Efficacy and Safety of Ibrutinib in Indian Patients with Relapsed or Refractory Chronic Lymphocytic Leukemia and Mantle Cell Lymphoma: Cases from a Named Patient Program
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(04): 508-515
DOI: DOI: 10.4103/ijmpo.ijmpo_43_17
Abstract
Context: This named patient program evaluated the safety and efficacy of ibrutinib, a selective inhibitor of Bruton's tyrosine kinase in Indian patients with relapsed/refractory chronic lymphocytic leukemia (CLL, with/without chromosome 17 deletion [del17p]) and mantle cell lymphoma (MCL). Subjects and Methods: The eight enrolled patients (relapsed/refractory CLL: n = 6 [4/6 patients with del17p] and relapsed/refractory MCL: n = 2) had median age of 55 years (range, 52–60) and had received a median of 3 (CLL patients) and 4 (MCL patients) prior therapies. Patients received once-daily dose of ibrutinib (420 mg: CLL, 560 mg: MCL). Results: In CLL patients, the median time to response was 3 months (range, 0.5–7) and five of six patients had partial response (PR) whereas one achievedcomplete response (CR). Median time on treatment was 11.5 months (range, 8–14); five patients continued treatment and one was recommended stem cell transplantation (SCT). Of the two MCL patients, one achieved PR and one showed CR and advanced to SCT. In CLL patients, the median (range) hemoglobin level improved from 9.8 g/dL (7.2–11) at baseline to 12.0 g/dL (9.5–13.2) and median (range) platelet count improved from 150,000 cells/μL (21,000–195,000) at baseline to 190,350 cells/μL (130,000–394,000) at the time of analysis (July 2016). Most adverse events (AEs) reported were infections (n = 2). No Grade 3-4 or serious AEs, dose reductions, or treatment discontinuation due to AEs were reported. Conclusions: In this first real-world experience in Indian patients, ibrutinib demonstrated therapeutic efficacy in relapsed/refractory CLL (with/without del17p) and MCL. Safety results were consistent with the current known profile of ibrutinib.
Keywords
B-cell malignancies - Bruton's tyrosine kinase inhibitor - efficacy - ibrutinib - Indian - safetyPublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used forcommercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Context:
This named patient program evaluated the safety and efficacy of ibrutinib, a selective inhibitor of Bruton's tyrosine kinase in Indian patients with relapsed/refractory chronic lymphocytic leukemia (CLL, with/without chromosome 17 deletion [del17p]) and mantle cell lymphoma (MCL).
Subjects and Methods:
The eight enrolled patients (relapsed/refractory CLL: n = 6 [4/6 patients with del17p] and relapsed/refractory MCL: n = 2) had median age of 55 years (range, 52–60) and had received a median of 3 (CLL patients) and 4 (MCL patients) prior therapies. Patients received once-daily dose of ibrutinib (420 mg: CLL, 560 mg: MCL).
Results:
In CLL patients, the median time to response was 3 months (range, 0.5–7) and five of six patients had partial response (PR) whereas one achieved complete response (CR). Median time on treatment was 11.5 months (range, 8–14); five patients continued treatment and one was recommended stem cell transplantation (SCT). Of the two MCL patients, one achieved PR and one showed CR and advanced to SCT. In CLL patients, the median (range) hemoglobin level improved from 9.8 g/dL (7.2–11) at baseline to 12.0 g/dL (9.5–13.2) and median (range) platelet count improved from 150,000 cells/μL (21,000–195,000) at baseline to 190,350 cells/μL (130,000–394,000) at the time of analysis (July 2016). Most adverse events (AEs) reported were infections (n = 2). No Grade 3-4 or serious AEs, dose reductions, or treatment discontinuation due to AEs were reported.
Conclusions:
In this first real-world experience in Indian patients, ibrutinib demonstrated therapeutic efficacy in relapsed/refractory CLL (with/without del17p) and MCL. Safety results were consistent with the current known profile of ibrutinib.
