Efficacy of Mobile Phone Technology for Managing Side Effects Associated with Chemotherapy among Cancer Patients: A Systematic Review and Meta-Analysis
CC BY-NC-ND 4.0 ? Indian J Med Paediatr Oncol 2021; 42(06): 518-527
DOI: DOI: 10.1055/s-0041-1740120
Abstract
Background?To establish the evidence related to the efficacy of mobile phone technology for managing side effects of chemotherapy and improved quality of life among patients with cancer.
Methods?Articles published in peer-reviewed journals were included in this review. Randomized control trials (RCTs) and non-randomized control trials (non-RCTs) consisting of mobile-based interventions (mobile application, smart phone App-based interventions or guidelines to manage side-effects of chemotherapy or mobile health services), and adult cancer patients (aged 18 or above years) as participants who were undergoing chemotherapy and received mobile phone-based interventions as an interventional group versus control/comparator group who were getting routine or usual care were included in this systematic review. Databases such as Scopus, Science Direct, Cochrane library, PubMed, and Google Scholar were systematically searched between 2007 and 2020. Using the Cochrane risk of bias tool, the methodological quality of the included studies was evaluated by two independent authors.
Results?We included 10 trials, involving 1467 cancer patients and the number of participants ranged from 50 to 457. All trials measured the side effects of chemotherapy as the main outcome and three trials measured the quality of life as the main outcome.
Ten trials included for narrative synthesis showed a significant decrease in chemotherapy side effects and considerable improvement in the quality of life in the interventional group than in the comparison group. Meta-analysis of four RCTs containing 803 subjects concluded a significant improvement (p?<?0.0001) in the quality of life.
A significant improvement in the quality of life was revealed by random effects model (SMD?=?0.31, 95% CI: 0.17, ?0.46) and a significant difference (Z?=?4.37,?p?<?0.001) was identified between experimental and control groups.
Conclusion?Current review strengthens the evidence that utilizing mobile-phone based technology has favorable effects on improving the quality of life by minimizing side-effects associated with chemotherapy among cancer patients.
Keywords
chemotherapy - side-effects - mobile apps/smartphone applications - cancer patient
Systematic Review Registration No
International Prospective Register for Systematic Reviews (Prospero Registration No-CRD42020152520).
Publication History
11 December 2021 (online)
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background?To establish the evidence related to the efficacy of mobile phone technology for managing side effects of chemotherapy and improved quality of life among patients with cancer.
Methods?Articles published in peer-reviewed journals were included in this review. Randomized control trials (RCTs) and non-randomized control trials (non-RCTs) consisting of mobile-based interventions (mobile application, smart phone App-based interventions or guidelines to manage side-effects of chemotherapy or mobile health services), and adult cancer patients (aged 18 or above years) as participants who were undergoing chemotherapy and received mobile phone-based interventions as an interventional group versus control/comparator group who were getting routine or usual care were included in this systematic review. Databases such as Scopus, Science Direct, Cochrane library, PubMed, and Google Scholar were systematically searched between 2007 and 2020. Using the Cochrane risk of bias tool, the methodological quality of the included studies was evaluated by two independent authors.
Results?We included 10 trials, involving 1467 cancer patients and the number of participants ranged from 50 to 457. All trials measured the side effects of chemotherapy as the main outcome and three trials measured the quality of life as the main outcome.
Ten trials included for narrative synthesis showed a significant decrease in chemotherapy side effects and considerable improvement in the quality of life in the interventional group than in the comparison group. Meta-analysis of four RCTs containing 803 subjects concluded a significant improvement (p?<!--?0.0001) in the quality of life.
A significant improvement in the quality of life was revealed by random effects model (SMD?=?0.31, 95% CI: 0.17, ?0.46) and a significant difference (Z?=?4.37,?p?<!--?0.001) was identified between experimental and control groups.
Conclusion?Current review strengthens the evidence that utilizing mobile-phone based technology has favorable effects on improving the quality of life by minimizing side-effects associated with chemotherapy among cancer patients.
