Endometrial stromal sarcoma: A review of the literature
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2012; 33(01): 1-6
DOI: DOI: 10.4103/0971-5851.96960
Abstract
Endometrial stromal sarcomas are rare malignant tumors of the uterus, and most of the information available in literature is based on small series or case reports. A proper preoperative diagnosis is difficult and in most cases the diagnosis is confirmed after hysterectomy for a presumed benign disease. Endometrial sampling, ultrasound, and magnetic resonance imaging can provide diagnostic clues. Total hysterectomy with bilateral salpingo-oopherectomy is the main line of management and for early disease complete cure is a reality. Ovarian conservation may be possible in young women with early stage disease and the role of lymphadenectomy is controversial. Adjuvant hormone therapy in the form of progesterone, gonadotropin releasing hormone analogues, and aromatase inhibitors are found to be effective in preventing recurrences. Hormone therapy, radiotherapy and surgical excision of the metastasis are recommended for recurrences.
Publication History
Article published online:
13 April 2022
© 2012. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Endometrial stromal sarcomas are rare malignant tumors of the uterus, and most of the information available in literature is based on small series or case reports. A proper preoperative diagnosis is difficult and in most cases the diagnosis is confirmed after hysterectomy for a presumed benign disease. Endometrial sampling, ultrasound, and magnetic resonance imaging can provide diagnostic clues. Total hysterectomy with bilateral salpingo-oopherectomy is the main line of management and for early disease complete cure is a reality. Ovarian conservation may be possible in young women with early stage disease and the role of lymphadenectomy is controversial. Adjuvant hormone therapy in the form of progesterone, gonadotropin releasing hormone analogues, and aromatase inhibitors are found to be effective in preventing recurrences. Hormone therapy, radiotherapy and surgical excision of the metastasis are recommended for recurrences.
INTRODUCTION
Endometrial stromal sarcomas (ESS) are very rare malignant tumors that make around 0.2% of all uterine malignancies. They resemble endometrial stromal cells in the proliferative stage. The annual incidence of ESS is 1–2 per million women. Compared to other uterine malignancies, ESS affects younger women and the mean age is 42 to 58 years.[1] They account for less than 10% of the uterine mesenchymal neoplasm and 10 to 25% of affected women are premenopausal.[1] ESS is an indolent tumor with local recurrences and distant metastasis can occur even 20 years after initial diagnosis.
Cytogenetics
The origin and biology of stromal sarcomas are poorly understood. Recently, a specific translocation t(7;17) (p15;q21) with involvement of two zinc finger genes juxtaposed with another zinc finger protein 1 and joint juxtaposed with another Zinc protein 1 was described in most of the ESS.[2] There is a relation between chromosomal aberrations andendometrial sarcomas. Chromosomal deletion on 7p was the most common finding (55.6%) in ESS and may play a role in tumor development and progression.[3] These tumors are diploid with a low S-phase fraction.[1]
PATHOLOGY
The pathogenesis of ESS is unknown, but exposure to tamoxifen, unopposed estrogens, and conditions such as polycystic disease of ovary are implicated.[4] In the latest 2003 WHO classification, endometrial stromal tumors are divided into (a) endometrial stromal nodule (ESN). (b) Low-grade endometrial stromal sarcoma (LGESS) or ESS. (c) Undifferentiated endometrial or uterine sarcoma (UES). In this classification the differentiation between low-grade and undifferentiated tumors is not made on mitotic count but on the basis of nuclear pleomorphism and necrosis. The features of these tumors are described below in the following Table 1 and Figure 1.
