Abstract
Gastric cancer remains one of the most important malignancies worldwide in terms of incidence and mortality. The treatment is based on the combination of local surgery and radiation therapy as well as systemic chemotherapy and targeted molecules. Fluoropyrimidines and particularly 5-fluorouracil (FU) represent still the backbone for gastric cancer chemotherapy and new molecular versions of this molecule have been brought to clinical practice in order to improve benefits and reduce adverse effects. S-1 is an oral prodrug of 5-FU, which has demonstrated high effectiveness for gastric cancer treatment and a favorable safety profile. Currently, there are geographic differences in the treatment of gastric cancer and in the use of S-1, which is a mainstay of gastric cancer management in Eastern countries, but is not part of the standard care in the rest of the world. In this review, we gathered data from phase I, II, and III trials of S-1 in gastric cancer, in order to define its real benefit-risk ratio and assess whether geographic differences in S-1 use are justified by unchangeable factors.
Keywords: Gastric cancer treatment, oral fluoropyrimidines, S-1
INTRODUCTION
Gastric cancer is the fourth most common malignant disease and the second leading cause of cancer-related death worldwide.[1] In East Asian countries, the screening for gastric cancer is routine due to the highest rates of incidence.[2] The treatment of gastric cancer is optimized by the fine combination of surgery, radiotherapy, chemotherapy, and molecularly targeted medicines. Surgery represents the foremost intervention for curable gastric cancer and the extent of lymph node dissection is an important variable, which shows significant distinctions worldwide. In East Asia D2 dissection has been routinely performed since the 1960s,[3] while in most of the Western countries D1 dissection is preferred. Two prospective randomized trials performed in the Netherland and the UK[4,5] showed that D1 dissection was associated with less mortality and morbidity compared to D2 dissection. However, some issues were identified in these trials such as an inadequate D2 dissection and common execution of distal pancreatectomy and splenectomy, which is currently considered unneeded.[6] Moreover, a 15 years follow-up of the Dutch D1D2 trial, showed that D2 lymphadenectomy is associated with lower loco-regional recurrence and gastric-cancer-related death rates than D1 surgery.[7] Recent studies have demonstrated that D2 gastrectomy could produce benefits in Western patients when conducted in experienced Western centers[8,9,10] and currently in Europe spleen preserving D2 resection is recommended and available in high-volume centers. On the other hand, in Japan, a clinical trial comparing D1 versus D2 would be considered inappropriate today. Partly depending on this discrepancy in the surgical modalities, the complementary chemotherapeutic and radiation treatments for gastric cancer are not applied homogeneously in the different geographical areas. In Europe, perioperative chemotherapy with epirubicine, cisplatin and infusion fluorouracil (ECF) without radiation is frequently used in gastric cancer patients based on the results of a phase III trial, which showed how this approach signifi cantly improves progression-free and overall survival (OS).[11] In the USA, postoperative radiochemotherapy was evaluated by conducting a phase III trial in patients with gastric cancer which underwent R0 resection, demonstrating an improvement of recurrence-free survival, and OS in 10 years follow-up.[12] In Japan, adjuvant therapy with S-1 for 1-year is a standard of care after it showed effectiveness in all subgroups of patients who underwent D2 resection in a phase III study.[13] Investigators in China and Korea conducted a phase III trial showing that postoperative therapy with capecitabine/oxaliplatin improves both disease-free survival (DFS) and OS with respect to surgery alone in patients, which underwent D2 resection for stage IIA-IIIB gastric cancer.[14] In the locally advanced and metastatic gastric cancer, systemic chemotherapy based on platinum-fluoropyrimidine combinations is the standard of care almost everywhere in the world. New molecular versions of these drugs, such as oral fluoropyrimidines and oxaliplatin, have already shown noninferiority while decreasing toxicity.[15] At the failure of the first line chemotherapy, other agents such as taxanes and irinotecan, can improve survival.[16,17] Targeted therapies are acquiring an important part in advanced gastric cancer (AGC) treatment, such as the addition of trastuzumab to the fluoropyrimidine-platinum combination in first line for HER-2 positive patients[18] or the anti-angiogenic monoclonal antibody ramucirumab beyond first line as a single agent and in combination with Paclitaxel.[19,20] Asian studies report better gastric cancer survivals compared to Western ones[11,13,21,22] and a recent retrospective investigation conducted on Asian and non-Asian individuals in the USA by controlling for the imbalance of known prognostic and treatment factors, found that the survival advantage of the Asian population continued to be present.[23] Due to this facts, in this paper we are going to highlight once more the East-West differences in the surgical approach and chemotherapy by focusing, eventually, on the role of the systemic treatment with the new oral fluoropyrimidine S-1, which has showed contrasting clinical benefit rates and toxicity profiles in these two different areas. Our conjecture is that the factors, which determine the divergent outcomes in these different population can be divided into changeable and unchangeable elements. We will try to identify some of these factors by reviewing trials of gastric cancer treated with S-1 conducted separately on Asian and Caucasian populations and eventually extrapolate data that could suggest the different “causality proportion” of the changeable versus the unchangeable elements in bringing the different outcomes in the S-1 treatment.
