. A number of mutations have been found in lung cancer which has been used as potential therapeutic targets. One such mutation is EGFR mutation which is targeted by TKIs and provides scope for better management of advanced lung cancer patients. The incidence of EGFR mutation has been found higher in Asian population as compared to Western population and hence should be sought in all NSCLCs particularly in non-smoker females with Stage III and IV disease.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.


 PDF
PDF  Views
Views  Share
Share

