Diabetes, Epstein-Barr virus and extranodal natural killer/T-cell lymphoma in India: Unravelling the plausible nexus
CC BY-NC-ND 4.0 · Indian J Med Paediatr Oncol 2016; 37(01): 6-13
DOI: DOI: 10.4103/0971-5851.177002
Abstract
The International Diabetes Federation Diabetes Atlas estimates a staggering 590 million people affected with diabetes mellitus (DM) within the next two decades globally, of which Type 2 DM will constitute more than 90%. The associated insulin resistance, hyperinsulinemia, and hyperglycemia pose a further significant risk for developing diverse malignant neoplasms. Diabetes and malignancy are multifactorial heterogeneous diseases. The immune dysfunction secondary to Type 2 diabetes also reactivates latent infections with high morbidity and mortality rates. Epstein-Barr virus (EBV), a ubiquitous human herpes virus-4, is an oncogenic virus; its recrudescence in the immunocompromised condition activates the expression of EBV latency genes, thus immortalizing the infected cell and giving rise to lymphomas and carcinomas. Extranodal natural killer/T-cell lymphoma (ENKTCL), common in South-East Asia and Latin America; is a belligerent type of non-Hodgkin lymphoma (NHL) almost invariably associated with EBV. An analysis of articles sourced from the PubMed database and Google Scholar web resource until February 2014, suggests an increasing incidence of NHL in Asia/India and of ENKTCL in India, over the last few decades. This article reviews the epidemiological evidence linking various neoplasms with Type 2 DM and prognosticates the emergence of ENKTCL as a common lymphoreticular malignancy secondary to Type 2 diabetes, in the Indian population in the next few decades.
Publication History
Article published online:
12 July 2021
© 2016. Indian Society of Medical and Paediatric Oncology. This is an open access article published by Thieme under the terms of the Creative Commons Attribution-NonDerivative-NonCommercial-License, permitting copying and reproduction so long as the original work is given appropriate credit. Contents may not be used for commercial purposes, or adapted, remixed, transformed or built upon. (https://creativecommons.org/licenses/by-nc-nd/4.0/.)
Thieme Medical and Scientific Publishers Pvt. Ltd.
A-12, 2nd Floor, Sector 2, Noida-201301 UP, India
Abstract
The International Diabetes Federation Diabetes Atlas estimates a staggering 590 million people affected with diabetes mellitus (DM) within the next two decades globally, of which Type 2 DM will constitute more than 90%. The associated insulin resistance, hyperinsulinemia, and hyperglycemia pose a further significant risk for developing diverse malignant neoplasms. Diabetes and malignancy are multifactorial heterogeneous diseases. The immune dysfunction secondary to Type 2 diabetes also reactivates latent infections with high morbidity and mortality rates. Epstein-Barr virus (EBV), a ubiquitous human herpes virus-4, is an oncogenic virus; its recrudescence in the immunocompromised condition activates the expression of EBV latency genes, thus immortalizing the infected cell and giving rise to lymphomas and carcinomas. Extranodal natural killer/T-cell lymphoma (ENKTCL), common in South-East Asia and Latin America; is a belligerent type of non-Hodgkin lymphoma (NHL) almost invariably associated with EBV. An analysis of articles sourced from the PubMed database and Google Scholar web resource until February 2014, suggests an increasing incidence of NHL in Asia/India and of ENKTCL in India, over the last few decades. This article reviews the epidemiological evidence linking various neoplasms with Type 2 DM and prognosticates the emergence of ENKTCL as a common lymphoreticular malignancy secondary to Type 2 diabetes, in the Indian population in the next few decades.