ORAL FLUOROPYRIMIDINES AND S-1
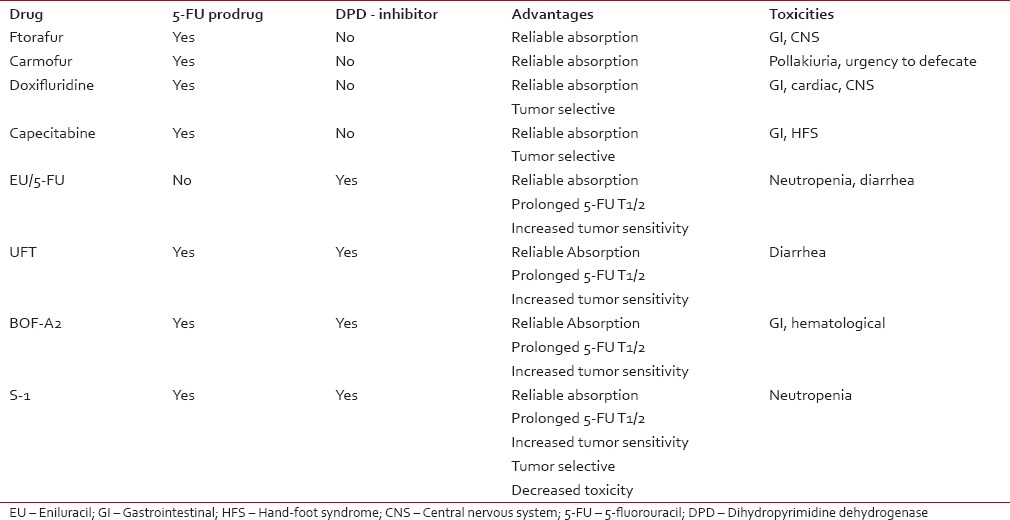
Fluoropyrimidines are emblematic of a rational anti-cancer drug design and 5-fluorouracil (5-FU) has become the most widely used among them since it was first introduced in 1957.[24] In are summarized the metabolic pathways and action mechanisms of 5-FU. Only 1-3% of the original dose of 5-FU will engage in the intracellular anabolic pathways[25] that generate its effective cytotoxic metabolites. The central factor in 5-FU catabolism is dihydropyrimidine dehydrogenase (DPD), an enzyme expressed mostly in the liver cells but also in intestine mucosa and various other tissues. 5-FU is catabolized by DPD finally leading to the formation α-fluoro-β-alanine [] which is the main responsible for the toxicity related to 5-FU.[26,27] Early clinical use of 5-FU relied on simple intravenous (i.v.) administration in bolus until the superiority of i.v. continuous infusion was demonstrated in terms of response rate (RR), OS, and toxicity.[28] However, this led to the inconvenience of pumps and implantable catheters; consequently, the oral administration of 5-FU was assessed having as a rationale the potentiality to approximate or even improve the pharmacokinetics of continuous infusion. The oral prodrugs of 5-FU have been developed by refashioning biochemically the molecule into compounds which are not substrates for DPD and are, therefore, absorbed intact through the gastrointestinal (GI) mucosa needing to be activated subsequently into 5-FU by enzymes. The first three prodrugs which signed the passage to oral formulations are represented by carmofur, doxifluridine, and tegafur (ftorafur) []. Ftorafur (tegafur; R,S-1-1 (tetrahydrofuran-2-yl)-5-FU) is the main ancestor of S-1, the novel oral fluoropyrimidine we are going to focus on in our paper, but it has a limited clinical use on its own due to the important neurological and GI toxicities.[29] The combination of oral fluoropyrimidines, in the form of prodrugs, with agents which exert a targeted inhibition of DPD determines a prolongation of 5-FU half-life in plasma, a possible circumvention of 5-FU resistance due to DPD overexpression by cancer cells,[30] a better oral absorption, an increased sensitivity to the drug as well as the elimination of some toxic metabolites.[31] The application of a second modulation to the 5-FU prodrug/DPD inhibitor [] finally brought to S-1, a combination of tegafur and two 5-FU modulators, gimeracil (5-chloro-2,4-dihydroxypyridine [CDHP]), and oteracil (oxonate), in a molar ratio of 1:0.4:1 [], that enhances the anticancer activity and reduces the GI toxicity of 5-FU (C14). Orally-administrable tegafur was developed as a drug which showed an excellent absorbability, a slight conversion to 5-FU in the GI tract and a gradual conversion to 5-FU by cytochrome p450 enzymes in the hepatocytes.[32,33] Subsequently, CDHP was reported as one of the strongest inhibitors of DPD[34] and some further studies investigated the possibility of decreasing the GI toxicity of 5-FU without decreasing its antitumor activity by concomitant administration of a new molecule called oxonate, which localizes in GI mucosa and selectively inhibits the orotate phosphoribosyltransferase (OPRT), limiting 5-FU phosphorylation to FUMP, therefore reducing GI toxicity effects.[35] Based on these conclusions, tegafur and CDHP were given per OS concurrently to sarcoma-bearing rats at different molar ratios, and then oxonate was given orally during the consecutive administration of the tegafur-CDHP mixture and the final proposed formulation, called S-1, consisting of tegafur, CDHP, and oxonate at a 1:0.4:1 ratio, showed antitumor selective activity.[36] According to the findings of a Japanese phase I study, the recommended dose and administration for phase II trials were determined as twice daily administration of 75 mg/body for 28 consecutive days with 14 days rest (1 course).[37] Due to the significant differences between Asian and Caucasian patients in terms of Tegafur metabolism, phase I studies comprising only Western patients established lower recommended dose for S-1 (50-60 mg/m2/day for 4 weeks every 5 weeks).[38,39] Phase II studies have reported high RRs (35-50%) in different advanced solid tumors (gastric, colorectal, breast, and head and neck tumors).[40,41,42] In we listed all the oral fluoropyrimidine compounds assessed in the clinical setting.
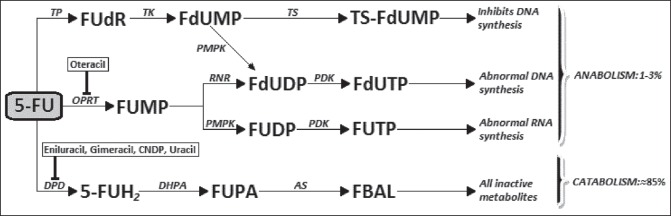
| Fig. 1 Metabolism of 5-fluorouracil and action site of DPD-inhibitors and oteracil. DPD: Dihydropyrimidine dehydrogenase; CNDP: Cyano dihydroxypyrimidine; 5-FUH2: Dihydrofluorouracil; FUPA: α-fluoroureidopropionic acid; FBAL: α-fluoro-β-alanine; FUMP: Fluorouridine monophosphate; FUDP: Fluorouridine diphosphate; FUTP: Fluorouridine triphosphate; TP: Thymidine phosphorylase; FUdR: Fluorodeoxyuridine; TK: Thymidine kinase; FdUMP: Fluorodeoxyuridine monophosphate; TS: Thymidylate synthase; FdUDP: Fluorodeoxyuridine diphosphate; FdUTP: Fluorodeoxyuridine triphosphate
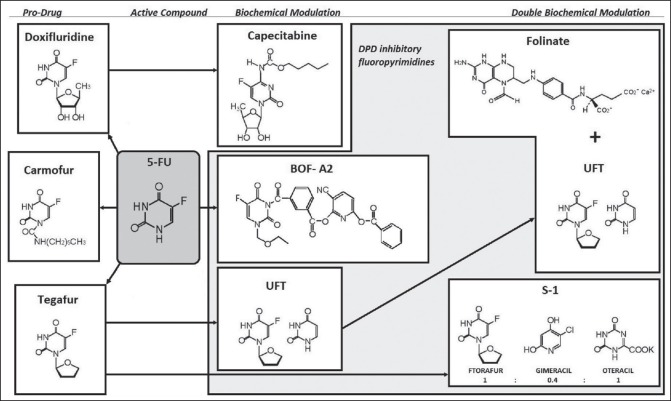
| Fig. 2 Evolution of oral fluoropyrimidines
Table 1
Oral fluoropyrimidines in comparison
S-1 IN GASTRIC CANCER
Advanced gastric cancer
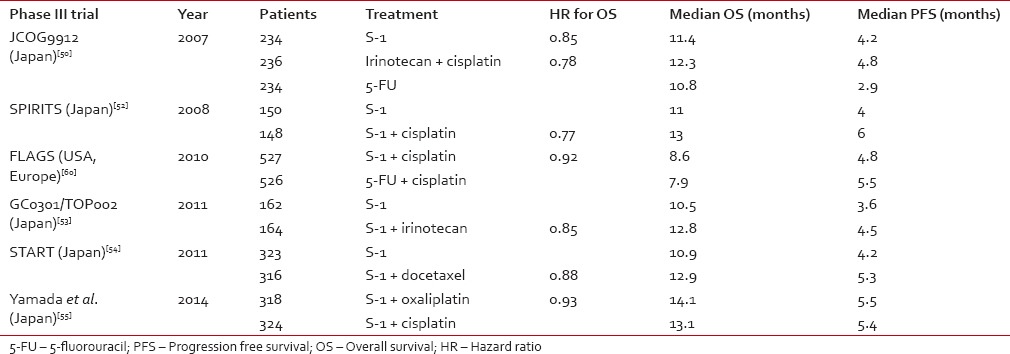
