Effectiveness of Three Prognostic Scoring Systems in Predicting the Response and Outcome in Pediatric Chronic Myeloid Leukemia Chronic Phase on Frontline Imatinib
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2017; 38(03): 282-286
DOI: DOI: 10.4103/ijmpo.ijmpo_104_16
Abstract
Introduction: The Sokal and Hasford (Euro) scores were developed in the chemotherapy and interferon eras and are widely used as prognostic indicators in patients with chronic myeloid leukemia (CML). Recently, European Treatment and Outcome Study (EUTOS) scoring system was introduced. Data on risk stratification in pediatric CML population was lacking due to its rarity (<3 class="b" xss=removed>Objective: To study the effectiveness in predicting the response and outcome with three prognostic scores in pediatric CML-chronic phase patients on front line Imatinib. Materials and Methods: We retrospectively analyzed the hospital records of newly diagnosed CML CP patients (aged ≤18 years) from 2006 to 2010 for their risk score, cytogenetic response at 18 months and event free survival (EFS) at the end of 4 years. Events include loss of hematological response, loss of cytological response, progression to accelerated/blast phase (AP/BC). All received free Imatinib under Gleevac international patient assistance program. Results: Data of 106 children was analyzed with median age of 13.5 (ranged 5-18 years) and male preponderance (M:F = 1.14:1). The distribution of children was 63%, 32% and 5% in Sokal low, intermediate and high risk respectively, 50%, 43% and 5% in Hasford/Euro low, intermediate and high risk respectively, 71% and 29% in EUTOS low and high risk respectively. The overall cumulative complete hematological response at the end of 3 month was 94%, and complete cytogenetic response at 12 months was 75%. The CCyR at 18 month was seen in 90%,74% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 84% and 86% EUTOS low and high risk groups respectively. The EFS at the end of 48 months was seen in 87%,79% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 86% and 80% EUTOS low and high risk groups respectively. Conclusion: None of the scoring systems predicted the response and outcome effectively in children with CML CP on front line Imatinib.
Keywords
Chronic myeloid leukemia chronic phase - Euro score - European Treatment and Outcome Study score and cytogenetic response - imatinib - Sokal scorePublication History
Article published online:
04 July 2021
© 2017. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Introduction:
The Sokal and Hasford (Euro) scores were developed in the chemotherapy and interferon eras and are widely used as prognostic indicators in patients with chronic myeloid leukemia (CML). Recently, European Treatment and Outcome Study (EUTOS) scoring system was introduced. Data on risk stratification in pediatric CML population was lacking due to its rarity (<3 class="b" xss=removed>Objective: To study the effectiveness in predicting the response and outcome with three prognostic scores in pediatric CML-chronic phase patients on front line Imatinib. Materials and Methods: We retrospectively analyzed the hospital records of newly diagnosed CML CP patients (aged ≤18 years) from 2006 to 2010 for their risk score, cytogenetic response at 18 months and event free survival (EFS) at the end of 4 years. Events include loss of hematological response, loss of cytological response, progression to accelerated/blast phase (AP/BC). All received free Imatinib under Gleevac international patient assistance program. Results: Data of 106 children was analyzed with median age of 13.5 (ranged 5-18 years) and male preponderance (M:F = 1.14:1). The distribution of children was 63%, 32% and 5% in Sokal low, intermediate and high risk respectively, 50%, 43% and 5% in Hasford/Euro low, intermediate and high risk respectively, 71% and 29% in EUTOS low and high risk respectively. The overall cumulative complete hematological response at the end of 3 month was 94%, and complete cytogenetic response at 12 months was 75%. The CCyR at 18 month was seen in 90%,74% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 84% and 86% EUTOS low and high risk groups respectively. The EFS at the end of 48 months was seen in 87%,79% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 86% and 80% EUTOS low and high risk groups respectively. Conclusion: None of the scoring systems predicted the response and outcome effectively in children with CML CP on front line Imatinib.
