Does Neoadjuvant Chemotherapy Increase the Survival in Patients with Locally Advanced Gastric Cancer Patients – A Real?World Evidence
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2020; 41(06): 832-840
DOI: DOI: 10.4103/ijmpo.ijmpo_188_20
Abstract
Background: In locally advanced gastric cancer (LAGC), perioperative chemotherapy has shown to improve the survival to a larger extent compared to surgery alone. In India, the treatment followed for gastric carcinoma widely varies based on the type of health-care provider and treatment access. There is a paucity of data on the role of neoadjuvant chemotherapy on survival among LAGC patients in the Indian context. Aim: The aim of this study was to compare the disease-free survival (DFS) and overall survival (OS) between neoadjuvant and adjuvant chemotherapies among LAGC patients. Subjects and Methods: This was a retrospective cohort study involving clinical record review of LAGC patients enrolled between 2015 and 2017 from four tertiary cancer centers in South India. The date for the following events, namely diagnosis, recurrence, death, and last day of visit, was extracted in a mobile-based open-access tool. The median duration of OS and DFS between the neoadjuvant and adjuvant groups was compared using Kaplan–Meier survival curves. Results: Of the 137 patients, 70 (51%) had received neoadjuvant chemotherapy followed by surgery and 67 (49%) had adjuvant chemotherapy following the surgery. The mean (standard deviation) age of participants was 55.4 (11.4) years. Seventy-eight percent of the patients were diagnosed at Stage 3 or 4. Regional lymph nodes were involved in 83.9%. The median duration of follow-up was 15 months. The OS in the neoadjuvant and adjuvant groups was 18.6 months and 8.3 months, respectively. Nonregional lymph node involvement and adjacent organ involvement had independently increased the risk of death. Conclusion: Among LAGC patients, the neoadjuvant chemotherapy indicated a better median and DFS compared to the adjuvant group. However, these findings were statistically not significant. The current study has contributed an important finding to the existing evidences of clinical practice in an Indian setting. Further large-scale studies are required to validate the promising trend of using neoadjuvant chemotherapy in LAGC.
Keywords
Collaborative Medical Oncology Group - D2 lymphadenectomy - gastric carcinoma - stomach neoplasm - Structured Operational Research and Training InitiativePublication History
Received: 24 April 2020
Accepted: 23 October 2020
Article published online:
14 May 2021
© 2020. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
Background: In locally advanced gastric cancer (LAGC), perioperative chemotherapy has shown to improve the survival to a larger extent compared to surgery alone. In India, the treatment followed for gastric carcinoma widely varies based on the type of health-care provider and treatment access. There is a paucity of data on the role of neoadjuvant chemotherapy on survival among LAGC patients in the Indian context. Aim: The aim of this study was to compare the disease-free survival (DFS) and overall survival (OS) between neoadjuvant and adjuvant chemotherapies among LAGC patients. Subjects and Methods: This was a retrospective cohort study involving clinical record review of LAGC patients enrolled between 2015 and 2017 from four tertiary cancer centers in South India. The date for the following events, namely diagnosis, recurrence, death, and last day of visit, was extracted in a mobile-based open-access tool. The median duration of OS and DFS between the neoadjuvant and adjuvant groups was compared using Kaplan–Meier survival curves. Results: Of the 137 patients, 70 (51%) had received neoadjuvant chemotherapy followed by surgery and 67 (49%) had adjuvant chemotherapy following the surgery. The mean (standard deviation) age of participants was 55.4 (11.4) years. Seventy-eight percent of the patients were diagnosed at Stage 3 or 4. Regional lymph nodes were involved in 83.9%. The median duration of follow-up was 15 months. The OS in the neoadjuvant and adjuvant groups was 18.6 months and 8.3 months, respectively. Nonregional lymph node involvement and adjacent organ involvement had independently increased the risk of death. Conclusion: Among LAGC patients, the neoadjuvant chemotherapy indicated a better median and DFS compared to the adjuvant group. However, these findings were statistically not significant. The current study has contributed an important finding to the existing evidences of clinical practice in an Indian setting. Further large-scale studies are required to validate the promising trend of using neoadjuvant chemotherapy in LAGC.