A variety of phase II trials have shown that fluoropyridines are the most active single agents in terms of RR for AGC.[43] with a RR of 31% for continuous infusion 5-FU and 38% for capecitabine.[44,45] In Japanese phase II studies, S-1 increased the RR up to 48%[46,47] and showed a suitable treatment option for outpatients as for its mild side effects. Different investigations have detected some predictive factors for the response to S-1 therapy in AGC such as the level of thymidylate synthase, peritoneal metastases, advanced rather than recurrent disease, and PS 0-1.[48,49] An important phase III trial showed that S-1 at the dose of 40 mg/m2, b.i.d., for 4 weeks, every 6 weeks is a noninferior substitute for standard infusional 5-FU therapy for AGC, with RR 28% and progression-free survival (PFS) 4.2 months.[50] Further investigations increased the benefit from S-1 based chemotherapy in AGC by introducing combinations with other anti-cancer agents of different action mechanism. A phase I/II trial showed a RR of 74% and OS of 383 days with acceptable toxicity for the association of S-1 with cisplatin.[51] This combination in a phase III study compared to S-1 alone yielded higher RR (54% vs. 31%), longer PFS (6 months vs. 4 months), and OS (13 months vs. 11 months), becoming the standard therapy in Japan.[52] Phase III trials also tested the combination S-1 plus Irinotecan and S-1 plus docetaxel, but benefit in OS was not significant compared to S-1 alone, even though there was a higher RR.[53,54] A recent phase III trial in Japan compared the combination S-1 plus cisplatin to the combination S-1 plus oxaliplatin and showed a higher safety profile for oxaliplatin combination, with a 50% reduction of G3-G4 hematologic toxicities and a slight gain in terms of median OS (14.1 months vs. 13.1 months).[55] Retrospective data suggests that S-1 plus cisplatin could be effective also for patients with gastric cancer that recurs after adjuvant S-1 chemotherapy (especially for those with a recurrence free interval more than 6 months) and as a second line where cisplatin plus 5-FU failed.[56,57] The experience with S-1 in countries different than Japan is limited. However, there has been interest in S-1 as an agent for AGC also in Western countries where a phase I study of Caucasian subjects defined a different optimal dose for S-1 in the cisplatin combination (25 mg/m2 bid for 3 weeks, 2 weeks rest).[58] A phase II study using this schedule in USA-European area generated a RR of 51% and a median OS 10.9 months.[59] This scheme was subsequently tested versus standard cisplatin plus 5-FU in a phase III study conducted in 24 countries with 86% of patients being Caucasian showing no significant differences in PFS, OS, and RR.[60] It is also important to mention a multicenter phase II study conducted in Japan, in which patients with HER-2 positive AGC received combination chemotherapy with S-1 and cisplatin plus the targeted molecule trastuzumab, yielding and RR of 68%, an OS of 16 months, and a PFS of 7.8 months.[61] In , we listed the most significant phase III trials for S-1 in AGC, worldwide. Choice of the recommended treatment regimen still depends on geographic, institutional, and personal preferences. However, S-1-based chemotherapy may be a good choice for AGC in all ethnicities because of longer survival times, good tolerance, and convenient use.[62]Table 2
Comparison of Asian and Western phase III trials for S-1 based chemotherapy in advanced gastric cancer
Curatively resected gastric cancer
A phase III trial was conducted in Japan in 2007 to appraise the benefits of S-1 (40 mg/m2 twice a day for 4 weeks every 6 weeks and continued for 1-year) as adjuvant treatment after curative resection of gastric cancer with D2 lymph node dissection, testifying a significant benefit in terms of OS compared to the surgery-alone group (71.7% vs. 60.1% at 5 years).[13] S-1 monotherapy might be insufficient in some groups of patients (especially in the stage IIIB highly advanced group), therefore, Phase II trials have validated the feasibility of S-1 combined respectively with cisplatin, docetaxel, and oxaliplatin in the adjuvant setting showing increased effectiveness and acceptable toxicities.[63,64,65] These studies indicate that adjuvant therapy with S-1 plus cisplatin or plus docetaxel may provide a survival benefit to patients with stage III gastric cancer[66,67] []. In particular, S-1 plus docetaxel is a promising option for patients curatively operated in stage IIIB.[68] However, a recent phase III trial was conducted in gastric cancer patients with T4a-T4b disease which underwent D2 surgery to assess the superiority of the sequential adjuvant treatment (paclitaxel → S-1) and the results showed that for this group of Asian patients sequential treatment does not increase DFS, therefore, the S-1 monotherapy should remain the standard treatment.[69] Some prognostic and predictive factors for the S-1 adjuvant chemotherapy have been suggested by small retrospective series and need to be validated in prospective phase III studies.[70,71,72,73,74] In patients with stage II/III gastric cancer who underwent D2 gastrectomy followed by adjuvant S-1 chemotherapy factors indicating poor prognosis include high tumor diameter, male sex, age ≥67 years, intestinal-type histology, lymph node ratio ≥16.7%, and open surgery.[70,71] However, in this regard, the subgroup analysis of the phase III ACTS-GS randomized trial according to sex, age, disease stage, histologic type showed no interaction between S-1 adjuvant treatment and any of these factors.[13] Predictive factors for relapse after S-1 adjuvant chemotherapy may include high tumor diameter and some molecular characteristics of tumor tissue such as a low OPRT levels (OPRT < 2.0 ng/mg protein) and expression of specific microRNAs (miR-92b, miR-422a, miR-4732-5p, miR-4758-3p).[72,73,74] Even though debatable earlier studies have suggested no significant differences between Asian and Caucasian patients with regard to genetic influences or distribution of prognostic factors,[75,76] it is currently well documented that Asian patients have a better prognosis. Hence, it can be hypothesized that more complex factors such as early diagnosis (with consequent shift toward adjuvant chemotherapy), stage migration, pathological definition, type of surgery, and modality of adjunct systemic therapy might have a role in these different outcome rates.Table 3
Comparison between S-1 and S-1 combination adjuvant treatments for curatively resected gastric cancer