Introduction
Chronic lymphocytic leukemia (CLL) and mantle cell lymphoma (MCL) are rare hematological malignancies (non-Hodgkin's lymphoma) that have a unique pattern of resistance rendering these cancers refractory to conventional chemotherapies.[1,2,3] CLL is commonly diagnosed in elderly men and has a slow and steady disease progression with a median overall survival (OS) of 10 years.[2] Further, cytogenetic disruptions associated with deletions of chromosome 17 (del17p) lead to early relapse and poor prognosis (median OS: 2–3 years) adding to the therapeutic challenges in CLL.[2,4] In contrast to CLL, MCL has a relatively aggressive clinical course exhibiting overt manifestations, rapid progression, higher relapse rates, and a median OS of 4–5 years.[3,5,6]
Updated molecular studies have implicated the role of exaggerated activation of B-cell antigen receptor (BCR) signaling pathways and subsequent uncontrolled proliferation of B lymphocytes in the pathogenesis of CLL, MCL, and related malignancies with a similar immunophenotype. Such studies have significantly assisted the evolution of targeted therapies and inhibition of the proto-oncogene Bruton's tyrosine kinase (BTK), which is one such approach to disintegrate the BCR signaling cascade.[7,8,9] The validated overexpression of BTK in the pathogenesis of CLL and MCL and its well-characterized role in the proliferation, differentiation, and survival of B cells supports the development of BTK-targeted interventions in the treatment model of lymphoid malignancies.[10,11,12]
Ibrutinib is a first-in-class, orally active, selective inhibitor of BTK that covalently binds to BTK and blocks the downward BCR signaling, thereby curbing the uncontrolled proliferation of B-cells. Ibrutinib is approved in several countries including India for the treatment of patients with relapsed/refractory CLL and MCL and in patients with CLL having del17p.[13,14,15] The US Food and Drug Administration (FDA) granted accelerated approvals for ibrutinib based on landmark phase 1b/2 and phase 2 studies conducted in patients with relapsed/refractory CLL and MCL.[16,17] Confirmatory support for the approval was obtained from a pivotal Phase 3 study, where ibrutinib improved the progression-free survival (PFS; 78% reduction in risk of progression or death vs. ofatumumab) and significantly (P = 0.005) prolonged the OS relative to ofatumumab (hazard ratio: 0.43, 95% confidence interval: 0.24; 0.79 and 57% reduction in risk of death), in patients with relapsed/refractory CLL.[18] Efficacy of ibrutinib in patients with relapsed/refractory MCL was shown in a Phase 2 study wherein an overall response rate of 68% and complete response (CR) rate of 21% (duration of response = 17.5 months) was observed.[17] In a Phase 3 study, ibrutinib significantly prolonged PFS (P < 0 href="https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5759073/#ref19" rid="ref19" class=" bibr popnode tag_hotlink tag_tooltip" id="__tag_659253716" role="button" aria-expanded="false" aria-haspopup="true" xss=removed>19] In addition, in patients with CLL/MCL receiving ibrutinib earlier in the line of therapy, significantly longer PFS and superior survival outcomes were observed underscoring the value of early intervention with ibrutinib.[20,21] More recently, ibrutinib has received US FDA approval for the first-line therapy in treatment-naïve patients with CLL based on the results from the Phase 3 RESONATE-2 study.[22]
A named-patient program (NPP) is a provision under which ethical, controlled, early access to new drugs approved in other countries is allowed for patients with life-threatening conditions, chronically ill, or seriously debilitated with no sustainable treatment option.[23] The patient data made available through such programs facilitate the synthesis of community- or country-specific preliminary information of efficacy and safety of new treatments in standard conditions of real-world clinical practice.
The current NPP was initiated before the approval of ibrutinib in India (in October 2015) to facilitate its early access for the treatment of patients with relapsed/refractory CLL and MCL and patients with CLL with del17p. Here, we report an early clinical experience of ibrutinib in the Indian patient population and summarize the safety, tolerability, and efficacy results in a real-world health-care setting.
Subjects and Methods
Study design and patients
This NPP was initiated in February 2015 and included patients started on ibrutinib treatment on or before February 2016. Patients with relapsed/refractory CLL (with or without del17p) and MCL who had received at least one prior therapy were eligible for participation. Consenting physicians had to register in the program before initiating their patients on ibrutinib. All patients had to procure import license from Drug Controller General of India for obtaining ibrutinib for personal use.[23]
Data collection
Ibrutinib was procured by 25 physicians for use in thirty patients across India. The information collected in the case report form (CRF) included patient demographics (age and sex), medical history, disease stage, details of prior and concomitant treatment, details of any chest X-ray, magnetic resonance imaging (MRI), computed tomography (CT) scans, and data for efficacy and safety assessments. Final data were collected by studying the completed CRF information elicited from individual physicians between June 2016 and August 2016. The time of analysis for all cases was July 2016.
Treatment
In accordance with the intended labeling use, patients were prescribed once-daily doses of ibrutinib 140 mg capsules at a dose of 420 mg (3 capsules) for CLL and 560 mg (4 capsules) for MCL until signs of unacceptable toxicity or disease progression emerged.[9,13] Dose reduction or discontinuation was allowed as per the CLL and MCL treatment guidelines that were provided by the manufacturer to the physicians before initiating treatment with ibrutinib.
Evaluations
Efficacy and safety evaluations were based on routine clinical practice guidelines and included clinical laboratory investigations, vital signs measurements, evaluation of remission; assessment of disease progression, and recording of adverse events (AEs) including serious and nonserious. Assessment of response was based on recommendations provided by the International Workshop on CLL for CLL and Revised Response Criteria for Malignant Lymphoma for MCL.[24,25] The information collected included molecular cytogenetics (fluorescence in situ hybridization [FISH] analysis), physical examination (size of liver, spleen, and lymph nodes), chest, abdominal, and pelvic CT scans, marrow aspirate and biopsy, standard hematologic tests (complete and differential blood count), and serum chemistry values. The treating physician reported changes in hematological values (including international normalized ratio), median time to response, progression-free treatment period, and classification of responses (CR, partial response [PR], progressive disease, or stable disease)[24] after initiation of treatment. Recording and classification of AEs were according to Cancer Institute's Common Terminology Criteria for Adverse Events, version 4.0.[26]
Results
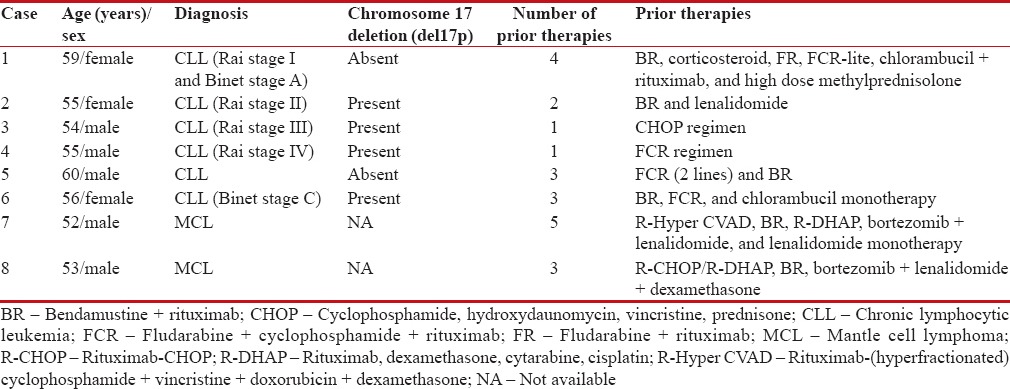
Only seven of the 25 physicians who registered patients in the ibrutinib NPP provided data for a total of eight cases. Thus, we present findings from six cases of relapsed/refractory CLL (4 of 6 with del17p) and two cases of relapsed/refractory MCL for whom complete information was available [Table 1].