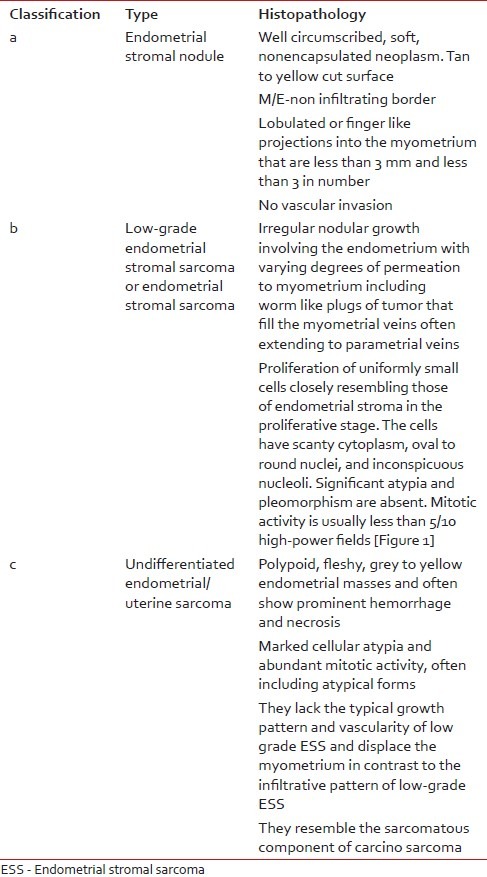
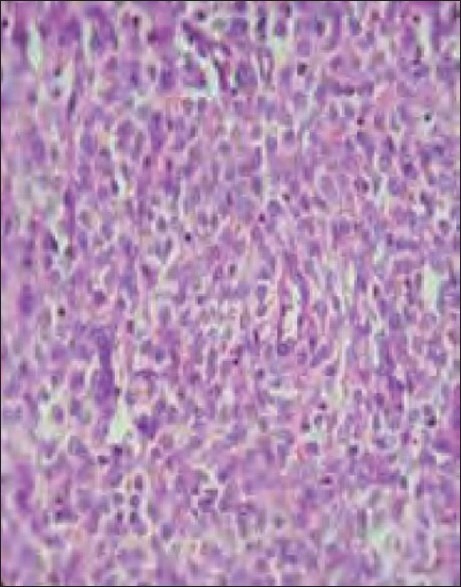
| Fig. 1 Endometrial stromal sarcoma uniform oval or spindle shaped neoplastic cells invading myometrium
The traditional classification of ESS into low-grade and high-grade categories has fallen out of favor, and high-grade tumors without recognizable evidence of a definite endometrial stromal phenotype are now termed undifferentiated endometrial sarcomas (UES) instead of high-grade ESS. UES represents a high-grade sarcoma that lacks specific differentiation and bears no histological resemblance to endometrial stroma and therefore the term ESS is now considered best restricted to neoplasm that are formally referred to as low-grade ESS. Therefore, in the review article ESS is used for previous LGESS and UES is used for previous high-grade endometrial stromal sarcoma (HGESS). Since myometrial and vascular invasion are the two features that help us to differentiate ESS from ESN and the UES resembles the sarcomatous component of carcino sarcoma, extensive sampling of the tissues is required for confirmation of diagnosis.[5]
DIAGNOSIS
The usual clinical presentation of ESS is abnormal uterine bleeding that occurs in about 90% of women and 70% cases show uterine enlargement. They can present with pelvic pain and dysmenorrhoea. An asymptomatic ESS occurs in 25% individuals. About 30 to 50% of the ESS has extra uterine spread at the time of the diagnosis.[1] Although the main tumor mass is almost always intramyometrial, most ESS involve the endometrium and uterine curettage may be helpful in preoperative diagnosis.[6,7] However, when the lesion is completely within the myometrium, the scrapings may not be helpful. Due to the great similarity of ESS with normal endometrium, it may be impossible to diagnose it with certainty on curettage fragments, and the definitive diagnosis can be made only on a hysterectomy specimen. Rarely ESS is initially present at an extra uterine site, most commonly the ovary. It can be a primary or metastatic lesion often from an occult tumor of the endometrium or from a previously undiagnosed case where a hysterectomy was done for a benign leiomyoma of the uterus.
Radiology
Ultrasound imaging is not reliable and can lead for the incorrect diagnosis of adenomyosis or uterine leiomyoma. Trans vaginal color Doppler shows low impedance flow compared to other benign tumors. Magnetic resonance imaging can be useful for a preoperative diagnosis. The important imaging feature that suggests ESS is the presence of bands of low-signal intensity within the area of myometrial invasion. This is due to the worm-like permeation of tumor cells into the myometrium. Another feature is continuous extension of the lesion into the adjacent structures along the vessels, fallopian tubes, ligaments, and the ovaries.[8,9]
Immunohistochemistry
Immunohistochemistry will help in the detection of tumor markers specific for ESS. CD10 is a cell-surface neutral endopeptidase, seen originally on immature lymphoid cells. Recently, CD10 expression has been shown in several nonhematopoieticneoplasm, including endometrial stromal sarcomas. Strong and/or diffuse positivity for CD10 is found in ESS, which are helpful in distinguishing these tumors from histological mimics like cellular leiomyoma that are generally negative.[10] However, literature search did not show the utility of CD10 for a preoperative diagnosis. Immunomarkers such asdesmin, h-caldesmon, oxytocin receptors, CD10, and inhibin are useful in distinguishing cellular leiomyoma. They express h-caldesmon, desmin, and oxytocin receptors while CD10 and inhibin expression is a feature of ESS.[11] ESS is almost always positive for both estrogen and progesterone receptors.
Differential diagnosis
This includes several soft-tissue neoplasms demonstrating arborizing vasculature, highly cellular leiomyoma, cellular endometrial polyp, low-grade mullarianadenosarcoma, and adenomyosis.[3] Extra genital ESS may be confused with gastrointestinal stromal tumors, hemangiopericytoma, lymphangiomyomatosis, or mesenchymal cystic hamartoma of the lung.[12,13] ESS of the ovary is difficult to distinguish from sex-cord stromal tumors.