NEOADJUVANT SETTING
The preoperative therapy may enhance the chance for cure of patients with resectable AGC. In this setting, a highly responsive cytotoxic treatment is needed. A phase II trial assessed the neoadjuvant combination S-1 with cisplatin confirming feasibility without a marked increase of toxicities or notable influence over the surgical morbidity.[77] The survival analysis update of this study has been recently presented in the ASCO GI 2015 meeting showing that four courses of S-1 plus Cisplatin do not yield an advantage in terms of median OS at 3 years compared to the two courses arm, therefore, currently the recommended neoadjuvant chemotherapy for a future phase III trial would consist in two S-1/cisplatin courses. Retrospective analysis evidenced that limited peritoneal dissemination of gastric origin is highly sensitive to induction chemotherapy with S-1 plus cisplatin and resection after disappearance of peritoneal metastasis could cure some patients.[78] Furthermore, the neoadjuvant combination of S-1 with oxaliplatin was associated with increased rate of R0 resection and D2 lymph nodes dissection with acceptable adverse effects.[79] Multimodal therapy comprising combined preoperative docetaxel plus cisplatin plus S-1 therapy and gastrectomy with para-aortic lymph node dissection was effective and feasible in a phase II trial for AGC with para-aortic lymph node metastasis.[80] Another phase II trial assessed the efficacy of the combination S-1/oxaliplatin/docetaxel as a neoadjuvant therapy for locally AGC and showed that it could be performed safely with a high R0 resection rate.[81] According to preliminary studies, neoadjuvant chemoradiation with S-1 for stage IIIB patients and with S-1 plus cisplatin in AGC are also feasible and effective.[82,83] However, all the studies mentioned were conducted on Asian patients and efficacy of the same approaches needs still to be assessed on Caucasian patients.
S-1 toxicity overview
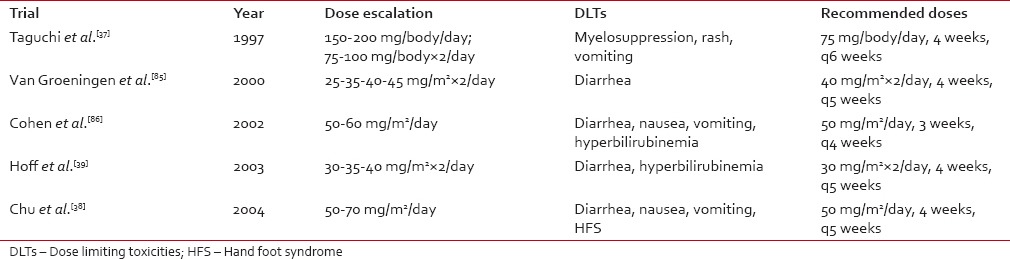
S-1 is a prodrug having intrinsic molecular mechanisms to optimize the side effects. There is evidence that toxicity may, in some extent, vary as a function of the ethnicity, disease characteristics, and patients comorbidities. Two phase I trials recommended an 80-120 mg daily dose of S-1 in Asian patients and concluded that myelosuppression was the most severe dose-related toxicity,[37,84] while trials conducted in Caucasian populations resulted in a recommended dose of 50-80 mg and diarrhea was the most severe dose-related toxicity[38,39,85,86] []. This difference can be partly explained by the evidence that there is a different expression of CYP 2A6 polymorphisms[87] in Western and Asian populations which causes Asians to convert FT to FU at a slower rate.[39] Regarding the toxicity of S-1 in combination, a phase I trial conducted in Western patients showed that the dose limiting toxicities (DLTs) for the combination with cisplatin were mainly GI.[58] Two Japanese phase I studies which investigated the combination of S-1 with docetaxel and paclitaxel, respectively, demonstrated that the DLTs were mostly hematological.[88,89] A significant factor influencing the GI toxicity of S-1 is the co-administration of food which determines a greater degradation of oteracil.[90] Moreover, an investigation in 2013 showed that the incidence of grade III-IV toxicities increased in patients who underwent a total gastrectomy compared to those which had a subtotal gastric resection.[91] There is also evidence that renal function might affect tolerability since adverse events seem to occur more frequently in the patients with creatinine clearance < 60 ml/min.[92]Table 4
Comparison of the DLTs and recommended doses of S-1 as a single agent in the main Japanese and Western phase I trials
CONCLUSION
We illustrated the main features of the role of surgery, radiotherapy, and especially chemotherapy in the treatment of gastric cancer in the advanced, adjuvant, and neoadjuvant settings by highlighting the divergences between East Asian countries and the West. With regard to the complementary chemotherapies, we focused mostly on the role of S-1. S-1 therapy is well established for AGC and in the adjuvant setting in Asian patients and it might yield benefits also preoperatively. In the light of our investigations, it appears that S-1 could be useful in the clinical practice for the relatively high effectiveness and mild toxicities as monotherapy as well as in combination also in non-Asian patients. There are specific factors that influence the response of single individuals to S-1 in terms of clinical benefit and adverse effects. One group of factors might be patient-related including elements such as renal function, food co-administration, and genetic polymorphisms, which are at the base of the inter-population differences in terms of tolerability. The second group of factors is cancer-related and comprises the histopathology, type of resection, and stage. Most of these elements are unchangeable and do not show significant differences between different ethnic groups, however, they need to be validated in larger prospective studies. Hence, the different influence of changeable and unchangeable factors is debatable; nevertheless, modifiable factors such as the earlier diagnosis, the surgical approach, and adequate adjunct chemotherapy might be significant in determining the overall divergences in the outcomes of the gastric cancer treatment especially in relation to S-1 therapy. These major changeable factors and other possible minor ones should be the object of discussion as they may significantly influence the optimization of gastric management worldwide.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.REFERENCES
1.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. [PubMed] [Google Scholar]2.