Keywords
Collaborative Medical Oncology Group - D2 lymphadenectomy - gastric carcinoma - stomach neoplasm - Structured Operational Research and Training InitiativeIntroduction
Globally, gastric cancer ranks the fourth most common cancer and second most common cause of cancer-related mortality. Among all cancer-related deaths, 8.2%-of deaths occurred due to gastric cancer.[1] Despite the annual 1.45%-decrease in the incidence of gastric cancers, every year, an estimated one million gastric carcinomas are diagnosed worldwide[2] and are accountable for 783,000 deaths. More than 50%-of the new cases of gastric carcinoma occur in developing countries.[3] The recent Indian Council of Medical Research (ICMR) report based on Indian cancer registry has estimated the incidence of gastric cancer to be approximately 34,000 which is predicted to become 50,000 by 2020.[2] Approximately seven out of ten cases are diagnosed at an advanced stage.[1] The standard treatment for gastric cancer is complete curative resection of the tumor with a standardized D2 lymphadenectomy.[3] Despite curative resection, nearly 50%-of the patients recur with a median survival of 12 months.[4],[5]
Chemotherapy given during the perioperative period (neoadjuvant and adjuvant chemotherapies) was found to influence the recurrence pattern and survival in locally advanced gastric cancer (LAGC) patients.[6],[7] Neoadjuvant chemotherapy may potentially downstage the tumor, treat the micrometastasis, and prevent the new onset of metastatic lesions.[8] Evidence shows that both peri- and postoperative chemotherapies may increase the disease-free survival (DFS) and overall survival (OS) in LAGC patients.[9],[10] Evidence by Cunningham et al. based on perioperative chemotherapy trial had shown the 5-year survival rate of 36% -for perioperative chemotherapy arm compared to 23%-survival among patients who underwent surgery alone.[11]
Depending on the extent of the disease and the patient tolerance level, perioperative chemotherapy is given alone or in combinations with radiotherapy.[12] However, there is a regional difference in the preferred chemotherapy regimen in India due to various factors such as poor access to regional cancer centers (catering to large population), physician preference, affordability issues, and different clinical circumstances. There are several approaches being followed by health-care providers. There is a paucity of evidence in the Indian context, whether these varying treatment approaches with or without neoadjuvant chemotherapy will make a difference in disease progression and survival.[13] The recent ICMR guidelines emphasized the lack of quality evidence on neoadjuvant regimens to guide the standard of care.[14]
Hence, the present study was conducted to compare the effectiveness of neoadjuvant chemotherapy to adjuvant chemotherapy among patients with locally advanced stomach cancer in terms of DFS and OS in selected tertiary care cancer centers in South India.
Subjects and Methods
Study design
This was a retrospective multicentric cohort study involving the review of patients' clinical records.
Study setting
This study was conducted across four centers in South India. These study sites are functioning as corporate hospitals, and the treatment-related expenditures are paid by the patient. As a part of the hospital information system, these centers maintain the patient demographic and clinical characteristics in an electronic as well as paper-based format. The patient management group involves a team of multiple specialists including a medical oncologist, radiation oncologist, surgical oncologist, and surgical gastroenterologist.
The National Comprehensive Cancer Network guidelines are widely followed with the discretion of treating physicians. The process involved in patient care management for locally advanced gastric carcinoma is depicted in [Figure 1]. The figure explains the chemotherapy types, adjuvant and neoadjuvant chemotherapies, and the duration. The various regimens used in the study are epirubicin + oxaliplatin + capecitabine (EOX), capecitabine + oxaliplatin, 5-fluorouracil (5-FU) + leucovorin calcium, epirubicin + Adriamycin + cisplatin + 5-FU, cisplatin + 5-FU, and 5-FU + leucovorin + oxaliplatin + docetaxel. All the node-positive patients had received radiotherapy with the discretion of a multidisciplinary tumor board.
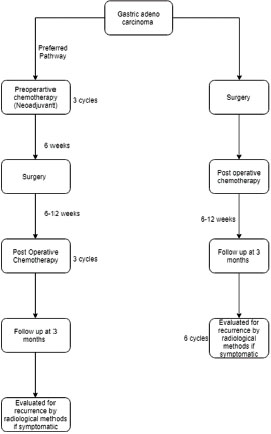
| Figure.1:Process involved in patient care management for the locally advanced gastric cancer patients who underwent neoadjuvant and adjuvant chemotherapies during 2015–2017
Study population
The study population included all locally advanced gastric carcinomas (Stage 2 or more) registered for treatment from January 2015 to December 2017 and attended a minimum one follow-up visit after 3 months of treatment in the abovementioned study sites. All eligible patients were followed till February 28, 2019. Patients with metastatic stomach cancer and those who did not undergo gastrectomy or with a previous history of chemoradiotherapy were excluded from the analysis.