Keywords
chemotherapy - side-effects - mobile apps/smartphone applications - cancer patient
Introduction
In many countries, mobile-phone based technology has become an integral part of healthcare system to render health services to the needy in the form of mobile health (mHealth), smartphone apps, or applications.[1] [2] Globally, cancer is accountable for around 10 million deaths in 2020.[3] There are different treatment modalities for cancer, and chemotherapy is one of the most commonly used treatment of choice for cancer.[4] The nature of chemotherapy is to kill the cancer cells along with healthy cells, which results in the development of certain side effects.[5] [6]
The most common side-effects of chemotherapy include nausea, vomiting, fatigue, diarrhea, lack of sleep, loss of appetite, pain, and hair loss.[7] [8] The quality of life among cancer patients is affected by these side effects and may lead to psychological distress.[6]
Utilization of mobile-phone based technology has been increasing in the healthcare sector.[9] [10] Lack of awareness on management of these side-effects at home is one of the challenging issues for cancer patients.[11] This issue can be resolved with the help of mobile-based technology as the mobile phones/smart phones have become a vital entity in our life. The mobile-based technology, such as smart phone apps/mHealth services, can provide information in the form of guidelines or instructions or education to the cancer patients to manage chemotherapy-associated side-effects.[12] [13]
A review conducted in 2018 recommended the need to develop more comprehensive interventions through mobile technology to meet the patients' needs in the form of guidelines and self-monitoring of side effects associated with chemotherapy.[14]
Use of mobile technology for self-care monitoring and reporting of the symptoms along with alert system that focus on severity of symptoms can enhance the living quality of cancer patients by reducing symptom burden of the chemotherapy.[15]
As per the background information and our knowledge, there are no existing meta-analyses on the intervention of utilizing mobile phone technology and its efficiency to heighten the living quality among cancer patients.
Results of this review will give intuition and support for the development of novel mobile-based interventions in the form of mobile apps/applications for progressing the quality of life. This review was decided to strengthen the evidence producing the evidence on determining the clinical outcomes of the patient's using mobile phone technology. The objective of this review is to establish the evidence related to the efficacy of mobile phone technology for managing side effects of chemotherapy and improving the quality of life among cancer patients.
Methods
The authors followed Joanna Briggs Institute Manual for Evidence Synthesis (Guidance for authors to conducting systematic reviews)[16] and the PRISMA guidelines for the preparation of this systematic review and meta-analysis.[17]
Eligibility Criteria
Inclusion criteria
a) Articles published in peer-reviewed journals.
b) Study designs: randomized control trials (RCTs) and non-randomized control trials (non-RCTs).
c) Interventions: Studies consisting of mobile-based interventions (mobile application, smart phone App-based interventions or guidelines to manage side-effects of chemotherapy or mHealth services.
d) Participants: The trials that included adult cancer patients (aged 18 or above years) who were undergoing chemotherapy, and received mobile phone-based interventions versus control/comparator group who were getting routine or usual care, across all types of cancers, gender, race, regions, and country.
e) Settings: Conducted in rural or urban areas or Hospitals or oncology units or clinical settings.
f) Outcomes: Studies were included if they described either a few or all side effects of chemotherapy such as nausea, vomiting, pain, mucositis, fatigue, sleep disturbances, diarrhea, constipation, dyspnea, urinary problems, hand foot syndrome, hair loss, and poor quality of life.
g) Language: Trials published in English language only.
Exclusion Criteria
a) Conference abstracts, databases containing only abstracts, books, and gray literature were excluded.
Information Sources
The databases such as Science Direct, Scopus, Cochrane Library, PubMed, and Google Scholar were searched for the eligible trials reported between 2007 and 2020. In addition to this, hand search of references was performed from related trials to identify the studies based on inclusion criteria.
Search Strategy
The search strategy was developed comprehensively using keywords in congruence with PICO terms (population, intervention, comparator, and outcome) to identify the relevant studies by following keywords: ?Cancer patients? AND chemotherapy AND ?mobile application? OR ?Mobile apps? OR mHealth AND ?side effects? OR ?symptom management? AND ?quality of life.?
The title and abstracts screening of the retrieved studies was done based as per the inclusion criteria. Duplicate trials were removed by screening the title and abstracts using Reference Manager (Zotero). The two authors (UP and SJN) independently performed screening of the retrieved abstracts based on pre-determined inclusion criteria, followed by screening of full-text articles.