Forman D, Burley VJ. Gastric cancer: Global pattern of the disease and an overview of environmental risk factors. Best Pract Res Clin Gastroenterol. 2006;20:633–49. [PubMed] [Google Scholar]3.
Kajitani T. The general rules for the gastric cancer study in surgery and pathology. Part I. Clinical classification. Jpn J Surg. 1981;11:127–39. [PubMed] [Google Scholar]4.
Bonenkamp JJ, Songun I, Hermans J, Sasako M, Welvaart K, Plukker JT, et al. Randomised comparison of morbidity after D1 and D2 dissection for gastric cancer in 996 Dutch patients. Lancet. 1995;345:745–8. [PubMed] [Google Scholar]5.
Cuschieri A, Fayers P, Fielding J, Craven J, Bancewicz J, Joypaul V, et al. Postoperative morbidity and mortality after D1 and D2 resections for gastric cancer: Preliminary results of the MRC randomised controlled surgical trial. The Surgical Cooperative Group. Lancet. 1996;347:995–9. [PubMed] [Google Scholar]6.
Biffi R, Chiappa A, Luca F, Pozzi S, Lo Faso F, Cenciarelli S, et al. Extended lymph node dissection without routine spleno-pancreatectomy for treatment of gastric cancer: Low morbidity and mortality rates in a single center series of 250 patients. J Surg Oncol. 2006;93:394–400. [PubMed] [Google Scholar]7.
Songun I, Putter H, Kranenbarg EM, Sasako M, van de Velde CJ. Surgical treatment of gastric cancer: 15-year follow-up results of the randomised nationwide Dutch D1D2 trial. Lancet Oncol. 2010;11:439–49. [PubMed] [Google Scholar]8.
Roviello F, Marrelli D, Morgagni P, de Manzoni G, Di Leo A, Vindigni C, et al. Survival benefit of extended D2 lymphadenectomy in gastric cancer with involvement of second level lymph nodes: A longitudinal multicenter study. Ann Surg Oncol. 2002;9:894–900. [PubMed] [Google Scholar]9.
Degiuli M, Sasako M, Ponti A, Soldati T, Danese F, Calvo F. Morbidity and mortality after D2 gastrectomy for gastric cancer: Results of the Italian Gastric Cancer Study Group prospective multicenter surgical study. J Clin Oncol. 1998;16:1490–3. [PubMed] [Google Scholar]10.
Degiuli M, Sasako M, Calgaro M, Garino M, Rebecchi F, Mineccia M, et al. Morbidity and mortality after D1 and D2 gastrectomy for cancer: Interim analysis of the Italian Gastric Cancer Study Group (IGCSG) randomised surgical trial. Eur J Surg Oncol. 2004;30:303–8. [PubMed] [Google Scholar]11.
Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de Velde CJ, Nicolson M, et al. Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med. 2006;355:11–20. [PubMed] [Google Scholar]12.
Smalley SR, Benedetti JK, Haller DG, Hundahl SA, Estes NC, Ajani JA, et al. Updated analysis of SWOG-directed intergroup study 0116: A phase III trial of adjuvant radiochemotherapy versus observation after curative gastric cancer resection. J Clin Oncol. 2012;30:2327–33. [PMC free article] [PubMed] [Google Scholar]13.
Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T, et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29:4387–93. [PubMed] [Google Scholar]14.
Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH, et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet. 2012;379:315–21. [PubMed] [Google Scholar]15.
Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. [PubMed] [Google Scholar]16.
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer — A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–14. [PubMed] [Google Scholar]17.
Ford HE, Marshall A, Bridgewater JA, Janowitz T, Coxon FY, Wadsley J, et al. COUGAR-02 Investigators. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7 [Epub 2013 Dec 10] [PubMed] [Google Scholar]18.
Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–97. [PubMed] [Google Scholar]19.
Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): An international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–9. [PubMed] [Google Scholar]20.
Wilke H, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, Hironaka S, et al. RAINBOW: A global, phase III, randomized, double-blind study of ramucirumab plus paclitaxel versus placebo plus paclitaxel in the treatment of metastatic gastroesophageal junction (GEJ) and gastric adenocarcinoma following disease progression on first-line platinum- and fluoropyrimidine-containing combination therapy rainbow IMCL CP12-0922 (I4T-IE-JVBE) J Clin Oncol. 2014;32(3_suppl):LBA7. [Google Scholar]21.
Nashimoto A, Nakajima T, Furukawa H, Kitamura M, Kinoshita T, Yamamura Y, et al. Randomized trial of adjuvant chemotherapy with mitomycin, fluorouracil, and cytosine arabinoside followed by oral fluorouracil in serosa-negative gastric cancer: Japan Clinical Oncology Group 9206-1. J Clin Oncol. 2003;21:2282–7. [PubMed] [Google Scholar]22.
Macdonald JS, Smalley SR, Benedetti J, Hundahl SA, Estes NC, Stemmermann GN, et al. Chemoradiotherapy after surgery compared with surgery alone for adenocarcinoma of the stomach or gastroesophageal junction. N Engl J Med. 2001;345:725–30. [PubMed] [Google Scholar]23.
Wang J, Sun Y, Bertagnolli MM. Comparison of gastric cancer survival between Caucasian and Asian patients treated in the United States: Results from the Surveillance Epidemiology and End Results (SEER) Database. Ann Surg Oncol. DOI 10.1245/s10434-015-4388-4. [PubMed] [Google Scholar]24.
Heidelberger C, Chaudhuri NK, Danneberg P, Mooren D, Griesbach L, Duschinsky R, et al. Fluorinated pyrimidines, a new class of tumour-inhibitory compounds. Nature. 1957;179:663–6. [PubMed] [Google Scholar]25.
Saif MW, Syrigos KN, Katirtzoglou NA. S-1: A promising new oral fluoropyrimidine derivative. Expert Opin Investig Drugs. 2009;18:335–48. [PubMed] [Google Scholar]26.
Okeda R, Shibutani M, Matsuo T, Kuroiwa T, Shimokawa R, Tajima T. Experimental neurotoxicity of 5-fluorouracil and its derivatives is due to poisoning by the monofluorinated organic metabolites, monofluoroacetic acid and alpha-fluoro-beta-alanine. Acta Neuropathol. 1990;81:66–73. [PubMed] [Google Scholar]27.
Matsubara I, Kamiya J, Imai S. Cardiotoxic effects of 5-fluorouracil in the guinea pig. Jpn J Pharmacol. 1980;30:871–9. [PubMed] [Google Scholar]28.
