Efficacy of Antiemetic Regimens for Prevention and Treatment of Chemotherapy-Induced Nausea and Vomiting in Patients of Breast Cancer Receiving Highly Emetogenic Chemotherapy
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(06): 819-824
DOI: DOI: 10.4103/ijmpo.ijmpo_200_20
Abstract
Background: Chemotherapy is fraught with serious and troublesome adverse effects, of which nausea and vomiting appears earliest and is the most disturbing. Therefore, this study was planned to investigate the antiemetic drug regimens used for chemotherapy-induced nausea vomiting (CINV) in patients with breast cancer receiving highly emetogenic chemotherapy (HEC). Subjects and Methods: An observational follow-up study was conducted to assess the efficacy of antiemetic regimens in breast cancer patients receiving HEC. A total of 71 newly diagnosed patients with breast cancer were included in the study. Patients were assessed for nausea by the visual analog scale, and a history of emetic episodes and need for rescue medication were recorded at 0 h, 6 h, 24 h, 48 h, and 120 h post-chemotherapy till three cycles. Results: The patients were prescribed a combination of ondansetron and dexamethasone (n = 23, n = 17, and n = 13 in first, second, and third cycle, respectively) or a combination of aprepitant, ondansetron, and dexamethasone (n = 48, n = 54 and n = 56 in the first, second, and third cycle, respectively). The intensity of nausea was higher for the patients who were prescribed ondansetron and dexamethasone regimen as compared to patients prescribed aprepitant additionally. Complete response, i.e., no emesis and no rescue medication, was higher in triple-drug regimen (52% vs. 0.4%, 63% vs. 17.6%, and 69% vs. 23% in three cycles, respectively). Conclusion: The control of CINV was better with a combination of aprepitant, ondansetron, and dexamethasone as compared to a regimen without aprepitant.
Keywords
5-HT3 receptor antagonist - breast cancer - chemotherapy-induced nausea and vomiting - highly emetogenic chemotherapy - NK-1 receptor antagonistPublication History
Received: 27 April 2020
Accepted: 22 August 2020
Article published online:
14 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: Chemotherapy is fraught with serious and troublesome adverse effects, of which nausea and vomiting appears earliest and is the most disturbing. Therefore, this study was planned to investigate the antiemetic drug regimens used for chemotherapy-induced nausea vomiting (CINV) in patients with breast cancer receiving highly emetogenic chemotherapy (HEC). Subjects and Methods: An observational follow-up study was conducted to assess the efficacy of antiemetic regimens in breast cancer patients receiving HEC. A total of 71 newly diagnosed patients with breast cancer were included in the study. Patients were assessed for nausea by the visual analog scale, and a history of emetic episodes and need for rescue medication were recorded at 0 h, 6 h, 24 h, 48 h, and 120 h post-chemotherapy till three cycles. Results: The patients were prescribed a combination of ondansetron and dexamethasone (n = 23, n = 17, and n = 13 in first, second, and third cycle, respectively) or a combination of aprepitant, ondansetron, and dexamethasone (n = 48, n = 54 and n = 56 in the first, second, and third cycle, respectively). The intensity of nausea was higher for the patients who were prescribed ondansetron and dexamethasone regimen as compared to patients prescribed aprepitant additionally. Complete response, i.e., no emesis and no rescue medication, was higher in triple-drug regimen (52% vs. 0.4%, 63% vs. 17.6%, and 69% vs. 23% in three cycles, respectively). Conclusion: The control of CINV was better with a combination of aprepitant, ondansetron, and dexamethasone as compared to a regimen without aprepitant.
Keywords
5-HT3 receptor antagonist - breast cancer - chemotherapy-induced nausea and vomiting - highly emetogenic chemotherapy - NK-1 receptor antagonistIntroduction
Cancer as a noncommunicable disease presents a tremendous burden on patients, their caregivers, and society. The currently estimated burden of cancer worldwide is touching to 18 million in 2018 and is going to reach up to 29.5 million by 2040, with the leading causes being tumors of the lung, breast, and prostate.[1]
In India, an estimated 1.15 million patients were diagnosed with cancer in the year 2018. The leading causes of cancer in males are cancers of the oral cavity and lip, followed by lung and stomach cancer. In females, the most common cancer site affected is the breast, followed by the oral cavity and lip and the cervix. An estimated 14% of all newly diagnosed cancer cases are of breast tumors.[2]
For the treatment of breast cancer, the mainstay of treatment is surgery to remove the tumor, followed by chemotherapy, radiotherapy, and hormonal therapy, depending on the tumor type and stage. The chemotherapy commonly prescribed to these patients is a combination of anthracycline–cyclophosphamide.