Data Collection Process
Quantitative data of the included studies were extracted independently by two authors based on JBI experimental studies data extraction tool.[16] The data extraction form consisted of details such as author, year of publication, location, study type, sample size/group, participants type and age, intervention details and duration, outcome measures, instruments used and study findings.
Data Items
Participants: The trials that included adult cancer patients (aged 18 or above years) who were undergoing chemotherapy, and received mobile phone-based interventions versus control/comparator group who are under routine or usual care.
Intervention: The experimental group cancer patients had received interventions based on mobile technology. Trials exploring the effectiveness of mobile-based interventions (mobile application, smart phone App-based interventions or guidelines to manage side effects of chemotherapy or mHealth services) on cancer patients undergoing chemotherapy were included.
Comparison: The comparator group included cancer patients undergoing chemotherapy and who received regular or routine care.
Outcome: In this systematic review, we analyze the side-effects of chemotherapy such as nausea, fatigue, vomiting, oral mucositis, depression, numbness, anxiety, hair loss, diarrhea. and quality of life. Meta-analysis was done for the quality of life.
Study Risk of Bias Assessment and Effect Measures
Using the Cochrane risk of bias tool, the methodological quality of the included studies was evaluated by two independent authors (UP and SJN). It included six domains as mentioned in [Fig. 1]. We found a 100% low-risk bias regarding random sequence generation, and 75% unclear bias was noted toward allocation concealment. The details regarding the percentages of risk of bias across all trials are reported in [Fig. 1].
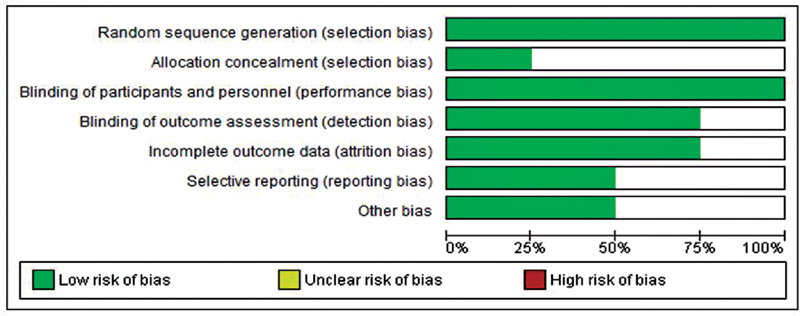
|?Fig. 1?Applications of artificial intelligence (AI) in oncology.|
Outcome of the Search
A total of 3,647 studies were found through electronic databases. Eight duplicated were removed using Reference Manager. After screening the title and abstracts, 3,622 studies were omitted as they did not meet the criteria of this review as per the PICO. Full-text articles assessed for the eligibility were 17, from which 7 articles were eliminated as they were unable to fulfil the inclusion criteria. The rational to exclude the full-text articles along with study selection and elimination process is mentioned in the PRSIMA flow chart ([Fig. 2]). Finally, 10 articles were involved for qualitative narrative synthesis, of which 4 RCTs were included for meta-analysis on the variable ?quality of life.?
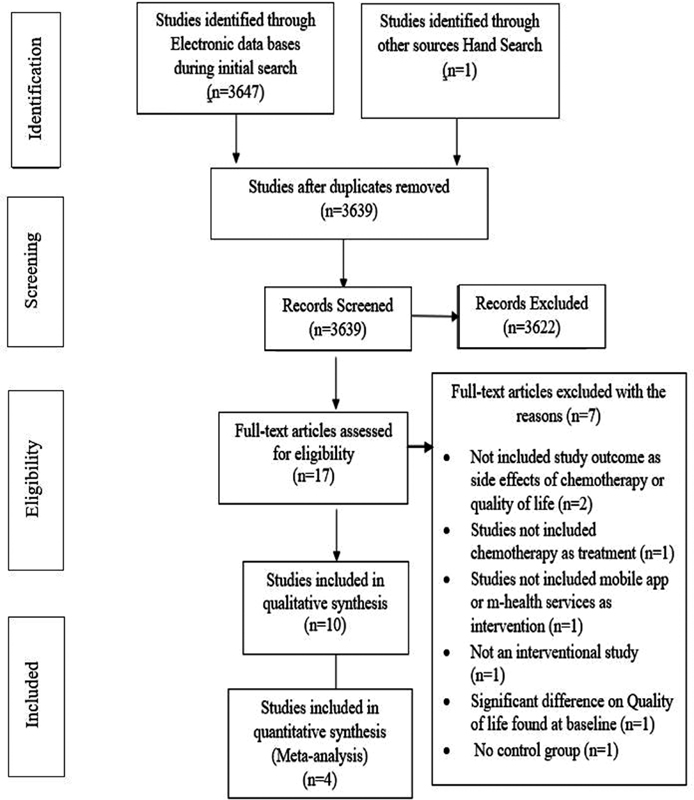
|?Fig. 2?Study selection process based on the PRISMA flow chart.|