Data collection
From each study site, investigators extracted the data in a structured data extraction pro forma. The pro forma included patient characteristics such as age, gender, stage of disease-based computed tomography abdomen and pelvis, and histopathological examinations. The study also included type of surgery, chemotherapy regimen used, timing of chemotherapy in relation to surgery (neoadjuvant or adjuvant) and number of cycles given, adverse events, date of each visit, and date of recurrence. The definition of the terms used in the present study such as lost to follow-up, radiological response, DFS, and OS is given in [Box 1].
|
AJCC – American Joint Committee on Cancer |
|||||||||
|
SUV |
6 |
||||||||
|
Locally advanced gastric carcinoma: Stage II-III as per AJCC staging manual 2017 |
Loss to follow-up: The time point after receiving either of the modalities is 6 months. When the study participant does not turn up for 6 months, it will be considered as loss to follow up |
Radiological response |
Complete response: Disappearance of all target lesions |
Partial response: >30 |
|||||
|
Characteristics |
Neoadjuvant therapy group, n (%) |
Adjuvant therapy group, n (%) |
P |
|---|---|---|---|
|
Total |
70(100) |
67 (100) |
|
|
Gender |
|||
|
Male |
53 (75.7) |
42 (62.7) |
0.098 |
|
Female |
17 (24.3) |
25 (37.3) |
|
|
Comorbidities |
|||
|
Diabetes |
13 (18.6) |
11 (16.4) |
0.740 |
|
Hypertension |
13 (18.6) |
11 (16.4) |
0.740 |
|
Coronary artery disease |
2 (2.9) |
5 (7.5) |
0.268 |
|
Others |
0 |
3 (4.5) |
0.114 |
|
Stage |
|||
|
Second stage |
13 (18.5) |
17 (25.4) |
0.520 |
|
Third stage |
51 (72.9) |
45 (67.2) |
|
|
Fourth stage |
6 (8.6) |
5 (7.5) |
|
|
Tumor site |
|||
|
Cardia |
23 (32.9) |
4 (6.0) |
0.001* |
|
Fundus |
16 (22.9) |
5 (7.5) |
0.012 |
|
Body |
25 (35.7) |
25 (37.3) |
0.846 |
|
Antrum |
20 (28.6) |
18 (26.9) |
0.824 |
|
Pylorus |
13 (18.6) |
22 (32.8) |
0.055* |
|
Lesser curvature |
14 (20.0) |
4 (6.0) |
0.021* |
|
Greater curvature |
6 (8.6) |
2 (3.0) |
0.275 |
|
Gastroesophageal junction |
11 (15.7) |
3 (4.5) |
0.046* |
|
Number of tumor sites |
|||
|
One |
35 (50.0) |
53 (79.1) |
0.001* |
|
Two |
17 (24.3) |
13 (19.4) |
|
|
Three |
14 (20.0) |
0 |
|
|
More than three |
4 (5.7) |
1 (1.5) |
|
|
Tumor size mean (standard deviation) |
4.88 (2.17) |
5.89 (3.62) |
0.070 |
|
Lymph node involvement |
|||
|
Nil |
5 (7.2) |
8 (12.5) |
0.494 |
|
Regional nodes |
62 (89.9) |
53 (82.8) |
|
|
Nonregional lymph nodes |
2 (2.9) |
3 (4.7) |
|
|
Preoperative imaging |
|||
|
Yes |
64 (97.0) |
52 (96.3) |
1.000 |
|
No |
2 (3.0) |
2 (3.7) |
|
|
Effect of treatment |
Adjuvant therapy group, n (%) |
Neoadjuvant therapy group, n (%) |
P |
|---|---|---|---|
|
pT1 – Tumor invades the lamina propria, muscularis mucosae, or submucosa; pT2 – Tumor invades the muscularis propria; pT3 – Tumor invades adventitia; pT4 – Tumor invades adjacent structures; pN0 – No regional lymph node metastasis; pN2 – Metastases in one or two lymph nodes; pN2 – Metastases in three to six lymph nodes; pN3 – Metastases in seven or more regional lymph nodes; R0 – No residual disease postsurgery; R1 – Microscopic residual disease postsurgery; R2 – Macroscopic residual disease postsurgery |