Objective:
To study the effectiveness in predicting the response and outcome with three prognostic scores in pediatric CML-chronic phase patients on front line Imatinib. Materials and Methods: We retrospectively analyzed the hospital records of newly diagnosed CML CP patients (aged ≤18 years) from 2006 to 2010 for their risk score, cytogenetic response at 18 months and event free survival (EFS) at the end of 4 years. Events include loss of hematological response, loss of cytological response, progression to accelerated/blast phase (AP/BC). All received free Imatinib under Gleevac international patient assistance program.
Results:
Data of 106 children was analyzed with median age of 13.5 (ranged 5-18 years) and male preponderance (M:F = 1.14:1). The distribution of children was 63%, 32%- and 5%-in Sokal low, intermediate and high risk respectively, 50%, 43%-and 5%-in Hasford/Euro low, intermediate and high risk respectively, 71%-and 29%-in EUTOS low and high risk respectively. The overall cumulative complete hematological response at the end of 3 month was 94%, and complete cytogenetic response at 12 months was 75%. The CCyR at 18 month was seen in 90%,74% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 84% and 86% EUTOS low and high risk groups respectively. The EFS at the end of 48 months was seen in 87%,79% and 83% among Sokal low, intermediate and high risk groups respectively, 83%, 86% and 83% among Hasford/Euro low, intermediate and high risk groups respectively, 86% and 80% EUTOS low and high risk groups respectively.
Conclusion:
None of the scoring systems predicted the response and outcome effectively in children with CML CP on front line Imatinib.
Introduction
The approval of multiple BCR-ABL-targeting tyrosine kinase inhibitors (TKIs) led to a therapeutic dilemma for clinicians in assigning upfront therapy. In an endeavor to guide and optimize treatment decisions, several risk score metrics have been formulated and used clinically to gauge the likely disease outcome. The Sokal and Hasford/Euro scores were developed in the chemotherapy[1] (1984) and interferon[2] eras (1998) and still they are widely used. Recently (2011), a new scoring system called European Treatment and Outcome Study (EUTOS) scoring system was formulated.[3]
Chronic myeloid leukemia (CML) in children accounts for 2%–3% of pediatric leukemias, making evidence-based recommendations difficult.[4] Imatinib is effective in children with CML in chronic phase (CML-CP) with response rates similar to that in adults.[5,6,7] Since the characteristics of CML in children seem to overlap extensively with what is described in adults, most of the pediatric algorithms are adapted from the treatment of CML in adults.[8] While there are several validated scoring systems for the adult CML population, none of them have been specifically validated in pediatric population. The present study has been aimed to analyze the effectiveness of the three risk scoring systems on the outcome of the pediatric CML-CP on imatinib.
Materials and Methods
Between years 2004 and 2011, consecutive newly diagnosed children (≤18 years) with BCR-ABL positive and/or Ph+ve CML-CP who received imatinib as first-line therapy were analyzed. Their hospital records were analyzed for demographic data, spleen size, white blood cell count, platelet count, differential count, disease phase, date of initiation of imatinib treatment, attainment of complete hematological remission (CHR), complete cytogenetic response (CCyR), and follow-up details for their outcome at the end of the 4th year. The three risk scores for all children were calculated using online calculator on the European LeukemiaNet website (http://www.leukemianet.org/content/leukemias/cml/cmlscore/index_eng.html). All children received free imatinib under the Glivec International Patient Assistance Program. They were started on imatinib at a dose of 260 mg/m2 after consent from parent/guardian for initiation of the treatment.
Those who did not undergo evaluation as per advice and whose follow-up data could not be retrieved were excluded from the study.
Events include loss of hematological response or cytogenetic response and progression to accelerated phase/blast crisis. Cytogenetic response was defined as per the guidelines of the European LeukemiaNet.[9] The outcome of individual risk groups was compared using Fisher's test.
Results
Data of 106 children were analyzed, with a median age of 13.5 (range 5–18 years) and male preponderance (male:female = 1.14:1) [Table 1]. The distribution of children was 63%, 32%, and 5% for Sokal low, intermediate, and high risk, respectively, 50%, 43%, and 5% for Hasford/Euro low, intermediate, and high risk, respectively, and 71% and 29% for EUTOS low and high risk, respectively. The cumulative CHR at the end of 3 months was 94%, and CCyR at 18 months was 85%.