Table 1
Demographics and pretreatment disease characteristics of patients
|
Cases of relapsed/refractory chronic lymphocytic leukemia
Case 1
A 59-year-old female developed CLL (Rai stage I and Binet stage A) in November 2011. Small palpable cervical and axillary lymph nodes were observed during physical examination and contrast-enhanced CT (CECT) of neck, chest, abdomen, and pelvis revealed significant cervical, retroperitoneal, paraaortic, and iliac lymphadenopathy without hepatosplenomegaly and FISH analysis revealed the absence of genetic abnormalities. No treatment or chemotherapy was initiated at this stage. In October 2012, the patient became febrile and developed generalized lymphadenopathy, left-sided pleural effusion, and lymphocytosis (97%) prompting initiation of bendamustine + rituximab treatment in November 2012. The patient relapsed again and received corticosteroids (August 2013), fludarabine + rituximab and low dose fludarabine + cyclophosphamide + rituximab (FCR-lite; August 2014) therapies. In November 2014, the patient was given chlorambucil + rituximab and high-dose methylprednisolone with no response. At this stage, her hemoglobin level was 9.8 g/dL and platelet count was 186,000 cells/μL.
Treatment with ibrutinib was started in June 2015. The patient showed response within 2 weeks (time to response) with improvements in major symptoms (lymphocytosis, fever, lymphadenopathy, and pleural effusion). In August 2015, the patient experienced hematuria and hematochezia resulting in interruption of ibrutinib treatment for 1 week. Following drug interruption, the bleeding stopped; however, lymphadenopathy increased immediately. Ibrutinib treatment (same dose, 420 mg once daily) was restarted and the patient responded within 3 weeks without recurrence of bleeding. After 5 months of ibrutinib treatment, the patient experienced multiple episodes of noninfective diarrhea and responded to short courses of steroids. Remission in major symptoms (fever and lymphadenopathy) was observed and patient achieved PR after 12 months of treatment and continued ibrutinib treatment. Hemoglobin level improved to 12.0 g/dL and platelet count increased to 286,000 cells/μL. As of July 2016, the redistribution lymphocytosis persisted, but the patient had normal health without any symptoms and resumed full-time occupation without any fatigue.
Case 2
A 55-year-old female diagnosed with CLL (Rai stage II) with del17p in September 2013 had 60% lymphocyte infiltration in the bone marrow. Cytogenetic examination revealed heterozygous TP53 deletion in 90%-cells and IgH (14q32) partial deletion in 85%-cells. The patient received no active treatment until she developed dyspnea in September 2014. Her chest radiograph showed mild right pleural effusion with basal atelectasis and CT revealed bilateral supraclavicular along with axillary nodes (1–2 cm), prevascular nodes (1–1.5 cm), lower mediastinum (1–2 cm), and portal and peripancreatic nodes (largest 3.4 cm × 2.7 cm). The patient was started on rituximab + bendamustine treatment from September 2014 and received five cycles till May 2015. She progressed soon after and was started on lenalidomide (5 mg), but it was discontinued after 15 days following neurological intolerance.
The patient was reassessed in October 2015. On physical examination, bilateral cervical and axillary adenopathy (<1>
Treatment with ibrutinib was started in December 2015. During 1st month of ibrutinib treatment, the patient developed a Grade 2 event of perioral herpes simplex virus (HSV) infection that was managed effectively with acyclovir 800 mg four times daily for 7 days that was reduced to 400 mg twice daily (ongoing) and prophylactic trimethoprim plus sulfamethoxazole combination, fluconazole, and oral chlorhexidine mouthwash. No hospitalization or interruption/reduction in ibrutinib dose was needed. In January 2016, hematology evaluation showed total leukocyte count of 187,700 cells/μL, 9% neutrophils, and 89% lymphocytes. After 5 months of treatment with ibrutinib, in May 2016, there were no palpable nodes, no signs of fatty liver, paraaortic or paracaval lymphadenopathy, enlarged nodes, or organomegaly. Hemoglobin level improved to 11.3 g/dL and platelet count increased to 186,000/μL; lymphocytosis and deranged differential count were persistent. The time to response was 7 months and the response was classified as PR with lymphocytosis. The patient has received 8 months of ibrutinib treatment and the treatment is ongoing as of July 2016.