|||
|
Total |
67 (100) |
70 (100) |
|
|
Radiological response |
|||
|
Complete response |
3 (5.8) |
12 (18.8) |
0.0001 |
|
Partial response |
19 (36.5) |
49 (76.6) |
|
|
Stable disease |
3 (5.8) |
2 (3.1) |
|
|
Progressive disease |
27 (51.9) |
1 (16) |
|
|
Missing* |
15 |
6 |
|
|
Type of gastrectomy |
|||
|
Partial gastrectomy |
47 (70.15) |
21 (30) |
0.0001 |
|
Total gastrectomy |
14 (20.90) |
37 (52.86) |
|
|
Sleeve resection |
1 (1.49) |
0 |
|
|
Esophagogastrectomy |
3 (4.48) |
5 (7.14) |
|
|
Others |
2 (2.99) |
7 (10.00) |
|
|
Number of chemotherapy cycles |
|||
|
<6> |
28 (46.67) |
13 (19.70) |
0.001 |
|
>6 |
32 (53.33) |
53 (80.30) |
|
|
<4> |
20 (33.33) |
8 (12.12) |
0.004 |
|
>4 |
40 (66.67) |
58 (87.88) |
|
|
Surgical stage |
|||
|
pT1 |
0 |
2 (2.86) |
0.551 |
|
pT2 |
14 (20.90) |
16 (22.86) |
|
|
pT3 |
39 (58.21) |
39 (55.71) |
|
|
pT4 |
14 (20.90) |
13 (18.57) |
|
|
Pathological node stage |
|||
|
pN0 |
6 (8.96) |
20 (28.57) |
0.027 |
|
pN1 |
24 (35.82) |
18 (25.71) |
|
|
pN2 |
20 (29.85) |
20 (28.57) |
|
|
pN3 |
17 (25.37) |
12 (17.14) |
|
|
Surgical response |
|||
|
R0 |
55 (91.67) |
64 (96.97) |
0.131 |
|
R1 |
5 (8.33) |
1 (1.52) |
|
|
R2 |
0 |
1 (1.52) |
|
|
Postoperative chemotherapy |
|||
|
Yes |
55 (82.1) |
55 (78.6) |
0.731 |
|
No |
5 (7.5) |
8 (11.4) |
|
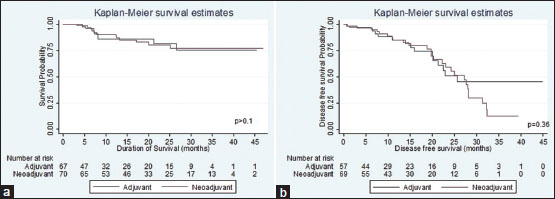
| Figure.2:(a) Comparison of overall survival between neoadjuvant and adjuvant chemotherapies in locally advanced gastric carcinoma, South India 2015–2017. (b) Comparison of disease-free survival between neoadjuvant and adjuvant chemotherapies in locally advanced gastric carcinoma, South India 2015–2017
Disease-free survival
|
Estimate |
Adjuvant therapy group, (n=67) |
Neoadjuvant therapy group, (n=70) |
|---|---|---|
|
CI – Confidence interval; HR – Hazard ratio |
||
|
Overall survival |
||
|
Median overall survival time (months) |
8.3 |
18.6 |
|
Number of deaths |
8 |
12 |
|
Duration followed (months) |
919.5 |
1440.7 |
|
Incidence rate for overall survival |
8.47/1000 person-months |
8.33/1000 person-months |
|
HR |
Reference |
0.97 (95% CI: 0.39-2.37) |
|
Median disease-free survival (months) |
10.3 |
13.3 |
|
Number of recurrence |
15 |
26 |
|
Duration followed (months) |
780.2 |
1014 |
|
Incidence rate for disease-free survival |
19.2/1000 person-months |
25.6/1000 person-months |
|
HR |
Reference |
1.25 (95% CI: 0.66-2.37) |
|
Variables |
Categories |
HR^ |
95% CI |
P |
HR^^ |
95% CI |
P |
|---|---|---|---|---|---|---|---|
|
^Unadjusted risk ratio; *P<0> |
|||||||
|
Group |
Adjuvant |
Reference |
Reference |
||||
|
Neoadjuvant |
1.25 |
0.66-2.37 |
0.49 |
1.46 |
0.64-3.32 |
0.372 |
|
|
Sex |
Male |
Reference |
Reference |
0.100 |
|||
|
Female |
0.58 |
0.28-1.22 |
0.15 |
0.44 |
0.17-1.17 |
||
|
Stage |
2 |
Reference |
|||||
|
3 |
1.55 |
0.6-4.0 |
0.36 |
- |
- |
- |
|
|
4 |
2.73 |
0.57-10.4 |
0.23 |
- |
- |
- |
|
|
Lymph node |
Nil |