Table 1
Patient characteristics (n=106)

|
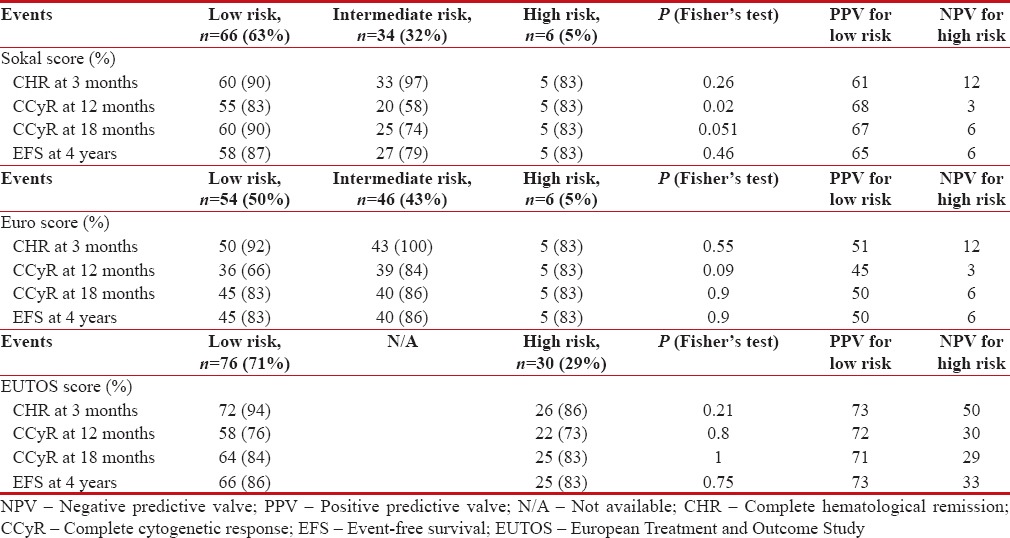
The CCyR at 18 months was attained in 90%, 74%, and 83%-among Sokal low, intermediate, and high-risk groups, respectively, 83%, 86%, and 83%-among Hasford/Euro low, intermediate, and high-risk groups, respectively, 84%- and 86% EUTOS low and high-risk groups, respectively. The event-free survival (EFS) at the end of 48 months was 87%, 79%, and 83%-among Sokal low, intermediate, and high-risk groups, respectively, 83%, 86%, and 83%-among Hasford/Euro low, intermediate, and high-risk groups, respectively, and 86% and 80%-EUTOS for low and high-risk groups, respectively. The response and outcomes among the risk groups were compared in Table 2.
Table 2
Outcome among the three risk groups
|
Discussion
With the increase in available treatment options for CML patients, there is gross unmet need in refining the prognostic risk score metrics which can aid in therapeutic decisions. The ideal risk score metric should clearly discriminate the risk groups with high sensitivity and specificity. It should be easy to apply and widely acceptable. Universally accepted risk scoring system facilitates the head-to-head comparison of trials and in framing conclusive guidelines.
Because the characteristics of CML in children seem to overlap extensively with what is described in adults, most of the pediatric algorithms are adapted from the treatment of CML in adults.[8] While there are several validated scoring systems for older CML population, none of them have been specifically validated in pediatric population.[10]
In the present study, EUTOS low-risk group children had higher chance of attaining CHR at 3 months, CCyR at 12, 18 months, and EFS at 4 years than high-risk children, but it was not statistically significant. Sokal low-risk group children had statistically significant better chance of attaining CCyR at 12, 18 months than high-risk children. Although low-risk cohort attained higher CHR at 3 months and EFS at 4 years, it was not statistically significant. Euro intermediate-risk group children had higher chance of attaining CHR at 3 months, CCyR at 12, 18 months, and EFS at 4 years than low- and high-risk children which was not statistically significant. Sokal risk groups showed statistically better differentiation in attaining CCyR at 12, 18 months than Euro and EUTOS scores. All three scoring systems have failed to predict the 4-year EFS.