INTRODUCTION
Diabetes mellitus (DM) has assumed near pandemic proportions with the International Diabetes Federation (IDF) Diabetes Atlas projecting a rise of 210 million cases of diabetes by 2035.[1] An upsurge in the incidence of Type 2 DM is observed in all countries, and this shall seriously impact the economic and health care situations in developing nations, which constitute approximately 4/5th of the total diabetic population.[1,2]
Among the South-East Asian (SEA) Nations, India is a home to approximately 65 million adults in the 20-79 years age range, with Type 2 diabetes.[2] Increasing prevalence of the prediabetic state with impaired fasting glucose (IFG) and impaired glucose tolerance test (IGT) has been observed in Rural India since 2000.[3] Sadikot et al. using the 1997 American Diabetes Association criteria found a prevalence rate of IFG in urban and rural Indian population to be 4.8% and 2.5%, respectively[4] and by using the World Health Organisation (WHO) (1999) criteria prevalence rate of IGT in the urban and rural population was 6.3% and 3.7% respectively.[5]
Further, an alarmingly high number of undiagnosed Type 2 diabetes has been reported by IDF. Worldwide in the year 2013, 174.8 million people were estimated to have undiagnosed diabetes. In the same year, number of cases of undiagnosed diabetes in India was approximately 31,920.[6] This augments the burden of diabetes cases in the Indian population.
Type 2 DM increases the risk for several neoplasms of diverse origin. The underlying mechanism for a higher risk of cancer in patients with diabetes is due to a compromised immune response. It is also associated with a definite predisposition to intercurrent infection and reactivation of latent infections secondary to altered immune function, and this reactivation of oncogenic virus is associated with documented increased risk of neoplasms.[7] Few of the neoplasms linked to infectious agents are restricted to distinct topographical areas. For instance, Epstein-Barr virus (EBV) associated nasopharyngeal carcinoma is prevalent among the Chinese[8] and extranodal natural killer/T-cell lymphoma (ENKTCL) is a common neoplasm in South-East Asia and Latin America.[9,10]
An increasing trend in the incidence of non-Hodgkin lymphoma (NHL) among all other lymphoreticular malignancies has been reported in the Asian continent, with a continuous rise in the Indian subcontinent over the last 30-40 years.[11] In India, the incidence rate of NHL is[12] 5.1/100,000 population. Few studies have suggested an increase in incidence of ENKTCL in Indian population. Sahni and Desai[13] reported an incidence rate of 1.07% for ENKTCL following a retrospective study in Tata Memorial Hospital, Mumbai, on 1250 reported cases of lymphoma for the year 2004, out of which 935 cases were of NHL. Subtyping of these cases was done according to the WHO classification of lymphoid neoplasms (2000). Based on the morphological and immunohistochemical evaluation, a total of 10 cases of ENKTCL were reported. In the same hospital from January 1995 to June 1998, 2773 cases of NHL were diagnosed, out of which 19 cases were of ENKTCL nasal/nasal type, i.e., 0.68% of all cases.[14] Thus, a rise of 0.39% of cases was seen over a 5-year period. Yeole[15] analyzed the incidence of NHL in Mumbai, Bengaluru, Chennai, New Delhi, and Bhopal over the last two decades from the records of urban registries under National Cancer Registry Programme of Indian Council of Medical Research, and found a significant spike in the incidence rate of NHL in both genders.
A methodical search of all the pertinent literature was performed using keywords such as “diabetes, EBV, reactivation, NHL, Asia, India.” The literature search was done using the PubMed database and Google Scholar web resource. The search was performed on articles published till February 2014. Literature was further intensified by cross references from the bibliography of relevant articles.
This paper explores the propensity of ENKTCL for a subset of patients with Type 2 DM harboring the EBV.