Meta-analysis Group in Cancer. Piedbois P, Rougier P, Buyse M, Pignon J, Ryan L, et al. Efficacy of intravenous continuous infusion of fluorouracil compared with bolus administration in advanced colorectal cancer. J Clin Oncol. 1998;16:301–8. [PubMed] [Google Scholar]29.
Au JL, Wu AT, Friedman MA, Sadée W. Pharmacokinetics and metabolism of ftorafur in man. Cancer Treat Rep. 1979;63:343–50. [PubMed] [Google Scholar]30.
Baker SD, Khor SP, Adjei AA, Doucette M, Spector T, Donehower RC, et al. Pharmacokinetic, oral bioavailability, and safety study of fluorouracil in patients treated with 776C85, an inactivator of dihydropyrimidine dehydrogenase. J Clin Oncol. 1996;14:3085–96. [PubMed] [Google Scholar]31.
Cao S, Rustum YM, Spector T. 5-Ethynyluracil (776C85): Modulation of 5-fluorouracil efficacy and therapeutic index in rats bearing advanced colorectal carcinoma. Cancer Res. 1994;54:1507–10. [PubMed] [Google Scholar]32.
Toide H, Akiyoshi H, Minato Y, Okuda H, Fujii S. Comparative studies on the metabolism of 2-(tetrahydrofuryl)-5-fluorouracil and 5-fluorouracil. Gan. 1977;68:553–60. [PubMed] [Google Scholar]33.
El Sayed YM, Sadée W. Metabolic activation of R,S-1-(tetrahydro-2-furanyl)-5-fluorouracil (ftorafur) to 5-fluorouracil by soluble enzymes. Cancer Res. 1983;43:4039–44. [PubMed] [Google Scholar]34.
Tatsumi K, Fukushima M, Shirasaka T, Fujii S. Inhibitory effects of pyrimidine, barbituric acid and pyridine derivatives on 5-fluorouracil degradation in rat liver extracts. Jpn J Cancer Res. 1987;78:748–55. [PubMed] [Google Scholar]35.
Shirasaka T, Shimamoto Y, Fukushima M. Inhibition by oxonic acid of gastrointestinal toxicity of 5-fluorouracil without loss of its antitumor activity in rats. Cancer Res. 1993;53:4004–9. [PubMed] [Google Scholar]36.
Shirasaka T, Shimamato Y, Ohshimo H, Yamaguchi M, Kato T, Yonekura K, et al. Development of a novel form of an oral 5-fluorouracil derivative (S-1) directed to the potentiation of the tumor selective cytotoxicity of 5-fluorouracil by two biochemical modulators. Anticancer Drugs. 1996;7:548–57. [PubMed] [Google Scholar]37.
Taguchi T, Inuyama Y, Kanamaru R, Hasegawa K, Akazawa S, Niitani H, et al. Phase I study of S-1. S-1 Study Group. Gan To Kagaku Ryoho. 1997;24:2253–64. [PubMed] [Google Scholar]38.
Chu QS, Hammond LA, Schwartz G, Ochoa L, Rha SY, Denis L, et al. Phase I and pharmacokinetic study of the oral fluoropyrimidine S-1 on a once-daily-for-28-day schedule in patients with advanced malignancies. Clin Cancer Res. 2004;10:4913–21. [PubMed] [Google Scholar]39.
Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, et al. Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res. 2003;9:134–42. [PubMed] [Google Scholar]40.
Baba H, Ohtsu A, Sakata Y, Mitachi Y, Sugimachi K, Taguchi T. Late phase II study of S-1 in patients with advanced colorectal cancer in Japan. Proc Am Soc Clin Oncol. 1998;17:277A. [Google Scholar]41.
Ohtsu A, Sakata Y, Horikoshi N, Mitachi Y, Sugimachi D, Taguchi T. A phase II study of S-1 in patients with advanced gastric cancer. Proc Am Soc Clin Oncol. 1998;17:262A. [Google Scholar]42.
Inuyama Y, Kida A, Tsukuda M, Kohno N, Satake B. Early phase II study of S-1 in patients with advanced head and neck cancer. S-1 Cooperative Study Group (Head and Neck Working Group) Gan To Kagaku Ryoho. 1998;25:1151–8. [PubMed] [Google Scholar]43.
Lordick F, Jäger D. Current status and future of chemotherapy and biochemotherapy in gastroesophageal cancers. Gastrointest Cancer Res. 2008;2:187–97. [PMC free article] [PubMed] [Google Scholar]44.
Moynihan T, Hansen R, Anderson T, Quebbeman E, Beatty P, Ausman R, et al. Continuous 5-fluorouracil infusion in advanced gastric carcinoma. Am J Clin Oncol. 1988;11:461–4. [PubMed] [Google Scholar]45.
Hong YS, Song SY, Lee SI, Chung HC, Choi SH, Noh SH, et al. A phase II trial of capecitabine in previously untreated patients with advanced and/or metastatic gastric cancer. Ann Oncol. 2004;15:1344–7. [PubMed] [Google Scholar]46.
Koizumi W, Kurihara M, Nakano S, Hasegawa K. Phase II study of S-1, a novel oral derivative of 5-fluorouracil, in advanced gastric cancer. For the S-1 Cooperative Gastric Cancer Study Group. Oncology. 2000;58:191–7. [PubMed] [Google Scholar]47.
Sakata Y, Ohtsu A, Horikoshi N, Sugimachi K, Mitachi Y, Taguchi T. Late phase II study of novel oral fluoropyrimidine anticancer drug S-1 (1 M tegafur-0.4 M gimestat-1 M otastat potassium) in advanced gastric cancer patients. Eur J Cancer. 1998;34:1715–20. [PubMed] [Google Scholar]48.
Miyazaki I, Kawai T, Harada Y, Moriyasu F. A predictive factor for the response to S-1 plus cisplatin in gastric cancer. World J Gastroenterol. 2010;16:4575–82. [PMC free article] [PubMed] [Google Scholar]49.
Lee SR, Kim HO, Yoo CH. Clinical outcomes of TS-1 chemotherapy for advanced and recurrent gastric cancer. J Korean Surg Soc. 2011;81:163–8. [PMC free article] [PubMed] [Google Scholar]50.
Boku N, Yamamoto S, Shirao K, Doi T, Sawaki A, Koizumi W, et al. Gastrointestinal Oncology Study Group/Japan Clinical Oncology Group. Randomized phase III study of 5-fluorouracil (5-FU) alone versus combination of irinotecan and cisplatin (CP) versus S-1 alone in advanced gastric cancer (JCOG9912) Journal of Clinical Oncology, ASCO Annual Meeting Proceedings (Post-Meeting Edition) 2007 25 (June 20 Supplement), LBA4513. [Google Scholar]51.