In this study, among the three risk scoring metrics, EUTOS score showed higher positive predictive valve in low-risk group for attaining CCyR at 12, 18 months and EFS at 4 years than Sokal and Euro low-risk groups. All the three risk scores had low negative predictive valve (NPV). EUTOS score showed higher NPV in high-risk group for attaining CCyR at 12, 18 months and EFS at 4 years than Sokal and Euro high-risk groups.
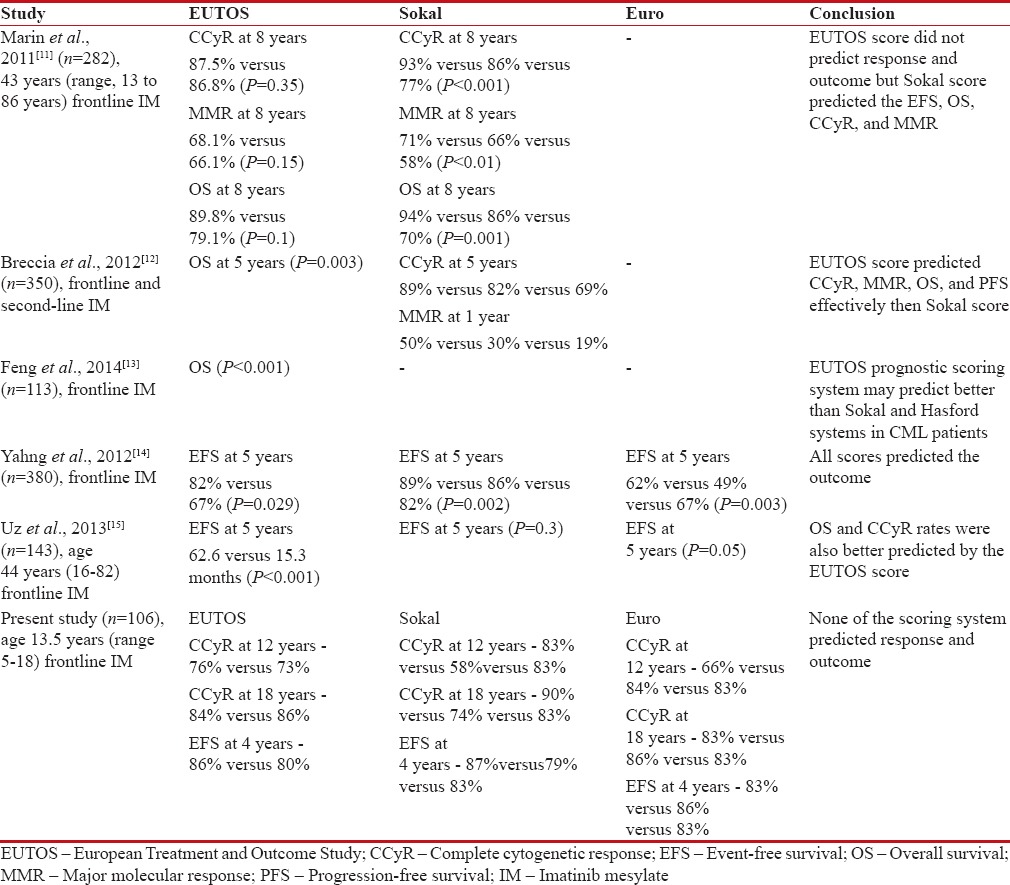
Previous studies comparing all the risk scoring metrics in adult population showed mixed results [Table 3]. Two studies compared Sokal and EUTOS scores. Marin et al.[11] concluded that there is no association between EUTOS score and overall survival (OS), progression-free survival (PFS), CCyR, and MMR. They also reported that Sokal score predicted the response and 8-year outcome. Breccia et al. retrospectively compared the Sokal and EUTOS scores and concluded that EUTOS scores were associated with CCyR, MMR, OS, and PFS.[12]
Table 3
Comparison with other studies
|
Three studies were reported so far comparing the three risk scores and outcome in adult CML patients. Uz et al. from Turkey reported that OS, EFS, and CCyR rates were better predicted by the EUTOS score than Euro/Hasford and Sokal systems in CML patients receiving frontline imatinib mesylate.[15] Yahng et al. from Korea reported that all three scores were found to be valid. Feng et al. reported the outcome in Chinese population that all three scoring systems were effective predictors of OS in CP-CML patients, and EUTOS scoring system may predict more accurately.[13]
The inconsistent results of these scoring systems in above studies could be explained by the relatively small sample size of high-risk group, all studies being the single-center studies, heterogeneous population with inclusion of second-line TKI, the errors due to manual measurement of spleen size, and the wide variation in the level of adherence with the treatment. Moreover, interracial differences in the pharmacokinetics and altered pharmacodynamics of imatinib in pediatric population may lead to differential response and outcome.[12] Although not assessed in the present study, these factors might influence the results of validation studies of the scoring systems used in CML. It would be of interest to investigate whether other biological or molecular determinants of the disease such as the expression or activity of human organic cation transporter or multidrug resistance phenotype may vary in this patient population compared to older population.