Case 3
A 54-year-old male with no history of any chronic disease or serious infection developed CLL (Rai Stage III) with del17p (in 80% interphase cells) in March 2015. No abnormalities were detected during physical examination and biochemistry investigations. Significant changes in periportal, paraaortic, pericaval, and iliac lymph nodes along with mild splenomegaly and hydroureteronephrosis due to paraaortic lymphadenopathy were noted during an abdominal ultrasound. Complete blood count showed severe anemia (hemoglobin 7.8 g/dL), low platelet count (116,000 cells/μL), and leukocytosis (total leukocyte count 128,900 cells/μL). Cytogenetic examination revealed monosomy 8, 21, and del17p. The patient received six cycles of cyclophosphamide, hydroxydaunomycin, vincristine, prednisone (CHOP) regimen till August 2015.
Ibrutinib treatment was initiated in September 2015. Patient achieved PR and time to response was recorded as 2 months. Few AEs were observed including sore throat, erythema, inflammation, and swelling on right tonsillar fossa that were managed effectively with intravenous antibiotics and no reduction in ibrutinib dose was needed. After nearly 8 months of treatment with ibrutinib (May 2016), hemoglobin increased to 13.1 g/dL, platelet count was 194,700 cells/μL, and the leukocyte count dropped to 72,160 cells/μL. The patient completed 11 months of ibrutinib treatment which is currently ongoing as of July 2016.
Case 4
A 55-year-old male was diagnosed with CLL in 2011–2012 and received fludarabine + cyclophosphamide + rituximab (FCR) therapy. In October 2015, he was diagnosed with relapsed/refractory CLL (Rai stage IV). Physical examination showed the absence of lymph nodes and hepatosplenomegaly. The patient had thrombocytopenia (platelet count 90,000 cells/μL) and lymphocytosis (>200,000 cells/μL). Manifestations of CLL were also noted in bone marrow aspirate. No signs of lymphadenopathy were noted in imaging studies. Cytogenetics at diagnosis did not reveal any abnormalities; however, del17p was detected at recurrence. At this stage, hemoglobin level was 11 g/dL and platelet count was 100,000 cells/μL.
Treatment with ibrutinib was initiated in October 2015. Patient achieved PR and time to response was reported as 3–4 months. After 10 months of treatment, the hemoglobin level improved to 12 g/dL and platelet count increased to 130,000 cells/μL. No significant AEs were reported during treatment with ibrutinib, except for mild depigmentation of skin (details of resolution not available). The patient completed 10 months of treatment with ibrutinib (as of July 2016) and is currently undergoing allogeneic SCT.
Case 5
A 60-year-old male was diagnosed with relapsed/refractory CLL in November 2015. Initial physical examination showed palpable neck and axillary nodes, lymphocytosis (>600,000 cell/μL) was reported in hematology testing, and bone marrow aspirates were also suggestive of CLL relapse. In 2009, the patient received six cycles of FCR and following first relapse in 2013, the patient was started on bendamustine + rituximab treatment. Following disease recurrence in 2015, the patient was reinitiated on FCR regimen and received two cycles. Around this time point, hemoglobin was 9.8 g/dL and platelet count was 195,000 cells/μL.
After failure of FCR therapy, treatment with ibrutinib was started in November 2015. Lymph nodes were reported to settle and patient “felt better” within the first 2 weeks of ibrutinib therapy. No AEs were reported during treatment with ibrutinib. Following 8 months of treatment on ibrutinib, the patient achieved PR. Hemoglobin level was 9.5 g/dL and platelet count improved to 394,000 cells/μL. Treatment with ibrutinib is currently ongoing as of July 2016.
Case 6
A 56-year-old female developed CLL (Binet stage C) with del17p since January 2015. Physical examination revealed the presence of cervical nodes (maximum 2 cm) and no significant changes were noted in ultrasound scans. Hematology examination showed severe anemia (hemoglobin: 7.2 g/dL) and thrombocytopenia (platelet count: 21,000 cells/μL). The patient received three prior chemotherapies including bendamustine + rituximab, FCR, and chlorambucil monotherapy.
Treatment with ibrutinib was started in August 2015. Time to response was 3 months and patient achieved CR. After 11 months of treatment, hemoglobin level increased to 13.2 g/dL and platelet count improved to 144,000 cells/μL. The patient did not experience any AEs during treatment with ibrutinib. Since 12 months, the patient is on ibrutinib therapy, which is ongoing as of July 2016.
Cases of relapsed/refractory mantle cell lymphoma
Case 1
A 52-year-old male without any major medical history of chronic disease or infection was diagnosed with relapsed/refractory MCL in March 2008. Enlarged axillary lymph nodes were observed during physical examination. Bone marrow biopsy revealed predominantly fibrocollagenous tissue and scanty marrow space with normocellular bone marrow with trilineage hematopoiesis without any abnormal lymphoid cells and 19% lymphocyte infiltration. In 2008, the patient received R-Hyper CVAD and six cycles of bendamustine + rituximab that was repeated from 2013 to January 2014. In 2014, a CECT of whole abdomen showed the presence of lobulated soft tissue density mass lesion with moderate homogeneous enhancement in retroperitoneum in midline extending from the level of upper part of spleen to the level of left renal vein inferiorly forming a large nodal mass. Multiple enlarged lymph nodes in retrocaval, paraaortic, and peripancreatic region and right iliac nodes were also observed. The patient additionally received six maintenance cycles of rituximab. In 2015, the patient received one cycle of rituximab, dexamethasone, cytarabine, cisplatin (R-DHAP) regimen and nearly 3 weeks each of bortezomib + lenalidomide and lenalidomide monotherapy.