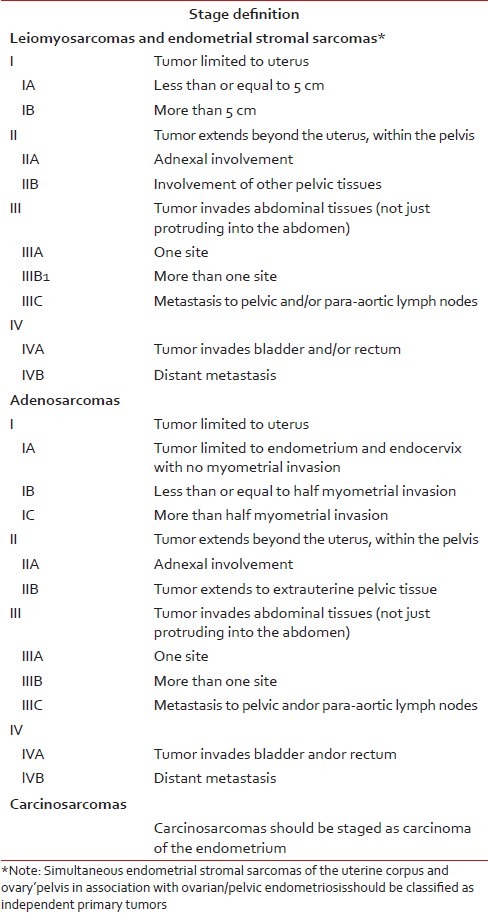
Prognostic factors are still controversial. Clinical factors such as age, race, parity, menopausal status, and pathological factors including tumor size, tumor stage, nuclear atypia, mitotic activity, tumor necrosis, lymphovascular space invasion, DNA ploidy proliferative activity, and expression of hormone receptors have been explored with varying outcomes. In multivariate analysis, older patients (age more than 50 years), black race, advanced stage, lack of primary surgery, nodal metastasis, high mitotic count more than 5 per 10 high-power fields, CD10 negative or low expression and lack of estrogen and progesterone receptors were independent prognostic factors for poor survival.[15,16] ESS and UES represent two distinct clinical entities and tumor's classification may be the most important prognostic factor.[7] However, ESS has a better life expectancy than other sarcomas.
Treatment
As for other sarcomas surgery is the most effective treatment for ESS. The efficacy of adjuvant therapy is not proven.
Surgery
Survival in patients with UES (previously called high-grade endometrial stromal sarcoma) appears to be related to the extent of residual disease after initial surgery and would suggest the necessity for aggressive cytoreduction as a main modality of treatment. However, the role of debulking surgery for ESS (formally known as “low-grade ESS”) remains unclear.[17] Immunohistochemical studies indicate a rich expression of estrogen and progesterone receptors (ER and PgR), and evidence shows that these tumors are hormonally responsive. Therefore, the standard surgical treatment considered total hysterectomy, with bilateral salpingo-ophorectomy, and hormone replacement therapy is contraindicated postoperatively.[18,19] However, various studies have shown bilateral salpingo-oophorectomy did not appear to affect time for recurrence or overall survival in stage-I ESS.[20–24] Considering the adverse effects of early surgical menopause, retention of ovarian function may be an option for premenopausal women with stage-I ESS.[25] In all other stages, total hysterectomy with bilateral salpingo-ophorectomy is recommended. There is a high rate of lymph node involvement reported in ESS. One study by Chan et al. showed nearly 10% of those who underwent lymph node dissection had nodal metastases, and they recommend lymphadenectomy for both prognostic and treatment purposes of ESS.[24] In addition, patients with positive nodal metastasis at the time of lymphadenectomy had significantly poorer survival (35.3%) compared with those with negative nodes (80.1%). Other studies also showed a higher rate of lymph node involvement in ESS.[26–29] Therefore, lymph node dissection clearly provides prognostic information and treatment guidance; however, the potential therapeutic value of lymph node dissection remains to be determined.