However, chemotherapy is fraught with a high frequency of serious and troublesome adverse effects that compromise not only the quality of life of the patient but also the patient compliance with the treatment. One of the most common and troublesome adverse effects is associated with nausea and vomiting, which appears earliest, and has a profound impact on the quality of life of the patient.[3]
Chemotherapy-induced nausea and vomiting (CINV) can be labeled as acute (within 24 h), delayed (beyond 24 h), anticipatory, breakthrough, and refractory, may present in all permutations and combinations, that are substantially challenging for the physician as well as to the patient.[4] Each chemotherapeutic agent used varies in its ability to induce emesis, with some drugs such as cisplatin, cyclophosphamide (dose >1500 mg/m2) and anthracycline- cyclophosphamide combinations being implicated as highly emetogenic and induce emesis in up to 90% of the patients receiving the drugs.[5]
It is imperative that nausea and vomiting occurring due to chemotherapy must be prevented and treated optimally. For the prophylaxis of CINV, the drugs primarily included are NK-1 receptor antagonists, such as aprepitant and fosaprepitant and 5HT3 receptor antagonists such as ondansetron, granisetron and palonosetron, and dexamethasone.[6] Apart from this, other drugs used are olanzapine, metoclopramide, domperidone, etc.[7]
There is a dearth of data on the efficacy of the treatment of CINV from the Indian subcontinent. There are no standard guidelines present from this region to suggest the appropriate drug therapy for the prevention and treatment of CINV.
Thus, the present study was undertaken to observe the antiemetics prescribed to prevent and treat CINV and their efficacy for patients with breast cancer planned to receive highly emetogenic chemotherapy (HEC).
Subjects and Methods
The study was conducted over a period of 12 months in the Day Care Ward of the Cancer Research Institute after obtaining written informed consent. The study was conducted in accordance with the principles of the Declaration of Helsinki and after obtaining clearance from the institutional ethics committee. A total of 71 patients diagnosed with breast cancer were included in the study and were followed up until the third cycle of chemotherapy.
Previously untreated patients (chemotherapy-naïve) female patients in the age group 18–65 years, newly diagnosed with breast cancer, post-surgery for tumor removal, and planned to receive HEC were included in the study after obtaining written informed consent.
Any patients planned to receive concurrent radiotherapy, patients with metastatic tumors, patients with a history of previous exposure to chemotherapy, and pregnant or lactating females were excluded from the study.
Demographic details including the age, weight, height, body mass index, and body surface area were recorded in case recording forms and any relevant medical and personal history was collected from the patients as well as caretakers. This included a history of motion sickness and morning sickness. The patients were prescribed antiemetic regimens as per the physician's preference and could be modified in subsequent cycles depending upon the experience of the patient.
The intensity of nausea was assessed based on a 10-point visual analog scale (VAS). 0 in the VAS corresponded to no nausea at all and 10 in the VAS corresponded to the worst possible nausea. The VAS responses were recorded at 0 h, 6 h, 24 h, 48 h, and 120 h postchemotherapy.
The patients were given an Emesis Diary and were instructed on how to fill the diary. The patients were instructed to record the frequency of vomiting episodes and any need for rescue medications. The frequency of vomiting episodes and the need for rescue medication were analyzed in the acute and delayed phase, as well as the overall phase for the different antiemetic regimens given to the patients.
A detailed analysis of the antiemetic drugs prescribed was done based on feedback given by the patient. Patients were similarly assessed at subsequent cycles, till three cycles of chemotherapy.
Data management and statistical analysis
All of the data from the case recording forms and questionnaire were entered in a master chart on Microsoft Excel for the analysis. The statistical analysis was based on standard descriptive statistical tests using Microsoft Excel and Statistical Package for the Social Sciences version 20.0 (SPSS, Inc.; Chicago, IL, USA) for Windows. The demographic data and VAS scores were represented as mean ± standard deviation and a history of morning and motion sickness was depicted in terms of frequency. The efficacy of the antiemetic regimes was assessed by comparison of the mean scores of VAS by the independent t-test. The chemotherapy regimens prescribed along with the antiemetic regimen used are depicted in frequency and percentage. The vomiting frequency, need for rescue medication, and complete response were expressed in frequency and percentages.