Treatment with ibrutinib was started in August 2015. The median time to response was 4 months and patient achieved PR. No AEs were observed during the treatment period. The patient has completed 12 months on ibrutinib treatment, which is currently ongoing as of July 2016.
Case 2
A 53-year-old male was diagnosed with relapsed/refractory MCL since September 2014. Generalized lymphadenopathy and palpable liver (3 cm below costal margin) were observed during initial physical examination in 2014. Abdominal ultrasound and chest CECT scans showed multiple discrete and homogenous enlarged lymph nodes in the cranial, mediastinal, mesenteric, subdiaphragmatic, and iliac regions (measuring up to 4.3 cm). Multiple metabolically active enlarged lymph nodes, splenomegaly, and mild ascites were reported during a positron emission tomography CT scan, and pleural effusion was noted in chest X-ray. Bone marrow aspirate revealed features of MCL and tumor biopsy results showed that the cells were positive for cd5, cd20, cyclin d1, and bcl2 and negative for cd3. The patient was planned for R-CHOP (rituximab-CHOP) alternating with R-DHAP regimen and received one cycle of R-CHOP (September 2014) followed by one cycle of R-DHAP (October 2014); however, the patient was intolerant to these first-line therapies. Between November 2014 and February 2015, the patient was given six cycles of rituximab + bendamustine, followed by third-line therapy with bortezomib + lenalidomide + dexamethasone (one cycle; April 2015 to May 2015). The patient was resistant to these therapies.
Treatment with ibrutinib was started in June 2015. The patient achieved CR within 2 months of initiation of therapy and was recommended for transplantation. No AEs were reported during the course of ibrutinib treatment. The patient received allogeneic peripheral blood stem cell transplant in August 2015.
Discussion
This NPP attempted to provide initial data for safety and therapeutic activity of ibrutinib in a real-world patient population with CLL and MCL from India. The safety profile of ibrutinib reported in these cases corroborated with the observations from larger clinical studies of ibrutinib.[17,18,19,22,27,28] The pattern of reported AEs commonly included infections and was similar to those observed in clinical studies (rates of all infections commonly ≥30%) of ibrutinib [Table 2].[18,29,30,31] None of AEs reported were of Grade 3–4 severity and all AEs of infections (HSV infection and tonsillitis) were manageable and no dose reductions or delays were recommended. Only one episode of bleeding (hematuria and hematochezia) related to platelet aggregation defect and raised partial thromboplastin time in a patient with CLL was reported. Both events resolved without recurrence upon interrupting ibrutinib treatment for one week. No other episodes of serious hemorrhage or bleeding occurred. The safety data from these real-world cases add to the available data that suggest the positive benefit-risk ratio of ibrutinib.[18,19,22]
Table 2
Summary of adverse events in patients with chronic lymphocytic leukemia

|
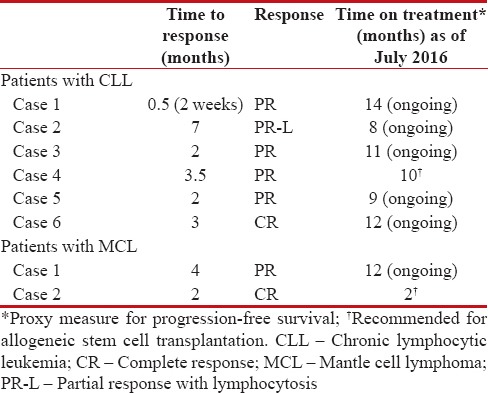
Patients described here had an advanced disease profile and were heavily pretreated (median of 3 [range: 1–5] prior therapies). Of the six patients with CLL, five (83%) achieved PR and one patient (17%) achieved CR [Figure 1]. Remission of B-cell symptoms such as fever and lymphadenopathy were also observed. The median time to response among CLL patients was 3 months (range: 0.5–7). The median (range) hemoglobin levels improved from 9.8 g/dL (7.2–11) at baseline to 12.0 g/dL (9.5–13.2) at the time of analysis. Similarly, median (range) platelet count also improved from 150,000 cells/μL (21,000–195,000) at baseline to 190,350 cells/μL (130,000–394,000) [Figure 2]. Among patients with MCL, one showed PR while the other patients achieved CR and proceeded to allogeneic SCT following 2 months of therapy [Table 3].