Reference |
2.30 |
0.31-17.08 |
0.418 |
||
|
involvement |
Regional nodes |
2.04 |
0.40-8.5 |
0.33 |
63.65 |
2.76-1465.90 |
0.009 |
|
Nonregional lymph nodes |
26.6 |
3.1-225.5 |
0.003* |
Reference |
|||
|
Adjacent organ |
No |
Reference |
- |
- |
- |
||
|
involvement |
Yes |
0.92 |
1.3-6.8 |
0.94 |
- |
- |
- |
|
Radiological |
Complete response |
Reference |
- |
- |
- |
||
|
response |
Partial response |
1.67 |
0.57-4.90 |
0.35 |
- |
- |
- |
|
Progressive disease |
1.58 |
0.29-8.77 |
0.6 |
- |
- |
- |
|
|
Stable disease |
1.44 |
0.45-4.62 |
0.54 |
- |
- |
- |
|
|
Node dissection |
D1 |
Reference |
Reference |
||||
|
D2 |
0.14 |
0.02-1.18 |
0.07 |
0.99 |
0.05-21.59 |
0.998 |
|
|
Surgical stage# |
1.29 |
0.79-2.12 |
0.3 |
||||
|
Pathological nodal |
pN0 |
Reference |
Reference |
||||
|
stage |
pN1 |
0.83 |
0.31-2.25 |
0.72 |
0.76 |
0.18-3.26 |
0.711 |
|
pN2 |
0.86 |
0.33-2.25 |
0.77 |
0.76 |
0.17-3.41 |
0.722 |
|
|
pN3 |
1.33 |
0.53-3.30 |
0.54 |
1.29 |
0.29-5.67 |
0.735 |
|
|
Surgical response |
R0 |
Reference |
Reference |
||||
|
R1 |
1.34 |
0.48-3.83 |
0.56 |
3.66 |
0.95-14.12 |
0.059 |
|
|
R2 |
8.66 |
1.1-6.70 |
0.04* |
- |
- |
- |
|
|
Grade of tumor# |
1.67 |
0.98-2.85 |
0.06 |
- |
- |
- |
|
|
Number of |
<6> |
Reference |
|||||
|
chemotherapy cycles |
>6 |
0.69 |
0.32-1.51 |
0.35 |
- |
- |
- |
|
<4> |
Reference |
- |
- |
- |
|||
|
>4 |
0.87 |
0.34-2.24 |
0.78 |
- |
- |
- |
|
|
Variables |
Categories |
HR^ |
95% CI |
P |
HR^^ |
95% CI |
P |
|---|---|---|---|---|---|---|---|
|
^Unadjusted risk ratio; *P<0> |
|||||||
|
Group |
Adjuvant |
Reference |
Reference |
||||
|
Neoadjuvant |
0.97 |
0.39-2.372 |
0.94 |
1.05 |
0.36-3.00 |
0.933 |
|
|
Sex |
Male |
Reference |
Reference |
||||
|
Female |
0.25 |
0.06-1.07 |
0.06 |
0.12 |
0.01-1.06 |
0.056 |
|
|
Stage |
2 |
Reference |
|||||
|
3 |
0.73 |
0.24-2.23 |
0.59 |
- |
- |
- |
|
|
4 |
1.07 |
0.19-5.83 |
0.94 |
- |
- |
- |
|
|
Lymph node |
Nil |
Reference |
Reference |
||||
|
involvement |
Regional nodes |
1.12 |
0.15-8.5 |
0.9 |
0.72 |
0.09-5.87 |
0.757 |
|
Nonregional lymph nodes |
20.8 |
2.18-197.9 |
0.008* |
9.14 |
0.71-117.4 |
0.089 |
|
|
Adjacent organ |
No |
Reference |
Reference |
||||
|
involvement |
Yes |
5.75 |
1.67-19.8 |
0.006* |
0.19 |
0.05-0.80 |
0.023 |
|
Radiological |
Complete response |
Reference |
- |
- |
- |
||
|
response |
Partial response |
1.54 |
0.34-7.04 |
0.58 |
|||
|
Progressive disease |
4.1 |
0.57-29.4 |
0.16 |
||||
|
Stable disease |
0.64 |
0.09-4.53 |
0.653 |
||||
|
Node dissection |
D1 |
Reference |
- |
- |
- |
||
|
D2 |
0.36 |
0.05-2.78 |
0.3 |
||||
|
Surgical stage |
pT1 |
Reference |
Reference |
||||
|
pT2 |
0.19 |
0.03-1.15 |
0.07 |
0.44 |
0.05-3.58 |
0.441 |
|
|
pT3 |
0.15 |
0.03-0.70 |
0.02* |
0.32 |
0.04-2.79 |
0.302 |
|
|
pT4 |
0.23 |
0.04-1.18 |
0.08 |
0.24 |
0.03-1.98 |
0.184 |
|
|
Pathological |
pN0 |
Reference |
- |
- |
- |
||
|
nodal stage |
pN1 |
0.14 |
0.03-0.66 |
0.01* |
|||
|
pN2 |
0.48 |
0.18-1.34 |
0.16 |
||||
|
pN3 |
0.23 |
0.06-0.87 |
0.03* |
||||
|
Grade of tumor# |
0.61 |
0.29-1.30 |
0.2 |
||||
|
Number of |
<6> |