Results
Seventy-one patients recently diagnosed with Carcinoma Breast who received adjuvant HEC were included in the study. All of these patients had undergone surgical tumor removal before starting chemotherapy. All of these patients had been prescribed chemotherapy, followed by hormone therapy and radiotherapy. Sixty-seven patients (94%) had been diagnosed with infiltrating ductal carcinoma of the breast. The mean age of the patients included in the study was 51.49 ± 10.81 years. The minimum age was 27 years and the maximum age was 65 years. Forty-one patients reported a positive history of morning sickness and 35 patients gave a positive history of motion sickness [Table 1].{Table 1}
|
Criteria |
Subjects (n=71) |
|---|---|
|
Values expressed as mean±SD. SD: Standard deviation |
|
|
Age (years) |
51.49±10.81 |
|
Weight (kg) |
63.28±11.22 |
|
Height (cm) |
155.48±6.05 |
|
Body surface area (m2) |
1.64±0.16 |
|
Body mass index (kg/m2) |
26.22±4.36 |
|
History of morning sickness |
41 |
|
History of motion sickness |
35 |
|
History of motion and morning sickness |
25 |
|
Regimen number |
Number of patients |
Regimen |
Dose (mg) |
|---|---|---|---|
|
PET – Positron emission tomography |
|||
|
1 |
50 |
Epirubicin |
154.2±9.18 |
|
Cyclophosphamide |
925±136.5 |
||
|
2 |
21 |
Adriamycin |
86.36±7.1 |
|
Cyclophosphamide |
872±82.2 |
||
|
Cycle |
Ondansetron + dexamethasone (%) |
Aprepitant kit + ondansetron + dexamethasone (%) |
||
|---|---|---|---|---|
|
8 + 8 mg |
16 + 16 mg |
8 + 8 mg |
16 + 16 mg |
|
|
Cycle 1 |
2 |
21 |
0 |
48 |
|
Cycle 2 |
2 |
15 |
0 |
54 |
|
Cycle 3 |
0 |
13 |
2 |
56 |
|
Total (213) |
4 (2) |
49 (23) |
2 (1) |
158(74) |
|
Time (h) |
Ondansetron + dexamethasone (n=23) |
Aprepitant + ondansetron + dexamethasone (n=48) |
P |
|---|---|---|---|
|
Independent t-test. Values expressed as mean±SD. SD: Standard deviation |
|||
|
At 0 |
0 |
0 |
|
|
At 6 |
5.30±1.69 |
2.85±1.01 |
0.000 |
|
At 24 |
6.13±1.18 |
4.56±1.24 |
0.000 |
|
At 48 |
5.57±1.04 |
3.40±1.16 |
0.000 |
|
At 120 |
2±0.67 |
1.65±0.93 |
0.109 |
|
Time (h) |
Ondansetron + dexamethasone (n=17) |
Aprepitant + ondansetron + dexamethasone (n=54) |
P |
|---|---|---|---|
|
Independent t-test. Values expressed as mean±SD. SD: Standard deviation |
|||
|
At 0 |
1.24±1.20 |
0.98±1.55 |
0.538 |
|
At 6 |
5.41±1.23 |
3.44±1.21 |
0.000 |
|
At 24 |
6.12±1.54 |
4.78±1.67 |
0.004 |
|
At 48 |
4.53±1.23 |
3.13±1.21 |
0.000 |
|
At 120 |
1.94±1.03 |
1.54±0.93 |
0.131 |
|
Time (h) |
Ondansetron + dexamethasone (n=13) |
Aprepitant + ondansetron + dexamethasone (n=58) |
P |
|---|---|---|---|
|
Independent t-test. Values expressed as mean±SD. SD: Standard deviation |
|||
|
At 0 |
0.92±1.12 |
0.71±1.20 |
0.554 |
|
At 6 |
5.15±1.21 |
3.17±1.30 |
0.000 |
|
At 24 |
6.69±1.75 |
3.98±0.98 |
0.000 |
|
At 48 |
4.31±1.55 |
2.69±0.86 |
0.003 |
|
At 120 |
2±1 |
1.41±0.96 |
0.05 |
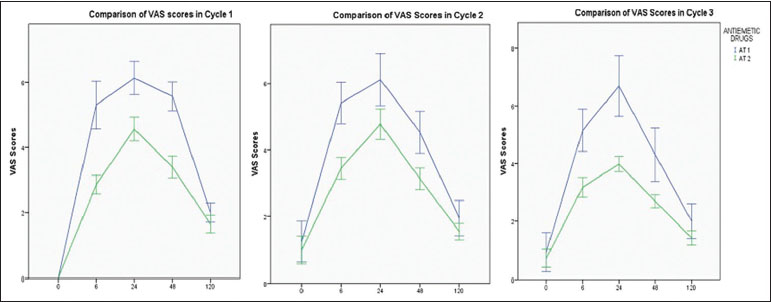
| Figure.1:Visual analog scale scores for intensity of nausea in all cycles in patients receiving antiemetic regimens for highly emetogenic chemotherapy (n = 71)
In the cycle 1 of chemotherapy, in the overall period (0–120 h), patients reporting with no episodes of vomiting were higher in patients receiving aprepitant in addition to ondansetron and dexamethasone as compared to patients who were not prescribed aprepitant (52%-in comparison to 0.4%). Similarly, in the acute phase (<24>24 h), 54%-of the patients said that they experienced no episode of vomiting when prescribed the aprepitant regimen, as compared to 13%-of patients on ondansetron and dexamethasone regimen. Similar results were observed in the second and third cycles as well [Table 5].