Koizumi W, Tanabe S, Saigenji K, Ohtsu A, Boku N, Nagashima F, et al. Phase I/II study of S-1 combined with cisplatin in patients with advanced gastric cancer. Br J Cancer. 2003;89:2207–12. [PMC free article] [PubMed] [Google Scholar]52.
Koizumi W, Narahara H, Hara T, Takagane A, Akiya T, Takagi M, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): A phase III trial. Lancet Oncol. 2008;9:215–21. [PubMed] [Google Scholar]53.
Narahara H, Iishi H, Imamura H, Tsuburaya A, Chin K, Imamoto H, et al. Randomized phase III study comparing the efficacy and safety of irinotecan plus S-1 with S-1 alone as first-line treatment for advanced gastric cancer (study GC0301/TOP-002) Gastric Cancer. 2011;14:72–80. [PMC free article] [PubMed] [Google Scholar]54.
Kim Y, Koizumi W, Lee K, Kishimoto T, Chung H, et al. Randomized phase III study of S-1 alone versus S-1 plus docetaxel (DOC) in the treatment for advanced gastric cancer (AGC): The START trial. J Clin Oncol. 2011;29(Suppl 4):7–10. doi:10.1200/JCO.2010.32.3022. PubMed: 21115871. [Google Scholar]55.
Yamada Y, Higuchi K, Nishikawa K, Gotoh M, Fuse N, Sugimoto N, et al. Phase III study comparing oxaliplatin plus S-1 with cisplatin plus S-1 in chemotherapy-naïve patients with advanced gastric cancer. Ann Oncol. 2015;26:141–8. [PubMed] [Google Scholar]56.
Shitara K, Morita S, Fujitani K, Kadowaki S, Takiguchi N, Hirabayashi N, et al. Combination chemotherapy with S-1 plus cisplatin for gastric cancer that recurs after adjuvant chemotherapy with S-1: Multi-institutional retrospective analysis. Gastric Cancer. 2012;15:245–51. [PMC free article] [PubMed] [Google Scholar]57.
Lv F, Liu X, Wang B, Guo H, Li J, Shen L, et al. S-1 monotherapy as second line chemotherapy in advanced gastric cancer patients previously treated with cisplatin/infusional fluorouracil. Int J Clin Exp Pathol. 2014;7:4274–9. [PMC free article] [PubMed] [Google Scholar] Retracted58.
Ajani JA, Faust J, Ikeda K, Yao JC, Anbe H, Carr KL, et al. Phase I pharmacokinetic study of S-1 plus cisplatin in patients with advanced gastric carcinoma. J Clin Oncol. 2005;23:6957–65. [PubMed] [Google Scholar]59.
Lenz HJ, Lee FC, Haller DG, Singh D, Benson AB, 3rd, Strumberg D, et al. Extended safety and efficacy data on S-1 plus cisplatin in patients with untreated, advanced gastric carcinoma in a multicenter phase II study. Cancer. 2007;109:33–40. [PubMed] [Google Scholar]60.
Ajani JA, Rodriguez W, Bodoky G, Moiseyenko V, Lichinitser M, Gorbunova V, et al. Multicenter phase III comparison of cisplatin/S-1 with cisplatin/infusional fluorouracil in advanced gastric or gastroesophageal adenocarcinoma study: The FLAGS trial. J Clin Oncol. 2010;28:1547–53. [PubMed] [Google Scholar]61.
Kurokawa Y, Sugimoto N, Miwa H, Tsuda M, Nishina S, Okuda H, et al. Phase II study of trastuzumab in combination with S-1 plus cisplatin in HER2-positive gastric cancer (HERBIS-1) Br J Cancer. 2014;110:1163–8. [PMC free article] [PubMed] [Google Scholar]62.
Yang J, Zhou Y, Min K, Yao Q, Xu CN. S-1-based vs non-S-1-based chemotherapy in advanced gastric cancer: A meta-analysis. World J Gastroenterol. 2014;20:11886–93. [PMC free article] [PubMed] [Google Scholar]63.
Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Feasibility study of adjuvant chemotherapy with S-1 plus cisplatin for gastric cancer. Cancer Chemother Pharmacol. 2011;67:1423–8. [PubMed] [Google Scholar]64.
Tamura S, Fujitani K, Kimura Y, Tsuji T, Matsuyama J, Iijima S, et al. Phase II feasibility study of adjuvant S-1 plus docetaxel for stage III gastric cancer patients after curative D2 gastrectomy. Oncology. 2011;80:296–300. [PubMed] [Google Scholar]65.
Yang L, Yang Y, Qin Q, Zhou A, Zhao J, Wang J, et al. Dose-finding study on adjuvant chemotherapy with S-1 plus oxaliplatin for gastric cancer. Mol Clin Oncol. 2014;2:93–98. [PMC free article] [PubMed] [Google Scholar]66.
Takahari D, Hamaguchi T, Yoshimura K, Katai H, Ito S, Fuse N, et al. Survival analysis of adjuvant chemotherapy with S-1 plus cisplatin for stage III gastric cancer. Gastric Cancer. 2014;17:383–6. [PubMed] [Google Scholar]67.
Fujitani K, Tamura S, Kimura Y, Tsuji T, Matsuyama J, Iijima S, et al. Three-year outcomes of a phase II study of adjuvant chemotherapy with S-1 plus docetaxel for stage III gastric cancer after curative D2 gastrectomy. Gastric Cancer. 2014;17:348–53. [PubMed] [Google Scholar]68.
Egawa T, Kemmochi T, Irino T, Mihara K, Okamura A, Etoh E, et al. Adjuvant chemotherapy with S-1 plus docetaxel for highly advanced gastric cancer patients. Gan To Kagaku Ryoho. 2012;39:2310–2. [PubMed] [Google Scholar]69.
Tsuburaya A, Yoshida K, Kobayashi M, Yoshino S, Takahashi M, Takiguchi N, et al. Sequential paclitaxel followed by tegafur and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial randomised controlled trial. Lancet Oncol. 2014;15:886–93. [PubMed] [Google Scholar]70.
Aoyama T, Yoshikawa T, Watanabe T, Hayashi T, Ogata T, Cho H, et al. Macroscopic tumor size as an independent prognostic factor for stage II/III gastric cancer patients who underwent D2 gastrectomy followed by adjuvant chemotherapy with S-1. Gastric Cancer. 2011;14:274–8. [PubMed] [Google Scholar]71.