Reference |
- |
- |
- |
- |
|
|
chemotherapy |
>6 |
0.19 |
0.08-0.46 |
0.001* |
- |
- |
- |
|
cycles |
<4> |
Reference |
Reference |
||||
|
>4 |
0.28 |
0.11-0.70 |
0.006* |
0.36 |
0.10-1.35 |
0.130 |
|

| Figure.1:Process involved in patient care management for the locally advanced gastric cancer patients who underwent neoadjuvant and adjuvant chemotherapies during 2015–2017

| Figure.2:(a) Comparison of overall survival between neoadjuvant and adjuvant chemotherapies in locally advanced gastric carcinoma, South India 2015–2017. (b) Comparison of disease-free survival between neoadjuvant and adjuvant chemotherapies in locally advanced gastric carcinoma, South India 2015–2017
References
- International Agency for Research on Cancer. GLOBOCAN Cancer Fact Sheets: Stomach Cancers. International Agency for Research on Cancer.2008
- Indian Council of Medical Research.Consensus Document for Management of Gastric Cancer.New Delhi: Indian Council of Medical Research; 2014
- Global Burden of Disease. GBD Compare IHME Viz Hub. Global Burden of Disease; 2013.
- Sitarz R, Skierucha M, Mielko J, Offerhaus GJ, Maciejewski R, Polkowski WP. Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Manag Res 2018; 10: 239-48
- Dikken JL, van de VeldeCJ, Coit DG, Shah MA, Verheij M, Cats A. Treatment of resectable gastric cancer. Therap Adv Gastroenterol 2012; 5: 49-69
- Park SC, Chun HJ. Chemotherapy for advanced gastric cancer: Review and update of current practices. Gut Liver 2013; 7: 385-93
- Mirza A, Pritchard S, Welch I. The postoperative component of MAGIC chemotherapy is associated with improved prognosis following surgical resection in gastric and gastrooesophageal junction adenocarcinomas. Int J Surg Oncol 2013; 2013: 781742
- Glynne-Jones R, Grainger J, Harrison M, Ostler P, Makris A. Neoadjuvant chemotherapy prior to preoperative chemoradiation or radiation in rectal cancer: Should we be more cautious? . Br J Cancer 2006; 94: 363-71
- Eom BW, Kim S, Kim JY, Yoon HM, Kim MJ, Nam BH. et al. Survival benefit of perioperative chemotherapy in patients with locally advanced gastric cancer: A propensity score matched analysis. J Gastric Cancer 2018; 18: 69-81
- Glatz T, Bronsert P, Schäfer M, Kulemann B, Marjanovic G, Sick O. et al. Perioperative platin-based chemotherapy for locally advanced esophagogastric adenocarcinoma: Postoperative chemotherapy has a substantial impact on outcome. Eur J Surg Oncol 2015; 41: 1300-7
- Cunningham D, Allum WH, Stenning SP, Thompson JN, Van de VeldeCJ, Nicolson M. et al Perioperative chemotherapy versus surgery alone for resectable gastroesophageal cancer. N Engl J Med 2006; 355: 11-20
- Earle CC, Maroun J, Zuraw L. Cancer Care Ontario Practice Guidelines Initiative Gastrointestinal Cancer Disease Site Group. Neoadjuvant or adjuvant therapy for resectable gastric cancer? A practice guideline. Can J Surg 2002; 45: 438-46
- Ministry of Health and Family Welfare Government of India. Achievements Under National Cancer Control Programme. Ministry of Health and Family Welfare Government of India