|
Cycle |
Regimen (n) |
Acute phase (<24> |
Delayed phase (24- 120 h) (%) |
Overall phase (0- 120 h) (%) |
|---|---|---|---|---|
|
At 1: Ondansetron + dexamethasone, At 2: Aprepitant + ondansetron + dexamethasone |
||||
|
Cycle 1 |
At 1 (23) |
11 (47.8) |
3 (13) |
1 (0.4) |
|
At 2 (48) |
44 (91.6) |
26 (54) |
25 (52) |
|
|
Cycle 2 |
At 1 (17) |
11 (64.7) |
3 (17.6) |
3 (17.6) |
|
At 2 (54) |
44 (81.4) |
37 (68.5) |
34 (63) |
|
|
Cycle 3 |
At 1 (13) |
8(61.5) |
4 (30.7) |
3 (23) |
|
At 2 (58) |
53 (91.3) |
46 (79.3) |
40 (69) |
|

| Figure.1:Visual analog scale scores for intensity of nausea in all cycles in patients receiving antiemetic regimens for highly emetogenic chemotherapy (n = 71)
References
- World Health Organization. Cancer Fact Sheet. WHO Media Centre. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/. [Last acessed on 2019 May 20].
- World Health Organisation. Cancer Fact Sheet India 2018. International Agency for Research on Cancer. Available from: http://gco.iarc.fr/today/data/factsheets/populations/356-india-fact-sheets.pdf. [Last accessed on 2019 May 14].
- Salihah N, Mazlan N, Lua PL. Chemotherapy -induced nausea and vomiting: exploring patients' subjective experience. J Multidiscip Healthc 2016; 9: 145-51
- Janelsins MC, Tejani MA, Kamen C, Peoples AR, Mustian KM, Morrow GR. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin Pharmacother 2013; 14: 757-66
- Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA. et al. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol 2017; 35: 3240-61
- Navari RM. Treatment of breakthrough and refractory chemotherapy induced nausea and vomiting. Biomed Res Int 2015; 2015: 1-6
- Navari RM. editor Management of Chemotherapy Induced Nausea and Vomiting: New Agents and New Uses of Current Agents. Switzerland: Springer; 2016
- Alfano CM, Leach CR, Smith TG, Miller KD, Alcaraz KI, Cannady RS. et al. Equitably improving outcomes for cancer survivors and supporting caregivers: A blueprint for care delivery, research, education, and policy. CA Cancer J Clin 2019; 69: 35-49
- Nurgali K, Jagoe RT, Abalo R. Editorial: Adverse effects of cancer chemotherapy: Anything new to improve tolerance and reduce sequelae?. Front Pharmacol 2018; 9: 245
- Ko Y, Gwee YS, Huang YC, Chiang J, Chan A. Costs and length of stay of drug-related hospital admissions in cancer patients. Clin Ther 2014; 36: 588-92
- Paul EP, Behanan A, Eapen BA, James A, Sherief SH, Palanisamy MK. et al. A study on evaluation of anti-emetics in the prevention of chemotherapy induced nausea and vomiting in cancer patients in a Tertiary Care Hospital. Indian J Pharm Pract 2017; 10: 9-16
- Paul EP, Behanan A, Eapen BA, James A, Sherief SH, Palanisamy MK. et al. A study on evaluation of anti-emetics in the prevention of chemotherapy induced nausea and vomiting in cancer patients in a Tertiary Care Hospital. Indian J Pharm Pract 2017; 10: 9
- Dranitsaris G, Mazzarello S, Smith S, Vandermeer L, Bouganim N, Clemons M. Measuring the impact of guideline-based antiemetic therapy on nausea and vomiting control in breast cancer patients with multiple risk factors. Support Care Cancer 2016; 24: 1563-9
- Molassiotis A, Lee PH, Burke TA, Dicato M, Gascon P, Roila F. et al. Anticipatory nausea, risk factors, and its impact on chemotherapy-induced nausea and vomiting: Results from the pan European emesis registry study. J Pain Symptom Manage 2016; 51: 987-93
- Hilarius DL, Kloeg PH, van der WallE, van den HeuvelJJ, Gundy CM, Aaronson NK. Chemotherapy -induced nausea and vomiting in daily clinical practice: A community hospital-based study. Support Care Cancer 2012; 20: 107-17
- Patil VM, Noronha V, Joshi A, Ramaswamy A, Gupta S, Sahu A. et al. Adherence to and implementation of ASCO antiemetic guidelines in routine practice in a tertiary cancer center in India. J Oncol Pract 2017; 13: e574-81


 PDF
PDF  Views
Views  Share
Share