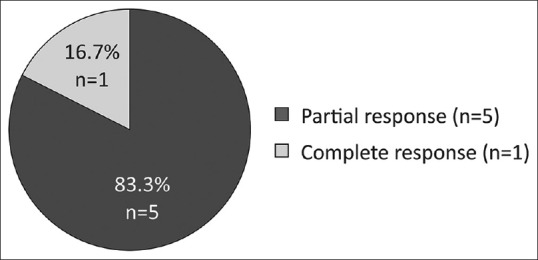
| Figure 1:Overall response rates in patients with chronic lymphocytic leukemia *Note: 1/5 PR responses reported were partial response with lymphocytosis
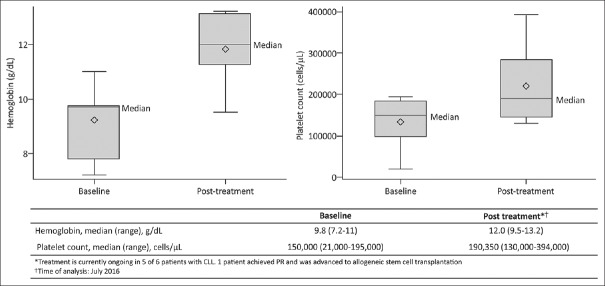
| Figure 2:Hematological findings in patients with chronic lymphocytic leukemia on ibrutinib treatmentBox represents 25th and 75th percentiles and the segment in the box represents median values. Whiskers represent minimum and maximum values. Diamond represents mean values
Table 3
Efficacy summary
|
It should be noted that 6 of 8 patients remained progression free and are continuing to receive ibrutinib, whereas the remaining two patients advanced to SCT. At the time of present analysis, the median duration of therapy was 11.5 months (range, 8–14) and with maintained ibrutinib therapy, further improvements in clinical responses can be anticipated over time. Overall, findings from these cases are consistent with high response rates and PFS rates reported in large prospective global clinical studies.[17,18,19,22,27,28] The trend of favorable outcomes was also similar to that presented in a recently published compassionate use program for ibrutinib in refractory CLL patients from Sweden.[32] Further, all four patients with del17p who had high-risk disease characteristics showed similar responses and remained progression free. These results are consistent with findings from other studies of ibrutinib, wherein treatment responses were found to be independent of genomic factors and comparable between patients with and without del17p.[18,31] These observations support available evidence that suggest ibrutinib to be a highly effective treatment option as a single agent for these CLL patients with inferior prognosis.[33,34]
It is noteworthy that the Indian patient population in this study represents a minority cohort encountered in real-life setting of clinical practice. As observed with similar population-based studies of rare hematological cancers, results from a real-world patient population are a valuable addition to the clinical data from large randomized studies and greatly aid comparison of outcomes and an analysis of benefit-risk relations.[32,35,36] Further, most patients had high-risk disease characteristics and were heavily pretreated with chemotherapy regimens suggesting poor prognosis. Thus, based on the favorable responses observed and the manageable safety profile, ibrutinib has proven to be a viable treatment option for continued use in these difficult-to-treat patients. In addition, these first case reports of treatment with ibrutinib in India did not reveal any influence of demographic variations.
Conclusion
In this first experience from a real-world setting in India, despite the acknowledged limitations (lack of patient monitoring and fewer patients) that are inherent to such retrospective analysis, ibrutinib monotherapy had a safety profile that was consistent with previous findings and demonstrated clinical benefits in patients with relapsed/ refractory CLL (with or without del17p) and MCL.
Financial support and sponsorship
This study was funded by Janssen, India. The sponsor also provided a formal review of the manuscript.
Conflicts of interest
Drs. Mishra and Nagrale are full-time employees of Janssen India (a Johnson & Johnson company). Drs. Agarwal, Bhurani, Shah, Sood, Singhal, Kamat, and Chezhian were the prescribing physicians who provided patient data for this study.
Acknowledgments
Medical writing assistance was provided by Priya Ganpathy, ISMPP CMPP™ and editorial assistance was provided by Sangita Patil, PhD, ISMPP CMPP™ (both SIRO Clinpharm Pvt. Ltd). This support was funded by Janssen, India. The authors thank all patients and their families who participated in this program and without whom this study would not have been accomplished.
References
- Ho AK, Hill S, Preobrazhensky SN, Miller ME, Chen Z, Bahler DW, et al. Small B-cell neoplasms with typical mantle cell lymphoma immunophenotypes often include chronic lymphocytic leukemias. Am J Clin Pathol 2009;131:27-32.
- Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M, ESMO Guidelines Working Group, et al. Chronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi50-4.
- McKay P, Leach M, Jackson R, Cook G, Rule S, British Committee for Standards in Haematology, et al. Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol 2012;159:405-26.
- Sellner L, Denzinger S, Dietrich S, Glimm H, Merkel O, Dreger P, et al. What do we do with chronic lymphocytic leukemia with 17p deletion? Curr Hematol Malig Rep 2013;8:81-90.
- Hoeller S, Zhou Y, Kanagal-Shamanna R, Xu-Monette ZY, Hoehn D, Bihl M, et al. Composite mantle cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A clinicopathologic and molecular study. Hum Pathol 2013;44:110-21.
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 2009;27:511-8.
- Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 2014;28:649-57.
- Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest 2012;122:3416-23.
- Bhatt V, Alejandro L, Michael A, Ganetsky A. The promising impact of ibrutinib, a bruton's tyrosine kinase inhibitor, for the management of lymphoid malignancies. Pharmacotherapy 2014;34:303-14.
- Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287-96.
- Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012;120:1175-84.
- ;Winer ES, Ingham RR, Castillo JJ. PCI-32765: A novel bruton's tyrosine kinase inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs 2012;21:355-61.
- Imbruvica (Ibrutinib) Capsules: Highlights of Prescribing Information; 2014. Available from: https://www.imbruvica.com/prescribing-information. [Last accessed on 2017 Jan 18].
- Cameron F, Sanford M. Ibrutinib:First global approval. Drugs 2014;74:263-71.
- ;Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015;386:743-800.
- O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: An open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014;15:48-58.
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16.
- Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213-23.
- ;Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8.
- Rule S, Dreyling M, Hess G, Auer R, Kahl B, Cavazos N, et al. Overall Survival Outcomes in Patients with Mantle-Cell Lymphoma (MCL) Treated with Ibrutinib in a Pooled Analysis of 370 Patients from 3 International Open-Label Studies. Available from: https://learningcenter.ehaweb.org/eha/2016/21st/135194/simon.rule.overall.survival.outcomes.in.patients.with.mantle-cell.lymphoma.html?f=m3. [Last accessed on 2016 Aug 25].