DIABETES: A CONTEMPORARY CONTAGION
DM is a heterogeneous multifactorial disease with a definite link to modern lifestyle. It is typified by elevated blood glucose levels and cascading systemic complications. Etiologically, two basic forms of DM are Type 1 and Type 2. Type 1 is the result of an autoimmune mediated destruction of beta-islets of Langerhans of pancreas, while Type 2 DM follows hyporesponsiveness of receptors to endogenous insulin and has a prevalence rate of 10% in urban and 4% in rural India.[16]
An intricate gene-environment interaction is associated with this metabolic disorder. Asian immigrants from low prevalence area to the Western world are at a higher risk of developing Type 2 diabetes suggesting a genetic predisposition associated with this polygenic disease, and thus supporting the “thrifty gene hypothesis” as proposed by Neel.[17]
Studies have also underscored the fact that compared to other ethnic groups in Asia; Asian Indians have a greater likelihood of diabetes irrespective of their country of residence.[2] This high rate has also been attributed to perinatal malnutrition.[18] Thus, the “Asian-Indian Phenotype” which is thought to be the result of some idiosyncratic biochemical and clinical attributes confers a higher risk for diabetes.[19]
Few candidate genes discovered for Type 2 diabetes are peroxisome proliferator activated receptor-ϒ gene, plasma cell glycoprotein gene polymorphism K121Q (ENPP1 K121Q), peroxisome proliferator-activated receptor-co-activator-1 alpha gene and insulin receptor substrate-2 gene. However, the principal locus associated with Type 2 diabetes in almost all the ethnic groups is transcription factor 7 like 2 genes.[20]
MECHANISMS LINKING TYPE 2 DIABETES AND CANCER
Numerous epidemiological studies and meta-analyses have reported an increased risk of various neoplasms viz., endometrial carcinoma, breast carcinoma, colorectal cancer, renal and bladder carcinoma, and NHL in Type 2 diabetes.[21] The studies have also proven that carcinoma in diabetics are less differentiated[22] and associated with increased metastasis and mortality rates.[23,24,25]
Genomic instability underlies the six hallmarks of cancer. The Warburg effect is now considered as the seventh hallmark.[26,27]
The six hallmarks of cancer[28] as described by Hanahan and Weinberg are as follows:
- Continuous proliferative signaling
- Evasion of growth suppressors
- Hostility to cell death
- Angiogenesis
- Possessing unlimited replicative capacity and
- Invasive/metastatic phenotype.
The genomic alterations subsequently foster the acquisition of capabilities necessary for tumor formation and its progression.[29]
Categorized as a chronic metabolic disorder, Type 2 diabetes is also the underlying cause of genomic instability secondary to telomere shortening.[30] Using the Southern blot technique to measure terminal restriction fragment, which determines telomere length, shortening of telomere in Type 2 diabetics of Asian-Indian origin has been demonstrated.[31] This shortening of telomere could be the consequence of increased insulin resistance (hyperinsulinemia) as observed in Type 2 diabetes[32] with associated hyperglycemia, increased oxidative stress and generation of reactive oxygen species (ROS).[33] Moreover, in vitro studies also unveil the liability of hyperinsulinemia to the neoplasm.[34,35]
Hyperinsulinemia
Insulin resistance and hyperinsulinemia are the characteristics of Type 2 diabetes. Hyperinsulinemia and hyperglycemia are the main channels via which diabetes can prompt neoplastic growth.[36] An experimental study in MKR transgenic mice model of Type 2 diabetes has shown that breast cancer development is induced by hyperinsulinemia, making cells more receptive to mitogenic and survival signals, thus implicating its tumor promoting activity.[37] Studies demonstrating that addition of insulin promote the growth of mammary carcinoma in rat, stresses on the growth stimulating properties of insulin.[38,39]
Individuals with Type 2 diabetes have increased the bioactivity of insulin like growth factor-1 (IGF-1) by escalating its synthesis and by decreasing IGF-I-binding proteins.[36,40] Chronic hyperinsulinemia as a consequence of insulin resistance in Type 2 diabetes instigates a set of metabolic reactions leading to increase in the production of IGF-1 by liver cells.[41] IGF-1, a multipotent growth factor has potent mitogenic properties that can promote carcinogenesis.[36,42] In an animal study model, IGF-1 has been found to play a pivotal role in tumor growth and liver metastasis.[43]
Various studies and meta-analyses have analogized higher level of IGF-1 with an increased risk of cancer.[41,44] This emphasizes the fact that hyperinsulinemia results in tumor development as a sequelae of overproduction of IGF-1 by hepatocytes. It has been shown that decreasing IGF-1 level by restricting calorie intake can decrease tumor growth and higher IGF-1 corresponds to enhanced tumor development and metastasis.[45,46,47]
Under normal circumstances, most of the insulin are directed toward the metabolic activity of the cell mediated by phosphoinositide 3-kinase (PI 3-kinase). However, in the condition of insulin resistance, compensatory hyperinsulinemia potentiates the mitogenic pathway (mitogen-activated protein kinases [MAPK] pathway) and impairs the PI 3-kinase pathway thereby enhancing cell proliferation[48,49] [Figure 1].