- ;Shrikhande SV, Sirohi B, Barreto SG, Chacko RT, Parikh PM, Pautu J. et al. Indian Council of Medical Research. consensus document for the management of gastric cancer. Indian J Med Paediatr Oncol 2014; 35: 239-43
- Sasako M, Sakuramoto S, Katai H, Kinoshita T, Furukawa H, Yamaguchi T. et al. Five-year outcomes of a randomized phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol 2011; 29: 4387-93
- Bang YJ, Kim YW, Yang HK, Chung HC, Park YK, Lee KH. et al. Adjuvant capecitabine and oxaliplatin for gastric cancer after D2 gastrectomy (CLASSIC): A phase 3 open-label, randomised controlled trial. Lancet 2012; 379: 315-21
- Ychou M, Boige V, Pignon JP, Conroy T, Bouché O, Lebreton G. et al. Perioperative chemotherapy compared with surgery alone for resectable gastroesophageal adenocarcinoma: An FNCLCC and FFCD multicenter phase III trial. J Clin Oncol 2011; 29: 1715-21
- Chelakkot PG, Ravind R, Sruthi K, Menon D. Treatment in resectable non-metastatic adenocarcinoma of stomach: Changing paradigms. Indian J Cancer 2019; 56: 74-80
- Hartgrink HH, van de VeldeCJ, Putter H, Songun I, Tesselaar ME, Kranenbarg EK. et al Neo-adjuvant chemotherapy for operable gastric cancer: Long term results of the Dutch randomised FAMTX trial. Eur J Surg Oncol 2004; 30: 643-9
- Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903-9
- Sharma A, Radhakrishnan V. Gastric cancer in India. Indian J Med Paediatr Oncol 2011; 32: 12-6
- Ahmed BM, Wani KA, Wani M. OP0003 Feasibility and efficacy of neoadjuvant chemotherapy in locally advanced gastric cancer: A randomised trial. Eur J Cancer 2015; 51: e1 -2
- Chalissery JR, Jose TA, Pillai S, Unni H, Varghese KM, Gopu GP. et al. Clinical impact of adjuvant chemotherapy and radiation for carcinoma stomach: Experience from a tertiary care center. J Can Res Ther. 2020
- Sharma A, Raina V, Lokeshwar N, Deo SV, Shukla NK, Mohanti BK. Phase II study of cisplatin, etoposide and paclitaxel in locally advanced or metastatic adenocarcinoma of gastric/gastroesophageal junction. Indian J Cancer 2006; 43: 16-9
- Shukla NK, Deo SV, Asthana S, Raina V, Dronamaraju SS. et al. Neonatal. Neonat 2013; 2: 4-7
- Kushwaha AK, Vidyarthi SK. Neoadjuvant chemotherapy in locally advanced stomach cancer: Our experience. J Carcinog Mutagen 2019; 10: 1-2
- Ibrahim M, Gilbert K. Management of gastric cancer in Indian population. Transl Gastroenterol Hepatol 2017; 2: 64
- Ostwal V, Sahu A, Ramaswamy A, Sirohi B, Bose S, Talreja V. et al. Perioperative epirubicin, oxaliplatin, and capecitabine chemotherapy in locally advanced gastric cancer: Safety and feasibility in an interim survival analysis. J Gastric Cancer 2017; 17: 21-32
- Chawla T, Thambudorai R, Ashok A, Roy B, Ghosh J, Ganguly S. et al. Perioperative chemotherapy with docetaxel, oxaliplatin, fluorouracil and leucovorin (FLOT) versus epirubicin, platinum and capecitabine or flourouracil (EOX/ECF) in resectable gastric or gastroesophageal junction adenocarcinoma: Safety and response data from India. Ann Oncol 2019; 30: 252-324


 PDF
PDF  Views
Views  Share
Share