Adjuvant therapy
For stage-I ESS, only postoperative observation is required. Hormone therapy may be an option in stage II to IV/recurrence/unresectable tumors’ postoperative hormone therapy with or without tumor directed radiotherapy (RT) [Figure 2].[30]
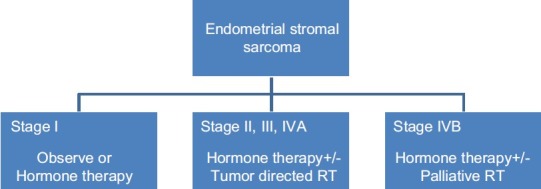
| Fig. 2 Adjuvant therapy
Hormone therapy
Hormone therapy has shown to be effective in ESS because ofestrogen and progestron receptors in it. Hormones include megestrol/medroxy progesterone, gonadotropin releasing hormone (GnRH) analogues, and aromatase inhibitors.[30] The mechanism of action of progestins is to bind progesterone receptors and down regulate gene transcription leading to decreased endometrial gland and stromal proliferation. GnRH agonists down regulate GnRH receptors in the anterior pituitary leading to a hypoestrogenic state. Estrogen deprivation is most specifically achieved using inhibitors that block the last stage in the biosynthetic sequence, that is, the conversion of androgens to estrogens by the aromatase enzyme. The new generation aromatase inhibitors such as letrazole, when given orally, inhibits peripheral aromatase and causes a marked reduction in circulating estrogens. ESS shows expression of aromatase enzymeand aromatase inhibitors such as letrazole and anastrozole can be used as adjuvant treatment.[31] Many case reports showed a disease free period of more than 10 years was obtained when treated with aromatase inhibitors after initial surgery.[32,33] In a study by Chu and colleagues, 75% of patients with stage-I disease did not recur if treated with adjuvant megestrol compared to 29% of similarly staged patients who did not receive adjuvant megestrol.[34] They recommend adjuvant megestrol 160 mg daily. Monthly intramuscular injections of leuprolide 7.5 mg can be given either alone or in combination with progesterone. The effective duration of preventive hormonal therapy is still undetermined. Various factors have been shown to influence hormone responsiveness. These are concentration of the sex steroid receptor, and relative expression of the progesterone receptor (PR) isoforms (PR-A and PR-B). The receptor concentration and the predominant isoform may vary in ESS originating in the uterus versus extra uterine sites, such as to make the latter less hormone responsive.[35–38] Tamoxifen, as well as hormonal replacement therapy containing estrogens are contraindicated in patients after treatment of ESS.[17,39–41] Advanced/metastatic ESS also can be treated with antioestrogen therapy, with an aromatase inhibitor or progestagen.[18] Maluf FC and Petal S suggest a dose of 2.5 mg letrazole daily for recurrent cases of ESS.[42] Due to the rarity of ESS, it is difficult to conduct prospective randomized clinical trials for determining the optimal treatment regimen. Treatment has been defined by the experience gained from retrospective case series and case reports.
Radiotherapy
RT in the form of brachytherapy with or without pelvic radiation can be used as adjuvant therapy. This will be useful for control of local recurrences but with limited effect on surveillance.[29,30] It is not recommended routinely in FIGO stage-I and stage-II disease. However, radiotherapycanbe considered for advanced or recurrent cases.[18,43]
Recurrent disease
Recurrences develop in one-third to one-half of patients with ESS and usually are limited to the pelvis and lower genital tract. Distant metastasis to lungs may occur after several years.[44,45] A growth stimulus by estrogens on residual tumor cells may contribute to recurrence. After oophorectomy, estrogens produced by peripheral tissues or exogenous administration in the form of hormone replacement therapy may be a reason for recurrences.[46] There is currently no standard therapy for patients with recurrent disease. Recurrent ESS has been treated with hormone therapy, radiation, surgical re-excision, or a combination of these modalities.[47] There are few case reports where the recurrent ESS was treated with etoposide, cyclophosphamide, and doxorubicin.[48] Even though chemotherapy is a mode of treatment in undifferentiated endometrial sarcoma, data supporting their efficiency in the case of recurrence of ESS are limited.
Follow-up and survival rates
At FIGO stage I, the 5-year survival rate for ESS is 54% to nearly 100% and at stage-IIit is 30%. For advanced disease (stage III and IV) the survival is only 11%. As these tumors have a tendency for late recurrence, long-term follow up is essential. It shall be once in 3 months for the first year and half-yearly for next 4 years. Thereafter annual follow up is recommended. Because of concern about radiation exposure, frequent routine asymptomatic surveillance imaging is not recommended after primary treatment.[29] The relapse free survival depends on the tumor stage, myometrial invasion, adjuvant treatment, and bilateral salpingoopherectomy.[49,50]
CONCLUSION
ESS is a rare uterine tumor. Because of the large variation in pathologic characteristics, combined with scarcity of patients, there is insufficient information about an optimal management. Study on prognostic factors is also not satisfactory. Hormone therapy is a new promising adjuvant treatment modality. Multianalysis from a large group of patients is necessary for predicting prognosis and to define proper treatment of endometrial stromal sarcoma.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES

| Fig. 1 Endometrial stromal sarcoma uniform oval or spindle shaped neoplastic cells invading myometrium

| Fig. 2 Adjuvant therapy


 PDF
PDF  Views
Views  Share
Share