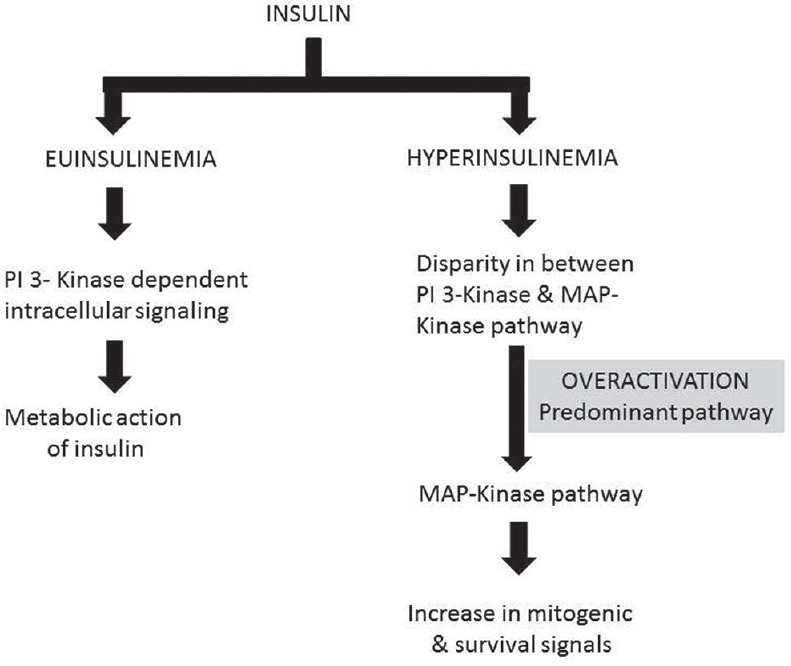
| Fig. 1 Proclivity for activation of mitogen-activated protein kinases pathway activation enhances cell proliferation under high insulin concentrations
Hyperglycemia
Before diagnosing clinical diabetes, hyperglycemia is the most pathognomonic clinical sign of diabetes reflected in impaired glucose tolerance test. The increase in cancer incidence has been found not only in diabetics but also in prediabetics.[50] Aggressiveness of tumor; thus associated morbidity and mortality increase linearly with hyperglycemia.[51]
Glucose is considered to be the source of energy for cells, then how and why does its increased availability nurture tumor evolution? In 1920s, Otto Heinrich Warburg made an independent observation that cancer cells prefer glycolysis over oxidative phosphorylation despite the presence of oxygen (Warburg effect or aerobic glycolysis).[22] This glycolytic phenotype has been demonstrated in many neoplasms which also is the basis of PET imaging technique using glucose analog tracer.[23] Although glycolysis is an inefficacious mean to generate adenosine triphosphate, its ability to provide carbon skeleton for synthesis of new molecules required for sustained cell proliferation, explains its partisanship by cancer cells.[22]
In Type 2 diabetes, compensatory hyperinsulinemia coexists with hyperglycemia. Proliferation and three-dimensional-migration assay discloses that diabetogenic glucose and insulin concentration fostered the multiplication and migration respectively, of tumor cell lines and also altered the gene activity of transcripts involved in regulating cell cycle.[52] Hyperglycemia results in excessive generation of ROS (superoxide) by the mitochondrial electron transport chain.[53] This raised level of ROS can further activate aerobic glycolysis in cancer cells[22] and damage cellular DNA, thus playing a key role in multistep carcinogenesis. ROS are prerequisites for the proliferation of cancer cells and anchorage-dependent growth via MAPK/ERK1/2 signaling pathway.[54,55]
Redox homeostasis regulates cytochrome c-mediated apoptosis, therefore excess of glutathione as an outcome of enhanced glucose metabolism results in decreased cancer cell apoptosis.[56] Furthermore, a shift in glucose metabolism elevates lactate levels and thus confers a selective growth advantage to cancer cells over normal cells.[52] Lactate also induces acidification of tumor milieu, thereby promoting tumor cell invasion and metastasis.[23,57]