Synthesis
The outcome measures of the trials that included chemotherapy-associated side-effects and quality of life between cancer patients who received mobile phone-based technology as intervention and the control group who were on routine care were differentiated. A meta-analysis on the quality of life was conducted to pool the results of RCTs using the Rev-Man v5.4 software. The intervention's effect size for the quality of life was estimated as a continuous outcome by pooling the standardized mean difference applying a random-effect model with 95% confidence interval (CI).?I?2?value was used to analyze the heterogeneity in the included trials. The quality evidence and the strength of the outcome (effect sizes: 0.8 and above-large, around 0.5 medium, around 0.2-small) were measured based on the GRADE approach guidelines.[18] [19] The findings are reported in [Table 1].
|
Efficiency of mobile phone-based technology compared to routine care for quality of life of cancer patients |
||||||
|
Patient or population: Chemotherapy cancer patient's setting: Intervention: Mobile phone-based technology Comparison: Routine care |
||||||
|
Outcomes |
Anticipated absolute effects* (95% CI) |
Relative effect (95% CI) |
No of participants (studies) |
Certainty of the evidence (GRADE) |
Comments |
|
|
Risk with routine care |
Risk with mobile phone-based technology |
|||||
|
Quality of life (QOL) assessed with EuroQol EQ-5D Index, EORTC QLQ-C30, World Health Quality of Life Breast Scale |
The mean quality of life ranged from ?1.86 to 82.2 |
SMD 0.31 higher (0.17 higher to 0.46 higher) |
? |
803 (4 RCTs) |
???? MODERATE a |
Mobile phone-based technology likely results in an increase in the quality of life. Moderate effect size. Statistically significant at p?<?0.00001. SMD of 0.317 higher represents the improved quality of life and considerable differences between groups. |
|
*The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; SMD: standardized mean difference |
||||||
|
GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect Explanation a. The presence of unclear risk of bias made the authors downgrade as serious |
||||||
Results
Characteristics of Patients
In this systematic review, 10 clinical trials involved 1,467 cancer patients and the number of participants ranged from 50 to 457. The mean age of the subjects was 60 years and both males and females were included, except for four RCTs, where the participants were only females.[20] [21] [22] [23] All trials measured the side effects of chemotherapy as the main outcome and three trials measured the quality of life as the main outcome. In all trials, the interventional group received mobile-based technology as an intervention and the control group was under routine/usual care. The details are mentioned in [Table 2.]