Ema A, Yamashita K, Sakuramoto S, Wang G, Mieno H, Nemoto M, et al. Lymph node ratio is a critical prognostic predictor in gastric cancer treated with S-1 chemotherapy. Gastric Cancer. 2014;17:67–75. [PubMed] [Google Scholar]72.
Sakurai Y, Kamoshida S, Furuta S, Sunagawa R, Inaba K, Isogaki J, et al. Predictive value of orotate phosphoribosyltransferase in chemoresistant patients with gastric carcinoma who underwent S-1-based neoadjuvant/adjuvant chemotherapy. Gan To Kagaku Ryoho. 2008;35:1147–55. [PubMed] [Google Scholar]73.
Wada T, Kunisaki C, Hasegawa S, Takagawa R, Momiyama M, Kosaka T, et al. Factors predictive of recurrence after surgery for gastric cancer followed by adjuvant S-1 chemotherapy. Anticancer Res. 2013;33:1747–51. [PubMed] [Google Scholar]74.
Omura T, Shimada Y, Nagata T, Okumura T, Fukuoka J, Yamagishi F, et al. Relapse-associated microRNA in gastric cancer patients after S-1 adjuvant chemotherapy. Oncol Rep. 2014;31:613–8. [PubMed] [Google Scholar]75.
McCulloch PG, Ochiai A, O’Dowd GM, Nash JR, Sasako M, Hirohashi S. Comparison of the molecular genetics of c-erb-B2 and p53 expression in stomach cancer in Britain and Japan. Cancer. 1995;75:920–5. [PubMed] [Google Scholar]76.
Bonenkamp JJ, van de Velde CJ, Kampschöer GH, Hermans J, Hermanek P, Bemelmans M, et al. Comparison of factors influencing the prognosis of Japanese, German, and Dutch gastric cancer patients. World J Surg. 1993;17:410–4. [PubMed] [Google Scholar]77.
Yoshikawa T, Tanabe K, Nishikawa K, Ito Y, Matsui T, Kimura Y, et al. Induction of a pathological complete response by four courses of neoadjuvant chemotherapy for gastric cancer: Early results of the randomized phase II COMPASS trial. Ann Surg Oncol. 2014;21:213–9. [PubMed] [Google Scholar]78.
Okabe H, Ueda S, Obama K, Hosogi H, Sakai Y. Induction chemotherapy with S-1 plus cisplatin followed by surgery for treatment of gastric cancer with peritoneal dissemination. Ann Surg Oncol. 2009;16:3227–36. [PubMed] [Google Scholar]79.
Li T, Chen L. Efficacy and safety of SOX regimen as neoadjuvant chemotherapy for advanced gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2011;14:104–6. [PubMed] [Google Scholar]80.
Oyama K, Fushida S, Kinoshita J, Makino I, Nakamura K, Hayashi H, et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, and S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. 2012;105:535–41. [PubMed] [Google Scholar]81.
Nagahama T, Ando M, Seki R, Fujiya K, Amagasa H, Takasaki J, et al. Preoperative chemotherapy for advanced gastric cancer. Gan To Kagaku Ryoho. 2013;40:2217–9. [PubMed] [Google Scholar]82.
Inoue T, Yachida S, Usuki H, Kimura T, Hagiike M, Okano K, et al. Pilot feasibility study of neoadjuvant chemoradiotherapy with S-1 in patients with locally advanced gastric cancer featuring adjacent tissue invasion or JGCA bulky N2 lymph node metastases. Ann Surg Oncol. 2012;19:2937–45. [PubMed] [Google Scholar]83.
Matsuda S, Takahashi T, Fukada J, Fukuda K, Kawakubo H, Saikawa Y, et al. Phase I study of neoadjuvant chemoradiotherapy with S-1 plus biweekly cisplatin for advanced gastric cancer patients with lymph node metastasis: -KOGC04- Radiat Oncol. 2014;9:9. [PMC free article] [PubMed] [Google Scholar]84.
Hirata K, Horikoshi N, Aiba K, Okazaki M, Denno R, Sasaki K, et al. Pharmacokinetic study of S-1, a novel oral fluorouracil antitumor drug. Clin Cancer Res. 1999;5:2000–5. [PubMed] [Google Scholar]85.
van Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, de Vries MJ, et al. Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol. 2000;18:2772–9. [PubMed] [Google Scholar]86.
Cohen SJ, Leichman CG, Yeslow G, Beard M, Proefrock A, Roedig B, et al. Phase I and pharmacokinetic study of once daily oral administration of S-1 in patients with advanced cancer. Clin Cancer Res. 2002;8:2116–22. [PubMed] [Google Scholar]87.
Yoshida R, Nakajima M, Nishimura K, Tokudome S, Kwon JT, Yokoi T. Effects of polymorphism in promoter region of human CYP2A6 gene (CYP2A6*9) on expression level of messenger ribonucleic acid and enzymatic activity in vivo and in vitro. Clin Pharmacol Ther. 2003;74:69–76. [PubMed] [Google Scholar]88.
Yoshida K, Hirabayashi N, Takiyama W, Ninomiya M, Takakura N, Sakamoto J, et al. Phase I study of combination therapy with S-1 and docetaxel (TXT) for advanced or recurrent gastric cancer. Anticancer Res. 2004;24:1843–52. [PubMed] [Google Scholar]89.
Hokita S, Aikou T, Miyazono F, Ishigami S, Aridome K, Maenohara S, et al. A phase I combination chemotherapy study of biweekly paclitaxel and S-1 administration in patients with advanced gastric cancer. Cancer Chemother Pharmacol. 2006;57:736–40. [PubMed] [Google Scholar]90.
Peters GJ, Noordhuis P, Van Groeningen CJ, Giaccone G, Holwerda U, Voorn D, et al. The effect of food on the pharmacokinetics of S-1 after single oral administration to patients with solid tumors. Clin Cancer Res. 2004;10:4072–6. [PubMed] [Google Scholar]91.
Chou WC, Chang CL, Liu KH, Hsu JT, Cheng WH, Hsu HC, et al. Total gastrectomy increases the incidence of grade III and IV toxicities in patients with gastric cancer receiving adjuvant TS-1 treatment. World J Surg Oncol. 2013;11:287. [PMC free article] [PubMed] [Google Scholar]92.
Aoyama T, Yoshikawa T, Hayashi T, Kuwabara H, Mikayama Y, Ogata T, et al. Risk factors for 6-month continuation of S-1 adjuvant chemotherapy for gastric cancer. Gastric Cancer. 2013;16:133–9. [PubMed] [Google Scholar]










 PDF
PDF  Views
Views  Share
Share