Hyperinsulinemia and hyperglycemia, the major characteristics of Type 2 diabetes can boost tumor development independently as well as synergistically. Being a metabolic hormone, increased availability of insulin via its mitogenic effects can promote malignancy, which is assisted in number of ways by high glucose concentration. Potentiating this effect is the associated genomic instability secondary to Type 2 diabetes.
PROSPECTS OF DIABETES TRIGGERING EPSTEIN-BARR VIRUS ASSOCIATED NATURAL KILLER/T-CELL LYMPHOMA
An increased incidence of various virus associated diseases has been reported in diabetes[36,58] with an increased frequency of infection and associated morbidity.[59] This is attributed possibly to impaired phagocytosis,[60] immune defects, decreased neutrophil activity, and cytokine production[61] [Figure 2]. Defective neutrophil functioning is due to faulty oxidative burst capacity as a result of impaired NADPH oxidase activity, thus reducing intracellular superoxide ion production, under high glucose concentration.[62]
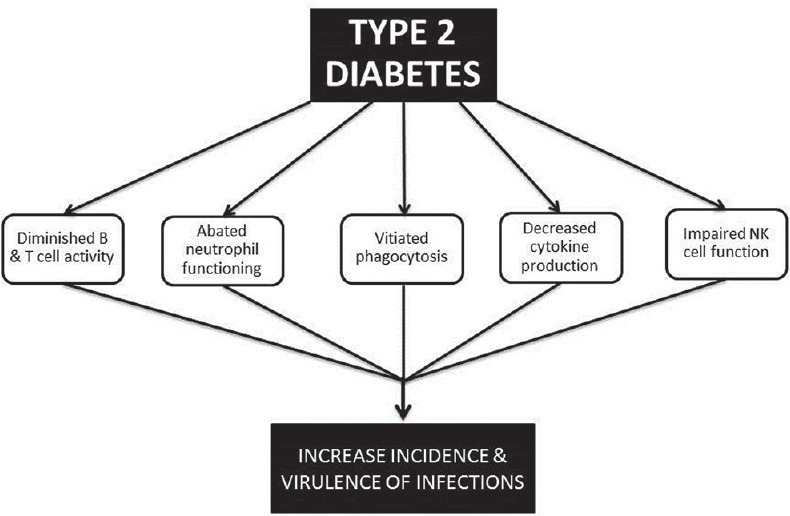
| Fig. 2 Underlying mechanisms in Type 2 diabetes, predisposing to infections/cancer
In patients with Type 2 diabetes, decreased expression of natural killer (NK) cell receptors NKp46 and NKG2D, capable of recognizing tumor ligands was found, along with functional inadequacy, i.e., decreased degranulation following stimulation.[63]
The overall effect of the feeble humoral and cell-mediated[58] immune response in Type 2 diabetes, is an increased susceptibility to diverse infections and also the considerable risk of reactivation of latent virus promoting virus replication and tissue tropism. Kumar et al. has demonstrated an increased mortality and high virus titer in diabetic mice compared to wild type when infected with West Nile virus.[64]
de Martel et al. in their analysis mentions that more than 2 million cases of cancer every year are attributed to infectious agents, which are categorized as carcinogenic to humans by the International Agency for Research on Cancer.[65] EBV, a Group 1 carcinogen[66] is an infectious ubiquitous virus affecting more than 90% of the population around the globe. Increased incidence of EBV-linked lymphoproliferative disorders has been documented in individuals with compromised immunity.[67,68]
EBV or human herpes virus 4 is a linear ds DNA virus; member of genus lymphocryptovirus, subfamily gammaherpesvirinae, family Herpes viridae. In vivo infection with EBV can vary from self-limited infectious primary disease to aggressive neoplasms[68,69] on reactivation following latency after a period of an asymptomatic infection. The virus persists as an episome within the cell instituting a latent infection.