|
Author, year and location |
Study type |
Sample size/group |
Participants with type of cancer |
Intervention details and duration |
Outcome measures |
Instruments |
Study findings |
|
Kearney et al, 2009, Scotland and England, United Kingdom |
RCT (advanced symptom management system (ASyMS) vs. standard care |
112, IG?=?56, CG?=?56 |
Breast cancer/lung cancer or colorectal cancer |
Mobile phone-based interventions providing remote monitoring with alerts based on severity and guidelines to manage chemotherapy related side effects (4 months) |
Sore throat, diarrhea, nausea, fatigue, sore throat |
CSAS |
A significant higher report of symptoms were observed in control group than the interventional group (p?<?0.05). |
|
Basch et al, 2016, New York, United States of America |
RCT (symptom tracking and reporting (STAR) vs. usual care) |
457, IG?=?227, CG?=?180 |
Breast, genito-urinary, gynecological or lung cancer |
Web-based symptom tracking and reporting (STAR) providing interface for self-reporting 12 regular symptoms come across during chemotherapy with automated e-mail alerts (6 months) |
Health Reheated Quality of life (HRQL) |
Euro-QoLEQ-5D Index |
Significant improvement in quality of life in interventional group. (p?<?0.05) |
|
Egbring et al, 2016, Zurich, Switzerland |
Three-arm RCT (regular physician support vs. mobile App vs. mobile App and physician) |
139, IG (App)?=?46, IG (App& Physician)?=?49 |
Breast cancer |
Novel mobile app and web-based application was given to group-B(App) and group-C (App and physician assistant) and group-A regular physician support (control group). The app contains features for reporting daily functional ability and severity of symptoms (4 months) |
Daily functional activity, symptoms |
ECOG & CTCAE |
Group C (App and physician) shown significant improvement of daily functional ability compared to others (p?<?0.05) and group B and group C reported more distinct symptoms compared to group A (p?<?0.05) |
|
Alboughobeish et al, 2017, Ahvaz, Iran |
Non-RCT (educational content in the form of mobile software vs. routine training |
50, IG?=?25, CG?=?25 |
Breast, genito-urinary, gastro-intestinal, gynecological, Hodgkin lymphoma, lung cancer, bone cancer |
Mobile software was given to interventional group which provides information regarding chemotherapy side effects and management recondensation's for nausea and vomiting and educational clips (1 month) |
Nausea and vomiting |
VAS and The Khavari Oncology Scale for Vomiting |
Nausea severity was declined in interventional group (p?<?0.05) but no change in control group and number of vomiting was decreased in interventional group (p?<?0.05) |
|
Di and Li, 2018 and Henan, China |
Non-RCT (medical App vs. standard care |
132 IG?=?65, CG?=?67 |
Naso-pharyngeal cancer |
Smart phone medical app given to the interventional group which contains features such as modules, re-examination reminder, knowledge-based information and online expert (6 months) |
Complications (oral mucositis, hearing loss, mouth opening difficulties, nasal congestion and quality of life |
EORTC- QLQ-C30 |
Complication were lower among interventional than control group (p?<?0.05), significant improvement in quality of life in interventional group (p?<?0.05) |
|
Kim et al, 2018 Seoul, Republic of Korea |
RCT (mobile game play group vs. conventional education group) |
70 IG?=?36, CG?=?40 |
Breast cancer |
A mobile game ILOVEBRESAT: given to the interventional group that provides education on preventing side and encouragement of social game playing and for control group education through brochure. (1 month) |
Quality of life, depression, anxiety and physical effects such as fatigue, nausea, numbness and hair loss |
World Health Quality of Life Breast Scale, Common Terminology Criteria for adverse events, BDI Score, Spielberger State-Trait Anxiety Scale |
Interventional group shows higher quality of life than control group (p?<?0.05). Interventional group reported a smaller number of adverse effects such as fatigue, nausea, numbness and hair loss (p?<?0.05) |
|
Greer et al, 2020 California, United States of America |
RCT (mobile App vs. standard care) |
181 IG?=?91, CG?=?90 |
Hematological, non-small cell lung, breast, high-grade glioma, gastro-intestinal, genito-urinary and melanoma |
A mobile app administered to the interventional group which contains features such as symptom reporting module and patient education |
Symptom burden and quality of life |
MD Anderson Symptom Inventory, Functional Assessment of cancer of therapy general |
Between interventional and control group no difference (p?>?0.05) was observed regrading symptom severity and quality of life |
|
Handa et al, 2020, Tokyo, Japan |
RCT (app group Vs No app group) |
102 IG?=?50, CG?=?52 |
Breast cancer |
Breast Cancer Patient Support System (BPSS) mobile app providing self-symptoms assessment, recommendation to for self-management of symptoms and when to consult the physicians (3 months) |
Anxiety, depression, side effects |
Anxiety and Depression scale |
Significant decrease in anxiety and depression scores (p?<?0.05) in interventional group compared to control group. App users reported more symptoms (side effects) compared to non-app users. |
|
Hou et al, 2020 Taipei, Taiwan |
RCT (BCSMS mHealth App vs. usual care) |
112 IG?=?53, CG?=?59 |
Breast cancer |
Breast cancer self-management support mHealth App which contains self-management model that provides education and patient centered disease management and initiates them to participate in self-care activities (3 months) |
Quality of life |
EORTC- QLQ-C30 and QLQ-BR23 |
Significant improvement in quality of life among app users compared non app users (p?<?0.05) |
|
Rico et al, Rio Grande Do Sul, Brazil |
RCT (text messages (SMS) vs. standard care) |
118 IG?=?59, CG?=?59 |
Breast, genito-urinary, gastro-intestinal, lung cancer |
Interventional group receives short message service which contains management guidelines on prevention of side-effects of chemotherapy |
Nausea, vomiting, diarrhea, sore throat |
EORTC-QLQ-C30 |
Compared to control group interventional group experienced fewer side effects (p?<?0.05) |
Effects of intervention: The quality of life was analyzed as a major outcome in this meta-analysis. The effectiveness of mobile-based technology interventions was calculated based upon the difference between intervention group and control group post test scores.