- Hillmen P, O'Brien SM, Byrd JC, Coutre S, Brown JR, Barr PM, et al. Outcomes with single-agent ibrutinib by prior line of therapy and following ibrutinib discontinuation in patients with cll: analyses from phase 3 studies. Available from: https://learningcenter.ehaweb.org/eha/2016/21st/133484/peter.hillmen.outcomes.with.single-agent.ibrutinib.by.prior.line.of.therapy.html?f=m3e968. [Last accessed on 2016 Aug 25].
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425-37.
- Patil S. Early access programs: Benefits, challenges, and key considerations for successful implementation. Perspect Clin Res 2016;7:4-8.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446-56.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86.
- National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010). 2010. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Last accessed on 2016 Aug 18].
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497-506.
- Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. Lancet Oncol 2015;16:169-76.
- ;Kim ES, Dhillon S. Ibrutinib: A review of its use in patients with mantle cell lymphoma or chronic lymphocytic leukaemia. Drugs 2015;75:769-76.
- de Claro RA, McGinn KM, Verdun N, Lee SL, Chiu HJ, Saber H, et al. FDA approval: Ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res 2015;21:3586-90.
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32-42.
- Winqvist M, Asklid A, Andersson PO, Karlsson K, Karlsson C, Lauri B, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: Data from 95 consecutive patients treated in acompassionate use program. A study from the swedish chronic lymphocytic leukemia group. Haematologica 2016;101:1573-80.
- Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: A single-arm, phase 2 study. Lancet Oncol 2014;15:1090-9.
- Stephens DM, Ruppert AS, Jones JA, Woyach J, Maddocks K, Jaglowski SM, et al. Impact of targeted therapy on outcome of chronic lymphocytic leukemia patients with relapsed del(17p13.1) karyotype at a single center. Leukemia 2014;28:1365-8.
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D'Amore F, et al. Real world data on primary treatment for mantle cell lymphoma: A Nordic lymphoma group observational study. Blood 2014;124:1288-95.
- Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014;124:1570-7.

| Figure 1:Overall response rates in patients with chronic lymphocytic leukemia *Note: 1/5 PR responses reported were partial response with lymphocytosis

| Figure 2:Hematological findings in patients with chronic lymphocytic leukemia on ibrutinib treatmentBox represents 25th and 75th percentiles and the segment in the box represents median values. Whiskers represent minimum and maximum values. Diamond represents mean values
References
- Ho AK, Hill S, Preobrazhensky SN, Miller ME, Chen Z, Bahler DW, et al. Small B-cell neoplasms with typical mantle cell lymphoma immunophenotypes often include chronic lymphocytic leukemias. Am J Clin Pathol 2009;131:27-32.
- Eichhorst B, Dreyling M, Robak T, Montserrat E, Hallek M, ESMO Guidelines Working Group, et al. Chronic lymphocytic leukemia: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2011;22 Suppl 6:vi50-4.
- McKay P, Leach M, Jackson R, Cook G, Rule S, British Committee for Standards in Haematology, et al. Guidelines for the investigation and management of mantle cell lymphoma. Br J Haematol 2012;159:405-26.
- Sellner L, Denzinger S, Dietrich S, Glimm H, Merkel O, Dreger P, et al. What do we do with chronic lymphocytic leukemia with 17p deletion? Curr Hematol Malig Rep 2013;8:81-90.
- Hoeller S, Zhou Y, Kanagal-Shamanna R, Xu-Monette ZY, Hoehn D, Bihl M, et al. Composite mantle cell lymphoma and chronic lymphocytic leukemia/small lymphocytic lymphoma: A clinicopathologic and molecular study. Hum Pathol 2013;44:110-21.
- Herrmann A, Hoster E, Zwingers T, Brittinger G, Engelhard M, Meusers P, et al. Improvement of overall survival in advanced stage mantle cell lymphoma. J Clin Oncol 2009;27:511-8.
- Cheng S, Ma J, Guo A, Lu P, Leonard JP, Coleman M, et al. BTK inhibition targets in vivo CLL proliferation through its effects on B-cell receptor signaling activity. Leukemia 2014;28:649-57.
- Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest 2012;122:3416-23.
- Bhatt V, Alejandro L, Michael A, Ganetsky A. The promising impact of ibrutinib, a bruton's tyrosine kinase inhibitor, for the management of lymphoid malignancies. Pharmacotherapy 2014;34:303-14.
- Herman SE, Gordon AL, Hertlein E, Ramanunni A, Zhang X, Jaglowski S, et al. Bruton tyrosine kinase represents a promising therapeutic target for treatment of chronic lymphocytic leukemia and is effectively targeted by PCI-32765. Blood 2011;117:6287-96.
- Woyach JA, Johnson AJ, Byrd JC. The B-cell receptor signaling pathway as a therapeutic target in CLL. Blood 2012;120:1175-84.
- ;Winer ES, Ingham RR, Castillo JJ. PCI-32765: A novel bruton's tyrosine kinase inhibitor for the treatment of lymphoid malignancies. Expert Opin Investig Drugs 2012;21:355-61.
- Imbruvica (Ibrutinib) Capsules: Highlights of Prescribing Information; 2014. Available from: https://www.imbruvica.com/prescribing-information. [Last accessed on 2017 Jan 18].
- Cameron F, Sanford M. Ibrutinib:First global approval. Drugs 2014;74:263-71.
- ;Global Burden of Disease Study 2013 Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990-2013: A systematic analysis for the global burden of disease study 2013. Lancet 2015;386:743-800.