However, apart from existing as an episome, EBV can also integrate with the genome as demonstrated in Namalwa, IB4, EB2, lymphoblastoid cell lines. This integration which can occur shortly after infection, as observed in mantle cell lymphoma cell lines, can result in chromosomal aberration and persuade oncogenicity in the juxtaposed genes.[66]
EBV replicates in epithelial cells and entrenches a latent infection in the lymphocytes. Latency and lytic cycle are two definite stages of EBV life cycle. Latency is the quiescent phase, which on reactivation switches to lytic stage. This virus shows four latency programs[69,70] varying from true latency (latency 0) to growth program (latency III) [Table 1].
Table 1
Different latency programs of Epstein-Barr virus

Latent proteins of EBV comprises of six EBV nuclear antigen (EBN1, EBN2, EBN3A, EBN3B, EBN3C, LP) and three latent membrane proteins (LMP1, LMP2A, LMP2B). EBV infected cells and associated tumors in immunodeficient individuals usually show expression of all these nine latency-associated viral proteins, referred to as latency III.[7,70,71]
EBV-associated neoplasms show a distinctively high incidence in some ethnic groups. It has been linked to African Burkitt's lymphoma, Cantonese cancer, which are rare in other parts of the world.[72,73]
Nasopharyngeal carcinoma, gastric cancer, hepatocellular carcinoma, adenocarcinoma of lung in female nonsmokers, and nasal NK/T-cell lymphoma are frequently seen in individuals of East/SEA ethnicity.[8] ENKTCL - nasal type, an aggressive lymphoproliferative disorder associated with EBV in almost all instances, is prevalent in South-East Asia, Central and South America.[9,10,74,75]
The immuno-incompetent state associated with EBV-positive lymphoproliferative malignancies.[69,72,76] Could be plausibly explained by switch in latency program of EBV in the immunocompromised state,[76] where T lymphocytes specific for EBV latent proteins cannot control the expansion of infected and activated B-cells as observed in lymphomatoid granulomatosis[77] and posttransplant lymphoproliferative disorders.[78]
Epstein-Barr virus and telomere
A study in transgenic mice has proven that LMP-1 of EBV is the principal transforming protein and can act as an oncogene by persuading expression of bcl-2, mcl-1, A20 and c-myc genes, and activating NF-ĸB, thus promoting lymphomagenesis.[79]
As for Type 2 diabetes, EBV infection also encourages genomic instability as a consequence of telomere dysfunction caused by EBNA; as was demonstrated in lymphoma cell lines. This EBNA causes telomere uncapping and shortening by inducing oxidative stress.[80] Short telomere has been implicated as a risk factor to develop aggressive NHL.[81] Aging is also another risk factor for NHL along with increased telomere attrition.[82]
Epstein-Barr virus associated extranodal natural killer/T-cell lymphoma-nasal type
ENKTCL is an EBV-associated NHL with an anatomical predilection for nasal cavity and upper aerodigestive tract.[9,10] Previously termed “angiocentric T-cell lymphoma” by Revised European American Lymphoma Classification,[83] the WHO Classification (2008) renamed it as “ENKTCL — nasal type.”[84]