In summary, with the increase in available treatment options for pediatric CML patients, there is gross unmet need in the risk stratification, which can aid in the therapeutic decisions. The ideal risk score metric should be simple, universally acceptable, and able to clearly discriminate the risk groups with high sensitivity and specificity. The present study did not validate the effectiveness of the available three risk scores in predicting the response and outcome but lowering the EUTOS score high-risk cutoff may result in better discrimination. Currently, the usefulness of these three risk scores in stratifying pediatric CML is uncertain. To resolve this issue, new prognostic models incorporating various clinical, molecular, and gene expression features need to be tested in a multicenter prospective study involving pediatric CML population over a long follow-up period.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
We would like to thank Max foundation, Novartis Oncology Access for free supply of Imatinib Mesylate (Glivec) in India.
References
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984;63:789-99.
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998;90:850-8.
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood 2011;118:686-92.
- Lakshmaiah KC, Bhise R, Purohit S, Abraham LJ, Lokanatha D, Suresh TM, et al. Chronic myeloid leukemia in children and adolescents: Results of treatment with imatinib mesylate. Leuk Lymphoma 2012;53:2430-3.
- Hu B, Savani BN. Impact of risk score calculations in choosing front-line tyrosine kinase inhibitors for patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Eur J Haematol 2014;93:179-86.
- Linga VG, Ganta RR, Kalpathi KI, Gundeti S, Rajappa SJ, Digumarti R, et al. Response to imatinib mesylate in childhood chronic myeloid leukemia in chronic phase. South Asian J Cancer 2014;3:203-5.
- Babu GK, Thanky A, Jacob LA, Suresh Babu MC, Dasappa L, Ganguly S. Outcome of young adults with chronic myeloid leukemia treated with upfront imatinib: A single institutional experience. J Appl Hematol 2015;6:157-61.
- Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program 2010;2010:368-76.
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041-51.
- Gurrea Salas D, Glauche I, Tauer JT, Thiede C, Suttorp M. Can prognostic scoring systems for chronic myeloid leukemia as established in adults be applied to pediatric patients? Ann Hematol 2015;94:1363-71.
- Marin D, Ibrahim AR, Goldman JM. European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol 2011;29:3944-5.
- Breccia M, Finsinger P, Loglisci G, Latagliata R, Mancini M, Salaroli A, et al. The EUTOS score identifies chronic myeloid leukeamia patients with poor prognosis treated with imatinib first or second line. Leuk Res 2012;36:e209-10.
- Feng G, Wang J, Jiang Y, Li Y, Ding M, Wang N, et al. Clinical significance of Sokal, Hasford and EUTOS prognostic scoring systems in chronic myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2014;35:743-6.
- Yahng SA, Jang EJ, Choi SY, Oh YJ, Bang JH, Park JE, et al. Comparison of Sokal, Hasford and EUTOS scores in terms of long-term treatment outcome according to the risks in each prognostic model: A single center data analyzed in 255 early chronic phase chronic myeloid leukemia patients treated with frontline imatinib mesylate. Blood 2012;120:2794.