- O'Brien S, Furman RR, Coutre SE, Sharman JP, Burger JA, Blum KA, et al. Ibrutinib as initial therapy for elderly patients with chronic lymphocytic leukaemia or small lymphocytic lymphoma: An open-label, multicentre, phase 1b/2 trial. Lancet Oncol 2014;15:48-58.
- Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med 2013;369:507-16.
- Byrd JC, Brown JR, O'Brien S, Barrientos JC, Kay NE, Reddy NM, et al. Ibrutinib versus ofatumumab in previously treated chronic lymphoid leukemia. N Engl J Med 2014;371:213-23.
- ;Dreyling M, Jurczak W, Jerkeman M, Silva RS, Rusconi C, Trneny M, et al. Ibrutinib versus temsirolimus in patients with relapsed or refractory mantle-cell lymphoma: An international, randomised, open-label, phase 3 study. Lancet 2016;387:770-8.
- Rule S, Dreyling M, Hess G, Auer R, Kahl B, Cavazos N, et al. Overall Survival Outcomes in Patients with Mantle-Cell Lymphoma (MCL) Treated with Ibrutinib in a Pooled Analysis of 370 Patients from 3 International Open-Label Studies. Available from: https://learningcenter.ehaweb.org/eha/2016/21st/135194/simon.rule.overall.survival.outcomes.in.patients.with.mantle-cell.lymphoma.html?f=m3. [Last accessed on 2016 Aug 25].
- Hillmen P, O'Brien SM, Byrd JC, Coutre S, Brown JR, Barr PM, et al. Outcomes with single-agent ibrutinib by prior line of therapy and following ibrutinib discontinuation in patients with cll: analyses from phase 3 studies. Available from: https://learningcenter.ehaweb.org/eha/2016/21st/133484/peter.hillmen.outcomes.with.single-agent.ibrutinib.by.prior.line.of.therapy.html?f=m3e968. [Last accessed on 2016 Aug 25].
- Burger JA, Tedeschi A, Barr PM, Robak T, Owen C, Ghia P, et al. Ibrutinib as initial therapy for patients with chronic lymphocytic leukemia. N Engl J Med 2015;373:2425-37.
- Patil S. Early access programs: Benefits, challenges, and key considerations for successful implementation. Perspect Clin Res 2016;7:4-8.
- Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: A report from the International Workshop on Chronic Lymphocytic Leukemia Updating the National Cancer Institute-Working Group 1996 guidelines. Blood 2008;111:5446-56.
- Cheson BD, Pfistner B, Juweid ME, Gascoyne RD, Specht L, Horning SJ, et al. Revised response criteria for malignant lymphoma. J Clin Oncol 2007;25:579-86.
- National Institutes of Health. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE); Version 4.0 Published: May 28, 2009 (v4.03: June 14, 2010). 2010. Available from: http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf. [Last accessed on 2016 Aug 18].
- Byrd JC, Furman RR, Coutre SE, Burger JA, Blum KA, Coleman M, et al. Three-year follow-up of treatment-naïve and previously treated patients with CLL and SLL receiving single-agent ibrutinib. Blood 2015;125:2497-506.
- Farooqui MZ, Valdez J, Martyr S, Aue G, Saba N, Niemann CU, et al. Ibrutinib for previously untreated and relapsed or refractory chronic lymphocytic leukaemia with TP53 aberrations: A phase 2, single-arm trial. Lancet Oncol 2015;16:169-76.
- ;Kim ES, Dhillon S. Ibrutinib: A review of its use in patients with mantle cell lymphoma or chronic lymphocytic leukaemia. Drugs 2015;75:769-76.
- de Claro RA, McGinn KM, Verdun N, Lee SL, Chiu HJ, Saber H, et al. FDA approval: Ibrutinib for patients with previously treated mantle cell lymphoma and previously treated chronic lymphocytic leukemia. Clin Cancer Res 2015;21:3586-90.
- Byrd JC, Furman RR, Coutre SE, Flinn IW, Burger JA, Blum KA, et al. Targeting BTK with ibrutinib in relapsed chronic lymphocytic leukemia. N Engl J Med 2013;369:32-42.
- Winqvist M, Asklid A, Andersson PO, Karlsson K, Karlsson C, Lauri B, et al. Real-world results of ibrutinib in patients with relapsed or refractory chronic lymphocytic leukemia: Data from 95 consecutive patients treated in acompassionate use program. A study from the swedish chronic lymphocytic leukemia group. Haematologica 2016;101:1573-80.
- Burger JA, Keating MJ, Wierda WG, Hartmann E, Hoellenriegel J, Rosin NY, et al. Safety and activity of ibrutinib plus rituximab for patients with high-risk chronic lymphocytic leukaemia: A single-arm, phase 2 study. Lancet Oncol 2014;15:1090-9.
- Stephens DM, Ruppert AS, Jones JA, Woyach J, Maddocks K, Jaglowski SM, et al. Impact of targeted therapy on outcome of chronic lymphocytic leukemia patients with relapsed del(17p13.1) karyotype at a single center. Leukemia 2014;28:1365-8.
- Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D'Amore F, et al. Real world data on primary treatment for mantle cell lymphoma: A Nordic lymphoma group observational study. Blood 2014;124:1288-95.
- Ellin F, Landström J, Jerkeman M, Relander T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood 2014;124:1570-7.


 PDF
PDF  Views
Views  Share
Share