A total of 10 trials were included for narrative synthesis that showed a significant decrease in symptoms/side effects and improved quality of life in the interventional group than in the comparison group.[11] [13] [20] [21] [22] [23] [24] [25] [26] [27]
Statistical Analysis
The meta-analysis of four RCTs containing 803 cancer subjects concluded that a significant improvement (p?<?0.0001) in the quality of life.
It shows that the mobile phone-based technology interventions were effective in enhancing the cancer patient's quality of life. A significant improvement in the quality of life was revealed by random effects model using standardized mean difference (SMD?=?0.31, 95% CI: 0.17, ?0.46) and a significant difference (Z?=?4.37,?p?<?0.001) was identified between experimental and control groups. The pooled studies were homogenous (p?=?0.46,?I?2?=?0%). Because the four RCTs used different tools for measuring the quality of life, the standardized mean difference with random effects model was used for effect measure ([Fig. 3]).
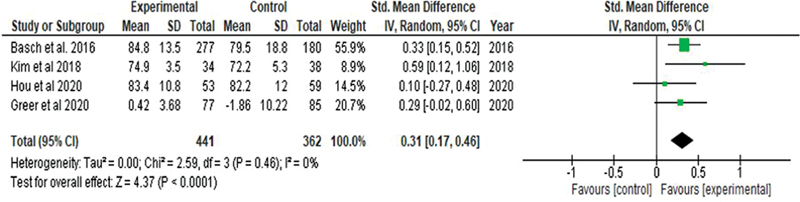
|?Fig. 2?Study selection process based on the PRISMA flow chart.|
Publication Bias
Publication bias was not evaluated as only four trials were incorporated for this meta-analysis.
Evidence of Quality
There was a moderate quality of evidence for the assessed quality of life due to an unclear risk of bias ([Table 1], [Fig. 1]).
Discussion
This systematic review gives evidence that mobile phone technology interventions (mobile applications, smart phone App-based interventions, or guidelines to handle the complications of chemotherapy or mHealth services) reduced the side effects/symptoms and improved the chemotherapy cancer patient's quality of well-being.
Our review included 10 trials published between 2007 and 2020, identified all studies reporting that chemotherapy-associated side-effects were reduced and enhanced the quality of life. All the studies showed favorable outcomes.
Four trials were incorporated in this meta-analysis to evaluate the effectiveness of mobile phone technology interventions among cancer patients regarding the changes in the quality of life. Out of four, two trials revealed significant favorable change in the quality of life, another two trials showed improvement in the quality of life but results were not significant. However, the pooled result of all four trials showed that the mobile phone technology interventions significantly improved the quality of life.
This systematic review identified that physical effects such as nausea, vomiting, diarrhea, sore throat (oral mucositis) fatigue, and psychological effects such as depression and anxiety were the most common side-effects experienced by the cancer patients under chemotherapy. These findings are congruent with those of previous studies that highlighted the common side effects experienced by the cancer patients under chemotherapy.[5] [6]
The outcome of our systematic review was supported with the previous systematic reviews that highlighted that mobile phone technology interventions had helped to minimize chemotherapy complications and improved cancer patient's quality of life.[7] [14]
The findings of this review will encourage healthcare professionals and organizations to develop innovative methods using mobile phone technology that can help cancer patients to manage the side effects associated with chemotherapy by themselves in their respective home settings. However, mobile phone-based technology alone may not enhance the well-being of cancer patients. The outcome of this review might disburse the opportunity for the usage of mobile phone-based technology for rendering the healthcare services to the cancer patients.


 PDF
PDF  Views
Views  Share
Share