- Uz B, Buyukasik Y, Atay H, Kelkitli E, Turgut M, Bektas O, et al. EUTOS CML prognostic scoring system predicts ELN-based 'event-free survival' better than Euro/Hasford and Sokal systems in CML patients receiving front-line imatinib mesylate. Hematology 2013;18:247-52.
References
- Sokal JE, Cox EB, Baccarani M, Tura S, Gomez GA, Robertson JE, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood 1984;63:789-99.
- Hasford J, Pfirrmann M, Hehlmann R, Allan NC, Baccarani M, Kluin-Nelemans JC, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst 1998;90:850-8.
- Hasford J, Baccarani M, Hoffmann V, Guilhot J, Saussele S, Rosti G, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: The EUTOS score. Blood 2011;118:686-92.
- Lakshmaiah KC, Bhise R, Purohit S, Abraham LJ, Lokanatha D, Suresh TM, et al. Chronic myeloid leukemia in children and adolescents: Results of treatment with imatinib mesylate. Leuk Lymphoma 2012;53:2430-3.
- Hu B, Savani BN. Impact of risk score calculations in choosing front-line tyrosine kinase inhibitors for patients with newly diagnosed chronic myeloid leukemia in the chronic phase. Eur J Haematol 2014;93:179-86.
- Linga VG, Ganta RR, Kalpathi KI, Gundeti S, Rajappa SJ, Digumarti R, et al. Response to imatinib mesylate in childhood chronic myeloid leukemia in chronic phase. South Asian J Cancer 2014;3:203-5.
- Babu GK, Thanky A, Jacob LA, Suresh Babu MC, Dasappa L, Ganguly S. Outcome of young adults with chronic myeloid leukemia treated with upfront imatinib: A single institutional experience. J Appl Hematol 2015;6:157-61.
- Suttorp M, Millot F. Treatment of pediatric chronic myeloid leukemia in the year 2010: Use of tyrosine kinase inhibitors and stem-cell transplantation. Hematology Am Soc Hematol Educ Program 2010;2010:368-76.
- Baccarani M, Cortes J, Pane F, Niederwieser D, Saglio G, Apperley J, et al. Chronic myeloid leukemia: An update of concepts and management recommendations of European LeukemiaNet. J Clin Oncol 2009;27:6041-51.
- Gurrea Salas D, Glauche I, Tauer JT, Thiede C, Suttorp M. Can prognostic scoring systems for chronic myeloid leukemia as established in adults be applied to pediatric patients? Ann Hematol 2015;94:1363-71.
- Marin D, Ibrahim AR, Goldman JM. European Treatment and Outcome Study (EUTOS) score for chronic myeloid leukemia still requires more confirmation. J Clin Oncol 2011;29:3944-5.
- Breccia M, Finsinger P, Loglisci G, Latagliata R, Mancini M, Salaroli A, et al. The EUTOS score identifies chronic myeloid leukeamia patients with poor prognosis treated with imatinib first or second line. Leuk Res 2012;36:e209-10.
- Feng G, Wang J, Jiang Y, Li Y, Ding M, Wang N, et al. Clinical significance of Sokal, Hasford and EUTOS prognostic scoring systems in chronic myeloid leukemia. Zhonghua Xue Ye Xue Za Zhi 2014;35:743-6.
- Yahng SA, Jang EJ, Choi SY, Oh YJ, Bang JH, Park JE, et al. Comparison of Sokal, Hasford and EUTOS scores in terms of long-term treatment outcome according to the risks in each prognostic model: A single center data analyzed in 255 early chronic phase chronic myeloid leukemia patients treated with frontline imatinib mesylate. Blood 2012;120:2794.
- Uz B, Buyukasik Y, Atay H, Kelkitli E, Turgut M, Bektas O, et al. EUTOS CML prognostic scoring system predicts ELN-based 'event-free survival' better than Euro/Hasford and Sokal systems in CML patients receiving front-line imatinib mesylate. Hematology 2013;18:247-52.


 PDF
PDF  Views
Views  Share
Share