The typical features of ENKTCL are necrosis following granulomatous-like lesions affecting the midface invading rapidly into adjoining hard and soft tissues.[85,86] FasL/Fas interaction in tumor cells and perforin-mediated pathway are possible molecular means along with angioinvasion and angiodestruction, for extensive local tissue involvement.[87,88] Hemophagocytic syndrome characterized by substantial phagocytosis of hematopoietic cells by macrophages may also be associated with ENKTCL.[89]
The majority of ENKTCL cases present with wide-ranging protean clinical manifestations mimicking numerous reactive and nonreactive processes.[90,91] Widespread necrosis, the sin-qua-non for this lymphoma; frequently leads to diagnostic dilemmas. Tumor cells are usually positive for CD56, CD2, cytoplasmic CD3 along with cytotoxic molecules viz., perforin, granzyme B-, and T-cell restricted intracellular antigen.[9,75]
CONCLUSION
The extensive epidemiological studies and evidences from laboratory studies on animal models suggest that hyperglycemia and insulin resistance has a strong association with increased incidence of various malignancies, related or unrelated to an infectious agent.
Among various cancer-linked pathogens, EBV has gained attention in past few decades. Its ubiquitous distribution has associated it with various neoplasms few of which are restricted to particular geographic regions. With unique life cycle and an ability to upgrade its latency state, this virus can survive for an entire lifetime in a healthy host and can lead to significant health care problems under conditions of immune dysfunction. Decreased immunity can lead to reactivation of this virus and hence provides a suitable milieu to express its latency genes as seen in posttransplant lymphoproliferative disorders.
With an upsurge in DM Type 2 cases; India will be the “diabetes capital of the world” in the next few decades. A dysregulated immune system secondary to Type 2 diabetes; predisposes to numerous complications one of which is reactivation of EBV. It is postulated that one of the reasons for the reported increase in the incidence of ENKTCL in the Indian studies could be the reactivation of EBV and subsequent progression to ENKTCL under the conditions of metabolic stress and debilitated immunity.
Finally, only more studies will validate this observation of a rise in the incidence of ENKTCL in the Indian subcontinent before considering it as an endemic lymphoreticular malignancy in the subset of diabetic population of this geographic region. Health care practitioners should carefully exclude various infectious/inflammatory diseases under the setting of diabetes and screen all patients for EBV; as EBV-linked ENKTCL can imitate various infectious processes.
This article reviews the epidemiological evidence linking various neoplasms with Type 2 DM and prognosticates the emergence of ENKTCL as a common lymphoreticular malignancy secondary to Type 2 diabetes, in the Indian population in next few decades [Figure 3].
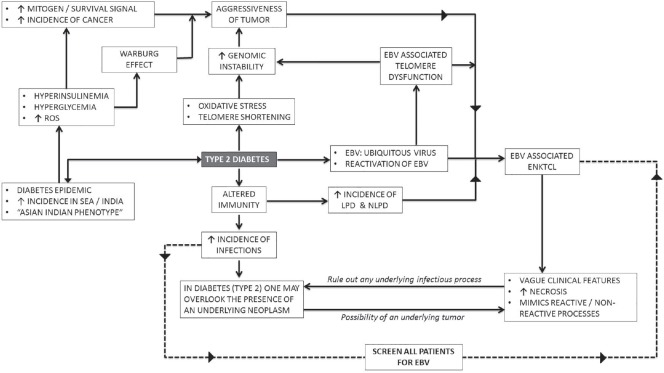
| Fig. 3 Flowchart depicting the nexus between Type 2 diabetes, Epstein-Barr virus, and extranodal natural killer/T-cell lymphoma
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES

| Fig. 1Proclivity for activation of mitogen-activated protein kinases pathway activation enhances cell proliferation under high insulin concentrations


 PDF
PDF  Views
Views  Share
Share

